ISSN: 0973-7510
E-ISSN: 2581-690X
Ten substrates viz., Rice husk alone 100%, Rice husk 50% + Pigeonpea husk 50%, Rice husk 50% +Sorghum grain 50%, Rice husk 50% + Cow dung 50%, Pigeonpea husk alone 100%, Pigeonpea husk 50% + Sorghum grain 50%, Pigeonpea husk 50% + Cow dung 50%, Sorghum grain alone 100%, Sorghum grain 50% + Cow dung 50% and Cow dung 100% were taken up for mass multiplication of five isolates (2 of T. harzianum, 2 of T. viride and 1 of T. virens) were selected for study to find out the best mycelium growth and conidial production. In T18 (T. harzianum) the best sporulation was recorded in Rice husk 50% + Sorghum grain 50% after 21 days of incubation. In T25 (T. harzianum), the best sporulation was found in sorghum grain only even after 28 days of incubation. In T26 (T. viride), best sporulation was observed in rice husk alone at 100%. In T30 (T. virens), the best sporulation was found in Rice husk alone (100%).
Biocontrol agent; mass multiplication; Organic substrates; Days; Trichoderma spp.
Biopesticides are now preferred than chemical pesticides because of several advantages like target specificity, no residual effect, promotion of crop growth and most significantly maintaining the ecological balance. The main agents currently employed as biocontrol agents include bacteria viz., fluoroscent pseudomonads (P. fluorescens and P. putida), Bacillus spp. and fungi viz., Trichoderma spp. Although several other bacteria and fungi have been reported to be effective biocontrol agents, Trichoderma spp. as bio-pesticides have better scope of commercial exploitation amongst fungi (Mukerji and Garg, 1993 a&b). The major limitation is the lacking of appropriate mass culturing techniques of T. harzianum and inadequate information on the suitability of carrier materials (Harman and Taylor, 1990). There are some reports, where T. harzianum has been formulated as bio-fungicides in various carrier materials including wheat bran, rice bran, maize bran, sawdust (Das et al., 1997 and Rajput et al., 2014); rice straw, chickpea bran, grass pea bran, rice course powder, black gram bran (Shamsuzzaman et al., 2003); cow dung, poultry manure, ground nut shell, black ash, coir waste, spent straw from mushroom bed, talc, vermiculite (Rettinassababady and Ramadoss, 2000). Therefore, the present study was undertaken to find out the effective local organic substrates and also made to enhance the conidial population of these substrates using nutritional supplements.
The mass multiplication is an important aspect for production and commercialization of biological product. This study was initiated for identification and development of cheaper substrates for mass multiplication of the antagonists for formulation. The substrates used, were rice husk, pigeonpea husk, sorghum grain, cow dung (fresh dried) and its combinations. Then in each 100 g rice husk alone plus 40 ml distilled water (water added on the basis of soaking capacity of the substrate) and standardized by adjusting the moisture level to 50 per cent (w/v) (Sangle et al., 2002) was taken into autoclavable polythene transparent bags (25 x 20 cm). Rice husk (50 g) plus pigeonpea husk (50 g) plus 50 ml distilled water, rice husk 50 g plus sorghum grain 50 g (sorghum grain boiled first up to soft broken grain) plus 20 ml water, 50 g rice husk plus 50 g dried cowdung powder plus 40 ml water. Next combination were used pigeonpea husk 100 g plus 60 ml water, then combination of pigeonpea husk 50 g plus 50 g boiled (soft) sorghum grain plus 50 ml water, pigeonpea 50 g plus 50 g dried cowdung powder plus 60 ml distilled water. Further alone 100 g sorghum grain (soft) and its combination 50 g sorghum grain (soft) plus 50 g cowdung and 40 ml water was added. Another 100 g cowdung alone plus 50 ml water were prepared.
All substrates was filled in bags, cotton plugs were placed on the open ends of these bags and then autoclaved at 15 psi for 1 h on two consecutive days. The bags were inoculated with two mycelial discs of 9 mm each, cut out from the periphery of 7 days old sporulating cultures of T. harzianum, T. viride and T. virens and mixed homogenously. Three replications per treatment were maintained and incubating at 25+1 0C under alternate 12 h light and 12 h darkness conditions.
