ISSN: 0973-7510
E-ISSN: 2581-690X
This study was conducted to develop an approach for reducing severe damage to grains caused by various insect pests using the biological control agent Cypovirus1 and silver nanoparticles, both alone and in combination. Four types of beetles that infest stored products, including drugstore beetle (Stegobium paniceum), saw-toothed grain beetle (Oryzaephilus surinamensis), warehouse beetle (Trogoderma variabile), and Cowpea weevil (Callosobruchus maculates), were collected from seven different areas in the Kingdom of Saudi Arabia, specifically Mecca regions. We determined the morphological characteristics of the pests in various grains. Corn borer pupae and larvae of corn plants infected with Cypovirus1 were collected from the stems of live and dead maize plants in fields in Mecca regions to isolate Cypovirus1. Infected samples were examined using triple antibody sandwich-enzyme-linked immunosorbent assay (TAS-ELIZA) and reverse transcription-polymerase chain reaction (RT-PCR). Transmission electron microscopy (TEM) analysis revealed virus-containing occlusion bodies inside infected cells of corn borer larvae with irregular shapes and dimensions of 2.2–4.9 µm. The effects of different concentrations of the virus activated by synthetic spherical silver nanoparticles with an average diameter of 36.32 nm on the larva of grain pests taken were evaluated, and all treatments led to an increased mortality percentage after 72 h compared to at 48 h. The treatment mixture consisting of 10/100 viral and 400 µg/kg AgNPs led to the highest average death rates of the four insect larvae at 72 h after treatment. Protein bands that were present in the virus-infected larvae of the four pests were absent from healthy larvae, indicating viral infection.
Biological Control, Cypovirus1, AgNPs, Insect Stored Products, KSA
Stored grains are attacked by approximately 30 types of stored grain pests, causing serious economic damage to harvested grains, including the drugstore beetle, saw-grain beetle, khapra beetle, and cowpea weevil.
The drugstore beetle is a common pest in grain worldwide because of its ability to adapt to many different environments,1 and can feed on various substances and grains. Because of these abilities, this beetle has been named as Stegobium paniceum.2,3
The saw-toothed grain beetle is distinguished by its dark brown color; its length ranges from 2.5 to 3.5 mm. This beetle belongs to the order Coleoptera and family Silvanidae. The saw-toothed grain beetle feeds on fried meats, chocolate, rolled oats, cereals, dried fruit, bran sugar, brown rice, and tobacco.4-7
The Khapra beetle, one of the five most important pests, is a serious exotic pest of grains and stored dry foodstuffs, and rankly highly as a target organism for exclusion by quarantine.8,9 Its distribution has been reviewed previously.10 The Khapra beetle belongs to the family Dermestidae.9
The family of the Coleoptera
Chrysomelidae includes many pests of harvested grains, the most important of which is the cowpea weevil.11 This major pest damages cowpea grains, specifically by reducing the grain germination rate and reducing the grain quality by reducing its weight. After the cowpea weevil lays its eggs on the cereal surface, larvae feed on the cotyledons of the grain.12
Many pesticides used to fumigate grain, including phosphine and methyl bromide, to combat grain pests have toxic effects on human health and negative impacts on the environment. Thus, in this study, we examined alternative approaches for grain pest control that are environmentally friendly with no effects on human health.13 We evaluated the effects of a bio-viral compound on the larvae of grain pests. Cypovirus1 has an icosahedral shape and single-shelled capsid and belongs to Reoviridae.14-19
The larvae show symptoms of viral infection at four days after Cypovirus1 inoculation, including sudden destruction of the central gastric tissue, causing the larvae to stop feeding and develop vomiting and diarrhea. These symptoms cause the larvae to die at approximately 10 days after viral infection.19-23
Synthetic spherical silver nanoparticles (AgNPs) with an average diameter of 36.32 nm can easily diffuse through the plasma membranes of cells, as its size is similar to those of cellular proteins.24,25 AgNPs affect the permeability of the plasma membrane of treated larval cells, their respiration, and their ability to molt and develop. These alterations leading to toxicity are easily observable.26
The objectives of this study were to investigate the effect of different concentrations of Crypovirus1 and synthetic spherical AgNPs on four different types of beetle larvae that infest stored food products.
Survey of four stored product beetle pests in Mecca regions
Four types of grain pests were collected in seven different areas in Mecca, for a total of 756 samples of drugstore beetle, saw-toothed grain beetle, warehouse beetle, and cowpea weevil. We collected 108 samples per location from four areas representing nine samples (three replicates) per area.
Morphological characteristics of the pests
The drugstore beetle,3 saw-toothed grain beetle,9 warehouse beetle,7 and cowpea weevil12 were separated based on the morphological characteristics of the adult insects and larvae.