One gram of the substrate from each treatment was drawn aseptically after thorough mixing within the bags at 7, 14, 21 and 28 days of incubation. Each sample was serially diluted with distilled sterile water adding 0.1% sterile Tween-X and the spore count was recorded (Sangle et al., 2005). The spores per g of the substrate were recorded by haemocytometer.
Spore load was calculated by the following formula.
Spore load in 1 ml solution = N x 1000
X
Where,
N = Total No. of spores counted /number of squares
X = Volume of solution between the cover glass and above the squares counted the
Square x depth of chamber.
Five isolates (2 isolates of T. harzianum, 1 isolates of T. viride and 1 isolate of T. virens) were selected for study to find out the best growth substrates for sporulation of the above isolates.
Isolate T18 (T. harzianum)
This isolate showed lower sporulation potential compared to the T13 isolate of T. viride (whereas a maximum of 209.17 x 109 spores/g were recorded in T13, the maximum in case of T18 was 177.50 x 109 spores/g). T18 showed good sporulation after 7 days of incubation in some of the substrates and their combinations (Table 1), the best being in rice husk alone (47.50 x 109 spores/g) and rice husk + sorghum grains (54.17 x 109spores/g). The second best substrates were rice husk + pigeon pea husk, rice husk + cow dung and sorghum grains alone. The least sporulation was observed in pigeon husk alone (5.83 x 109spores/g) and in cow dung alone. Sporulation reached the peak in most of the substrates and their combinations after 14 days of incubation; rice husk alone and its combination with either pigeon pea husk or sorghum grains were the best with the sporulation at par statistically and ranging from 150.83 to 177.50 x 109spores/g. After 14 days of incubation, the least sporulation was recorded in pigeon pea husk alone and cow dung alone. Similar trends continued after 21 and 28 days of incubation and there was no distinct advantage to incubate the substrate beyond 14 days as only marginal increases were noticed (Table.1 and Fig.4).
Table (1):
Sporulation of isolate No. T18 (T. harzianum) on organic substrates and their combinations.
| Treatment | No. of spores, days after inoculation ( x 109 spores / g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |||||
| Mean | + SD | Mean | + SD | Mean | + SD | Mean | +SD | |
| 1. Rice husk alone 100% | 47.50a | 2.50 | 177.50a | 16.39 | 169.17ab | 6.29 | 167.50a | 15.21 |
| 2. Rice husk 50% + Pigeonpea husk 50% | 31.67b | 6.29 | 150.83ab | 1.44 | 155.83ab | 6.29 | 146.67a | 22.55 |
| 3. Rice husk 50% + Sorghum grain 50% | 54.17a | 5.20 | 177.50a | 9.01 | 175.83a | 3.82 | 167.50a | 13.23 |
| 4. Rice husk 50% + Cow dung 50% | 35.00b | 11.46 | 145.00b | 5.00 | 154.17b | 7.64 | 150.83a | 1.44 |
| 5. Pigeonpea husk alone100% | 5.83d | 3.82 | 45.83de | 3.82 | 53.33de | 21.26 | 48.33d | 18.93 |
| 6. Pigeonpea husk 50% + Sorghum grain 50% | 18.33cd | 7.64 | 62.50d | 2.50 | 65.00d | 17.32 | 69.17c | 6.29 |
| 7. Pigeonpea husk 50% + Cow dung 50% | 12.50cd | 8.66 | 46.67de | 2.89 | 41.67e | 14.65 | 47.50d | 4.33 |
| 8. Sorghum grain alone 100% | 34.17b | 10.10 | 110.83c | 20.36 | 149.17b | 3.82 | 155.83a | 5.20 |
| 9. Sorghum grain 50% + Cow dung 50% | 24.17bc | 3.82 | 98.33c | 41.93 | 128.33c | 5.77 | 124.17b | 3.82 |
| 10. Cow dung 100% | 8.33d | 6.29 | 24.17e | 3.82 | 35.00e | 10.90 | 24.17e | 1.44 |
| CD at 5% | 12.14 | 27.43 | 19.31 | 19.99 | ||||
Isolate T25 (T. harzianum)
Data presented in Table 2 Figure 3 revealed that two substrates viz., rice husk + sorghum grain and sorghum grain alone showed highest sporulation (49.17 x 109 spores/g) in both the substrates after 7 days of incubation. However, statistically the other substrates, viz., rice husk alone, pigeon pea husk + sorghum grain, and sorghum grain + cow dung were at par with the above two substrates. Peak spore production reached after 14 days of incubation and rice husk alone or sorghum grains alone were the best. The sporulation tapered at 21 days and further at 28 days of incubation. Least sporulation was recorded in cow dung alone even at the end of 28 days incubation.
Table (2):
Sporulation of isolate No. T25 (T. harzianum) on organic substrates and their combinations.
| Treatment | No. of spores, days after inoculation ( x 109 spores / g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |||||
| Mean | + SD | Mean | + SD | Mean | + SD | Mean | +SD | |
| 1. Rice husk alone 100% | 44.17ab | 6.29 | 180.00a | 7.50 | 182.50ab | 5.00 | 173.33b | 3.82 |
| 2. Rice husk 50% + Pigeonpea husk 50% | 32.50bc | 5.00 | 168.33ab | 5.20 | 175.00b | 2.50 | 165.83bcd | 5.77 |
| 3. Rice husk 50% + Sorghum grain 50% | 49.17a | 3.82 | 164.17bc | 5.20 | 174.17b | 3.82 | 170.00bc | 7.50 |
| 4. Rice husk 50% + Cow dung 50% | 27.50cd | 4.33 | 150.83c | 5.20 | 160.83c | 2.89 | 155.83ef | 5.20 |
| 5. Pigeonpea husk alone100% | 31.67bc | 11.55 | 150.83c | 18.76 | 158.33c | 10.41 | 152.50f | 5.00 |
| 6. Pigeonpea husk 50% + Sorghum grain 50% | 40.83abc | 3.82 | 168.33ab | 5.20 | 163.33c | 1.44 | 163.33cde | 3.82 |
| 7. Pigeonpea husk 50% + Cow dung 50% | 16.67d | 10.41 | 120.83d | 7.22 | 130.00c | 6.61 | 129.17g | 5.20 |
| 8. Sorghum grain alone 100% | 49.17a | 3.82 | 171.67ab | 2.89 | 185.00a | 4.33 | 185.00a | 4.33 |
| 9. Sorghum grain 50% + Cow dung 50% | 35.83abc | 10.10 | 160.00bc | 4.33 | 157.50c | 5.00 | 158.33def | 3.82 |
| 10. Cow dung 100% | 16.67d | 10.41 | 60.83e | 10.10 | 59.17e | 1.44 | 55.00h | 2.50 |
| CD at 5% | 12.96 | 14.22 | 8.59 | 8.30 | ||||
Isolate T26 (T. viride)
This isolate of T. viride had high sporulation, though initially after 7 days incubation, the sporulation was similar to other Trichoderma isolates. Maximum sporulation was recorded in rice husk alone, rice husk + pigeon pea husk, rice husk + sorghum grain and sorghum grain alone (Table 4) after 7 days incubation; the least being in cow dung alone. After 14 days of incubation, the sporulation picked up and was maximum in this isolate at the end of 21 days of incubation, the best being rice husk alone (223.33 x 109spores/g) and rice husk + pigeon pea husk (219.17 x 109spores/g). The minimum sporulation was seen in case of cow dung alone even after 28 days incubation (69.17 x 109spores/g) (Table 3 and Fig 1).
Table (3):
Sporulation of isolate No. T26 (Trichoderma viride) on organic substrates and their combinations.