Infection of four pests in stored product beetles with Cypovirus1
Collection of infected samples
Pupae and larvae of maize infected with Cypovirus1 were collected from the stems of live and dead maize plants from fields in the Mecca regions. Other samples were collected from maize borer larvae Sesamia calamistis using bait comprised of molasses and yeast in the soil.27
Pathogen identification
The collected corn borer larvae showed symptoms of infection with the virus such as very slow movement, abstinence from feeding, vomiting, and diarrhea, as evaluated serologically by indirect enzyme-linked immunosorbent assay as described previously.28 We confirmed the presence of viral particles and inclusion bodies in ultra-thin sections of the larvae using transmission electron microscopy and Cypovirus1 particles was purified using Larsen and Duffus protocol.29,30
Virus production
Virus-infected corn borer larvae were ground in sterile distilled water, followed by filtration through filter paper to obtain the viral suspension, which was prepared at a concentration of 1 larva/mL. A viral dilution was prepared at a concentration of 103 from the prepared viral suspension, and 1 mL of the viral suspension was pipetted onto a small disc of artificial diet (15–20 mm3) provided to the larvae to be infected with the virus. The prepared food tablets mixed with the viral suspension were introduced to the larvae at 24 h and placed in small glass tubes fitted with cotton wool stoppers. The discs were removed and replaced every 24 h, and symptoms of infection were monitored the larvae until death or pupation.
Isolation of total genomic RNA
We used standard guanidium isothiocyanate methods to purify the viral polyhedral structure and extract genomic RNA.31 The dsRNA fragments were separated in a 1% agarose gel containing a 1-kbp marker and stained with ethidium-bromide.
Synthesis and collection of spherical AgNPs (avg. 36.32 nm) using 8.0 mM of tri-sodium citrate dehydrate (C6H5O7Na3)
Sodium citrate (C6H5O7Na3 solution; 8.0 M, 20 mL) heated at 60°C (as a reducing agent and surfactant) was added to 80 mL of AgNO3 solution after heating at 60°C (as a raw material for manufacturing spherical AgNPs). After stirring under heat at 60°C for 20 min, the samples were cooled to room temperature 24°C with constant stirring.32-35
The reaction mechanism is expressed in the following equation32:
4Ag+ + C6H5O7Na3 + 2H2O → 4Ag0 + C6H5O7H3 + 3Na+ + H+ + O2 ↑
The shape and size of the AgNPs were evaluated using electron microscopy. The spherical colloidal AgNPs were also characterized by radiation with 0.15405 nm Cu Kα radiation using X-ray diffraction (model D5000 Siemens, Munich, Germany). as described previously.32-35
Preliminary assays with the propagated virus
Bio-component of (CPV) was prepared by mixture 1mg of powder infected larvae with 999mg inactivated integrate containing of carborandum and talcum.
Effect of Cypovirus1 activated by AgNPs on four beetle pests of stored products
The bio-viral component (10 mg) was added to 100 and 1000 mg of mixed stored products. Additionally, 200 and 400 µg AgNPs were added to 1 kg of mixed stored products. Additionally, 10 mg/1000 mg of virus solution was added to 400 µg of AgNPs. 180 larvae per each concentration divided into 60 larvae were used (all assays were replicated three times). Mortality was recorded daily as a percentage. The mortality rates and severity of infection were determined at 48 and 72 h after treatment.
Symptoms of infestation of the medicinal beetle have been recorded on collected stored products samples including ground coffee beans, ground cumin, ground root of Curcuma longa, wormwood chamomile flowers, wheat flour, ground fennel seeds, meat spices (mixture of seven spices), cinnamon sticks crushed, rice grains, and ground fenugreek seeds.
Protein production pattern of all larva stages following treatment with Cypovirus1 activated with AgNPs
Protein extraction
1 mL Extraction buffer (40ml SDS10%, 20ml Glycerol 15%, 8ml Tris 1M (pH=8.8), 1ml EDTA 0.5M, H2O up to 100ml) added to the healthy and infected larvae at different life stages (0.5 g); these samples were ground in liquid nitrogen to extract protein. The samples were centrifuged 500xg for 10 min at 4°C and stored at -20°C.
Polyacrylamide gel electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed in a 12% (w/v) (pH 8.8) separating gel and 5% (w/v) (pH 6.8) stacking gel as described previously.36 add enough Bio-Safe Coomassie Stain to completely cover the gel. Let stain for 1 hour on a shaker. If the protein signal is low, stain overnight. The protein molecular marker was from Sigma (St. Louis, MO, USA) and contained Albumin from Bovine serum (weight 66.0kDa), albumin from Egg (weight 45.0kDa), phosphorylase from Rabbit Muscle (weight 36 kDa) and carbonic Anhydrase from Bovine Erythrocytes (weight 29.0kDa).