| Treatment | No. of spores, days after inoculation ( x 109 spores / g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |||||
| Mean | + SD | Mean | + SD | Mean | + SD | Mean | +SD | |
| 1. Rice husk alone 100% | 40.00a | 13.23 | 151.67ab | 9.46 | 223.33a | 23.76 | 204.17a | 8.04 |
| 2. Rice husk 50% + Pigeonpea husk 50% | 40.00a | 11.46 | 160.00a | 18.03 | 219.17a | 12.33 | 200.00ab | 2.50 |
| 3. Rice husk 50% + Sorghum grain 50% | 35.00ab | 2.50 | 159.17a | 8.04 | 191.67b | 19.09 | 183.33bc | 19.09 |
| 4. Rice husk 50% + Cow dung 50% | 23.33bc | 2.89 | 115.83b | 46.26 | 172.50bc | 16.39 | 179.17c | 3.82 |
| 5. Pigeonpea husk alone100% | 20.83c | 11.81 | 138.33ab | 26.73 | 141.67d | 10.41 | 125.00e | 5.00 |
| 6. Pigeonpea husk 50% + Sorghum grain 50% | 20.83c | 3.82 | 143.33ab | 10.10 | 177.50bc | 11.46 | 157.50d | 15.61 |
| 7. Pigeonpea husk 50% + Cow dung 50% | 20.83c | 3.82 | 149.17ab | 3.82 | 155.00cd | 18.03 | 158.33d | 19.09 |
| 8. Sorghum grain alone 100% | 45.83a | 3.82 | 144.17ab | 11.27 | 184.17b | 17.74 | 197.50abc | 4.33 |
| 9. Sorghum grain 50% + Cow dung 50% | 25.83bc | 6.29 | 142.50ab | 15.21 | 152.50cd | 9.01 | 147.50d | 9.01 |
| 10. Cow dung 100% | 1.67d | 1.44 | 70.83c | 9.46 | 75.83e | 1.44 | 69.17f | 6.29 |
| CD at 5% | 12.58 | 33.62 | 25.90 | 18.83 | ||||
Isolate T30 (T. virens)
Isolate No. T30 (T.virens)
This isolate showed better sporulation at 7 days of incubation (Table 4 and Fig 4) and most of the substrates and their combinations were at par except rice husk + cow dung, pigeon pea husk + cow dung and pigeonpea husk alone, which recorded a lower spore count. The sporulation picked up after 14 days of incubation and became maximum, or reached plateau after 21 days of incubation. After 21 days the best substrates were rice husk alone (182.50 x 109spores/g) and rice husk + pigeon pea husk (173.33 x 109spores/g). The sporulation only marginally increased after 28 days of incubation or reduced, and it was lowest in cow dung alone (42.50 x 109spores/g).
Table (4):
Sporulation of isolate No. T30 (T. virens) on organic substrates and their combination (T. virens).
| Treatment | No. of spores, days after inoculation ( x 109 spores / g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |||||
| Mean | + SD | Mean | + SD | Mean | + SD | Mean | +SD | |
| 1. Rice husk alone 100% | 40.00ab | 13.23 | 161.67ab | 19.42 | 182.50a | 9.01 | 170.83a | 7.22 |
| 2. Rice husk 50% + Pigeonpea husk 50% | 40.83ab | 3.82 | 164.17a | 10.10 | 173.33ab | 1.44 | 171.67a | 1.44 |
| 3. Rice husk 50% + Sorghum grain 50% | 33.33abc | 7.22 | 166.67a | 7.22 | 163.33bc | 11.27 | 171.67a | 2.89 |
| 4. Rice husk 50% + Cow dung 50% | 27.50bc | 4.33 | 135.00cd | 5.00 | 156.67c | 9.46 | 147.50c | 2.50 |
| 5. Pigeonpea husk alone100% | 34.17abc | 10.10 | 157.50ab | 15.61 | 159.17bc | 5.77 | 151.67c | 7.64 |
| 6. Pigeonpea husk 50% + Sorghum grain 50% | 38.33ab | 6.29 | 159.17ab | 10.10 | 157.50c | 6.61 | 149.17c | 3.82 |
| 7. Pigeonpea husk 50% + Cow dung 50% | 20.83cd | 3.82 | 116.67d | 10.41 | 118.33d | 9.46 | 110.00d | 15.21 |
| 8. Sorghum grain alone 100% | 44.17a | 6.29 | 157.50ab | 10.90 | 162.50bc | 6.61 | 166.67ab | 7.22 |
| 9. Sorghum grain 50% + Cow dung 50% | 37.50ab | 6.61 | 140.00bc | 13.23 | 160.00bc | 6.61 | 158.33bc | 5.20 |
| 10. Cow dung 100% | 12.50d | 6.61 | 45.83e | 7.22 | 43.33e | 10.10 | 42.50e | 4.33 |
| CD at 5% | 12.56 | 19.84 | 13.80 | 11.69 | ||||
Besides their effectiveness, the main hindrance in the widespread application of biocontrol agents like T. polysporum is their large scale availability for field use. Many workers have tried number of substrates such as rice grain, sorghum grain, millet grain, cotton cake, mustard cake, wheat straw, wheat bran, cotton waste, maize stover, rice straw, saw dust, sugarcane bagasse, sugarcane ash, farmyard manure (FYM) and wheat bran for mass multiplication of bio-control agents (Rettinassababady and Ramadoss, 2000; Saju et al., 2002). During the present studies sorghum grains followed by millet grains appeared as the most effective substrates in which highest populations of T. polysporum were recorded. Our results are in confirmation to those reported by Panday et al. (2012) who also found that sorghum grains produced significantly more population of Trichoderma species than other substrates.
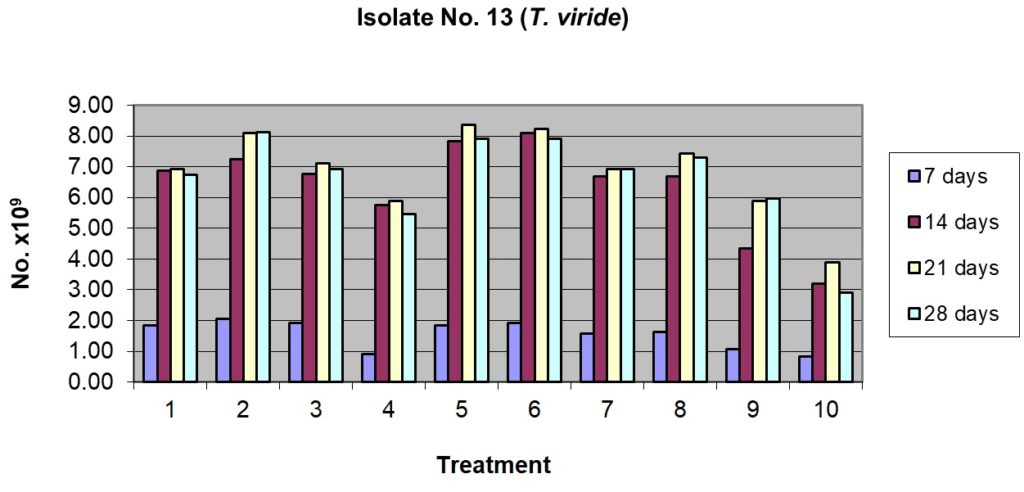
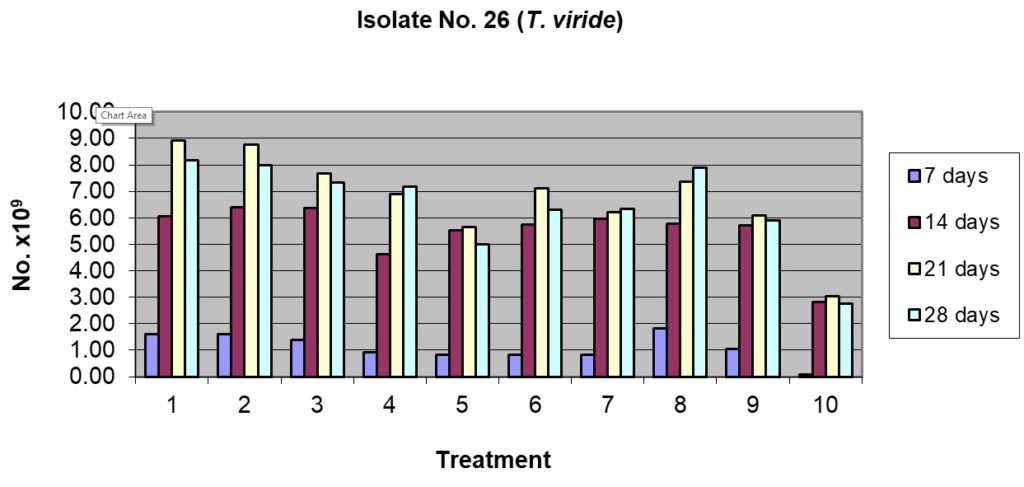
1= Rice husk alone 100%; 2= Rice husk 50% + Pigeonpea husk 50%
3= Rice husk 50% + Sorghum grain 50%; 4= Rice husk 50% + Cow dung 50%
5= Pigeonpea husk alone100%; 6= Pigeonpea husk 50% + Sorghum grain 50%
7= Pigeonpea husk 50% + Cow dung 50%; 8= Sorghum grain alone 100%
9= Sorghum grain 50% + Cow dung 50%; 10= Cow dung 100%
Figs. 1 & 2. Effect of sporulation of selective Trichoderma spp. isolates in combination with different nutrient substrates
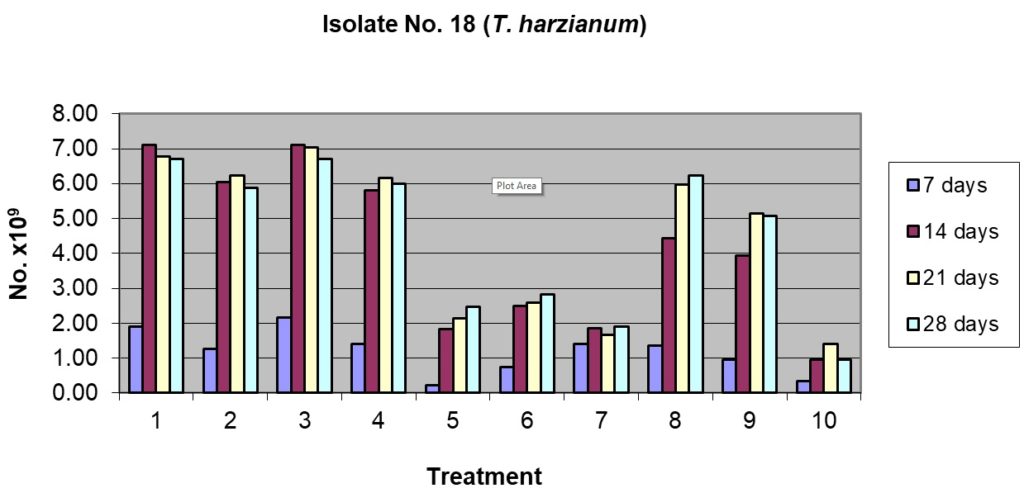
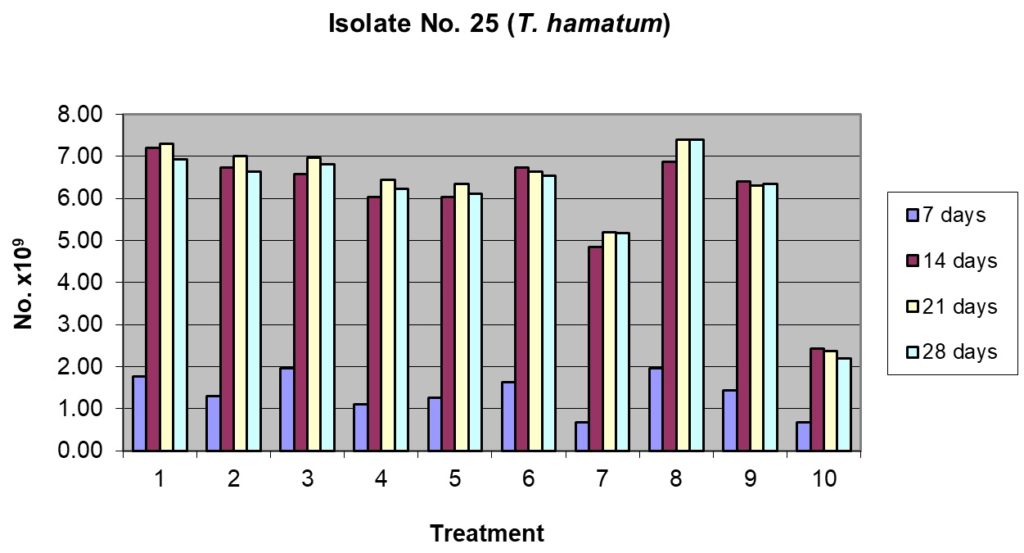
1= Rice husk alone 100%; 2= Rice husk 50% + Pigeonpea husk 50%
3= Rice husk 50% + Sorghum grain 50%; 4= Rice husk 50% + Cow dung 50%
5= Pigeonpea husk alone100%; 6= Pigeonpea husk 50% + Sorghum grain 50%
7= Pigeonpea husk 50% + Cow dung 50%; 8= Sorghum grain alone 100%
9= Sorghum grain 50% + Cow dung 50%; 10= Cow dung 100%
Figs. 3 & 4. Effect of sporulation of selective Trichoderma spp. isolates in combination with different nutrient substrates
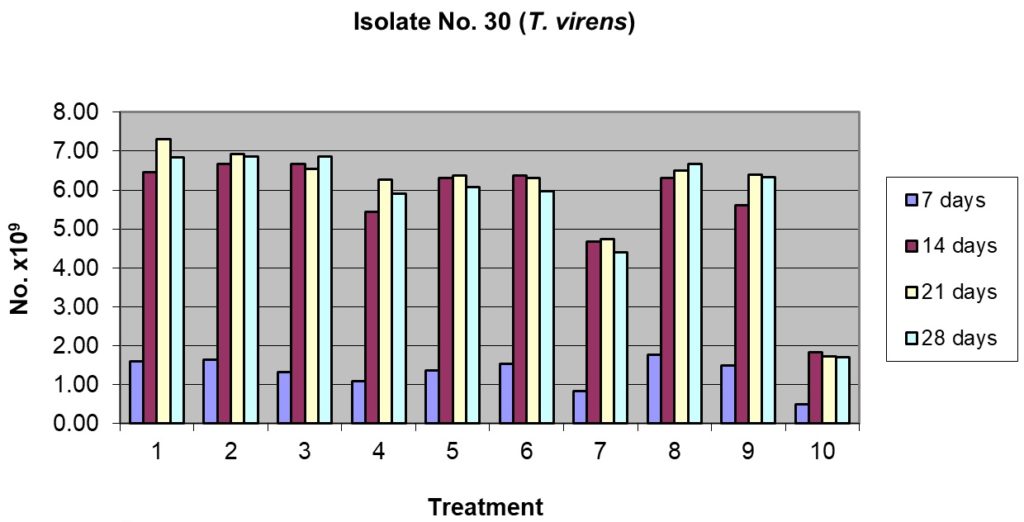
1= Rice husk alone 100%; 2= Rice husk 50% + Pigeonpea husk 50%
3= Rice husk 50% + Sorghum grain 50%; 4= Rice husk 50% + Cow dung 50%
5= Pigeonpea husk alone100%; 6= Pigeonpea husk 50% + Sorghum grain 50%
7= Pigeonpea husk 50% + Cow dung 50%; 8= Sorghum grain alone 100%
9= Sorghum grain 50% + Cow dung 50%; 10= Cow dung 100%
Fig. 5. Effect of sporulation of selective Trichoderma spp. isolates in combination with different nutrient substrates
The production and commercialization of biocontrol products is a dynamic process and the most critical obstacle is the paucity of methods for mass multiplication and delivery of antagonists (Papavizas, 1985). The mass culturing of Trichoderma harzianum was first attempted by Wells et al. (1972) using rye grass seeds + soil as substrate. Elad et al. (1980) prepared a medium consisting of wheat bran. Saw dust and tap water at the proporation of 3:1:4 for the mass multiplication of Trichoderma.
Kousalya and Jeyarajan (1990) used seven organic substrates, viz. farm yard manure, bio-gas slurry, coir waste, press mud, tapioca waste (product from sago industry), rice husk and pigeonpea husk were screened with and without additives, viz. urea and ammonium sulphate individually at 0.1 percent for the mass multiplication of T. harzianum. The above substrates were collected (200 g) sterilized in polypropylene bags and inoculated with the spore suspension of T. harzianum (x 108 cells / ml) @ 2 ml/ bag. The nitrogenous additives (urea and ammonium sulphate) were individually incorporated at 0.1 percent to the substrates before inoculation. The bags were incubated at 280C for 15 days. After incubation the samples were drawn and the population of T. harzianum was estimated through plating in Trichoderma selective medium, (with metalxy1 0.3 g, PCNB 0.2 g, and chloramphenicol 0.25 g) (Elad and Chet, 1983). Amongst the substrates, pigeonpea husk was found to be the best and recorded the maximum population of propagules (63.5 x 106 cfu /g) Followed by tapioca waste (56.2 x 106 cfu /g) and press mud (50.8 x 106 cfu /g). Addition of urea to the substrates reduced the multiplication and population of Trichoderma in all the cases, while ammonium sulphate stimulated its growth. The highest population was recorded in pigeonpea husk (129.5 x 106 cfu / g) followed by tapioca waste (101.6 x 106 cfu /g) and FYM (86.5 x 106 cfu / g). Though wheat bran is said to be the best substrate (Elad et al., 1980; Sangle et al., 2003), the relatively higher cost prohibits its use for mass multiplication of Trichoderma on large scale. All the substrates used are relatively cheaper than wheat bran. Kousalya and Jeyarajan (1990) tried various substrates for mass multiplication of Trichoderma spp. of which tapioca rind and waste were found to be the best followed by FYM and pressmud.
ACKNOWLEDGMENTS
The author are grateful to the Director, ICAR Research Complex for Eastern Region, Patna, India for providing facility under Institute Project for doing this research and is duly acknowledged.
- Mukerji K.G., Garg K.L. Biocontrol of Plant Diseases, Vol. I. CBS Publishers & Distributors, Delhi, 1993a; pp 198.
- Mukerji K.G., Garg K.L. Biocontrol of Plant Diseases, Vol. II. CBS Publishers & Distributors, Delhi, 1993b; pp 211.
- Harman, G.E., Taylor, A.G. Development of an effective biological seed treatment system. In D. Hornby (Ed.), Biological control of soil-borne plant pathogens. Wallingford, UK: C.A.B. International, 1990;pp 415-426.
- Das, B. C., Roy, S. K., Bora, L. C. Mass multiplication of Trichoderma species on different media. J. Agricl. Sci. Soc. North-East India, 1997; 10(1): 95-100.
- Rajput, A.Q., Khanzada M.A., Shahzad, S. Effect of Different Organic Substrates and Carbon and Nitrogen Sources on Growth and Shelf Life of Trichoderma harzianum, 2014; 16(4): 731-745.
- Shamsuzzaman, Islam, S. M. A., Hossain, I. Trichoderma culture and germination of sweet gourd seed. Bangl. J. Seed Sci. Technol., 2003; 7(1/2): 91-95.
- Rettinassababady, C., Ramadoss, N. Effect of different substrates on the growth and sporulation of Trichoderma viride native isolates. Agricul. Sci. Dig., 2000; 20(3):150-152.
- Sangle, U.R., Wuike, R.V., Gade, R.M. Influence of original moisture present in the substrates on the growth and sporulation of Trichoderma spp. Pestology, 2002; 26: 46-48.
- Sangle, U.R., Mittal R.K., Bambawale, O.M. Cultural variability in Trichoderma spp. isolated from North-Eastern Hills of Tripura and Mizoram. J. Mycopathol. Res., 2005; 43(2): 215-218.
- Saju, K.A., Anandraj, M., Sharma, Y.R. Of Farm production of Trichoderma harzianum using organic matter. Indian Phtypath., 2002; 55 (3): 277-281.
- Pandya J., Sabalpara, A.N., Chawda, S.K., Waghunde, R. Grain substrate evaluation for mass cultivation of Trichoderma harzianum. J. Pure Appl. Microbiol., 2012; 66(4): 2029-2032.
- Papavizas, G.C. Trichoderma and Gliocladium: Biology, ecology and potential for biocontrol. Annu. Rev. Phytopathol., 1985; 23: 23-54.
- Wells, H.D., Bell, D.K., Jaworski, C.C. Efficacy of Trichoderma harzianum as a biocontrol for Sclerotium rolfsii. Phytopathology, 1972; 62: 442-447.
- Elad, Y., Chet, I., Katan, J. Trichoderma harzianum, a biocontrol agent effective against Sclerotium rolfsii and Rhizoctonia solani. Phytopathology, 1980; 70: 119-121.
- Kousalya, G., Jeyarajan, R. Mass multiplication of Trichoderma spp. J. Biol. Control, 1990; 4: 70-71.
- Elad, Y., Chet, I. Improved selective media for isolation of Trichoderma spp. or Fusarium spp. Phytoparasitica, 1983; 11: 55-58.
- Sangle, U.R., Bambawale, O.M., Nasim, A., Singh, S.K. Substrate and Temperature Requirements for Sporulation of Sub-tropical isolates of Trichoderma spp. Annl. Pl. Protect. Sci., 2003; 11(2): 192-195.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