Statistical analysis
One-way analysis of variance (P ≤ 0.05) was used to calculate the remarkable assortment in the averages of the investigational treatments.37
Incidence of pest infestation of stored products from seven provinces in Mecca regions
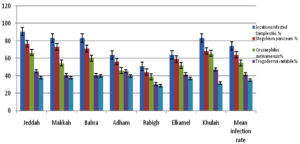
We found that 74.21% (561/756 samples) of stored products were infested with at least one pest. According to Figure 1, drugstore beetle showed the highest prevalence in stored products, with a mean infection rate of 64.29%. The highest rate of drugstore beetle infestation was observed in Jeddah (76.85%), whereas the lowest rate of drugstore beetle infestation was in Rabigh (44.44%). The infection rates of collected stored products samples including ground coffee beans, ground cumin, ground root of Curcuma longa, wormwood chamomile flowers, wheat flour, ground fennel seeds, meat spices (mixture of seven spices), cinnamon sticks crushed, rice grains, and ground fenugreek seeds are illustrated in Figure 2.1,2 The second most prevalent pest was the saw-toothed grain beetle, with an infection rate of 54.89%; its highest infection rate was in Jeddah (66.67%) and its lowest infestation rate was in Rabigh (38.89%).38,39 In addition, warehouse beetle exhibited a low infection rate in Rabigh (30.56%).38 Cowpea weevil showed a low infection rate in all regions (35.05%) (Figure 1).
Figure 1. Incidence of Drugstore beetle, Saw-toothed grain beetle, Ware house beetle, Cowpea weevil, and infestation stored products samples from seven provinces in Mecca regions, KSA
Figure 2. The domestic range is the most dangerous pest of stored products and grains, which is the drug beetle (a) Ground coffee beans infested with drugstore beetle, (b) Ground cumin infested with Drugstore beetle, (c) Ground cumin infested with Drugstore beetle, (d) Wormwood chamomile flowers infested with Drugstore beetle, (e) Wheat flour infested with drugstore beetle, (f) Ground fennel seeds infested with Drugstore beetle, (g) Meat spices (mixture of seven spices) infested with Drugstore beetle, (h) Cinnamon sticks crushed infested with Drugstore beetle, (i) rice grains infected with drugstore beetle, and (j) Ground fenugreek seeds infested with Drugstore beetle
Effect of Cypovirus1 and AgNP treatment on stored product beetles
Pathogen identification
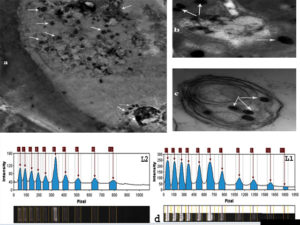
The presence of Cypovirus1 in the cytoplasm of corn borer larvae samples that clearly showed symptoms of viral infection was confirmed using transmission electron microscopy of ultrathin sections of these larvae, which showed typical icosahedral occlusion bodies and has a single-shelled capsid18 of Cypovirus1. Figure 3(a–c) show the irregular shape of the occlusion bodies (2.2–4.9 µm).40-42
Double strands RNA electrophoretic analysis
Figure 3(d) shows the results of Cypovirus1 genome analysis using 1% agarose gel electrophoresis. The genome contains 10 equimolar RNA segments with the sizes of 3,907, 3,716, 3,628, 3,249, 2,726, 1,914, 1,815, 1,256, 1,058, and 899 bp, respectively. based on comparison with size markers.40
Figure 3. (a), (b) and (c): Electron micrographs of typical cytoplasm polyhedral inclusion bodies from Stegobium paniceum showing inclusion bodies demonstrated that the occlusion bodies were of irregular shape and ranged from 2.4 to 5.3 µm in diameter. (d): Electrophoretic separation of Cypovirus1:1 total genome in 1% agarose gel. Lane 1: DNA molecular weight marker (1 kb); lane 2: Cypovirus1 genome segments. The arrows indicated that viral segments are Seg-1, 3,846 bp; Seg-2, 3,612 bp; Seg-3, 3,431 bp; Seg-4, 3,100 bp; Seg- 5, 2,972 bp; Seg-6, 2,523 bp; Seg-7, 2,115 bp; Seg-8, 1,756 bp; Seg-9, 1,275 bp; Seg-10, 754 bp. in size, respectively
Effect of Cypovirus1 and AgNPs on beetle larvae
Table 1 shows the effect of viral activity on the larvae of drugstore beetle, saw-toothed grain beetle, and warehouse beetle, in addition to the effects on adult cowpea weevil by determining the infection rates and mortality percentage at 48 and 72 h after treatment. We treated insect larvae with different concentrations of each viral component (10/100 and 10/1000) and AgNPs (200 and 400 µg/kg) and in combination (10/100 viral component with 400 μg/kg AgNPs). All treatments led to higher larvae mortality at 72 h than at 48 h. The treatment mixture consisting of 10/100 viral components and 400 µg/kg AgNPs led to the highest average death rates of larvae at 72 h (94.78%, 96.22%, 96.11%, and 92.78%) compared to those at 48 h (91.11%, 92.78%, 90.56%, and 86.67%) after treatment. Infection rates and the mortality percentage were increased at 72 h after treating the insect larvae with the viral component at concentrations of 10/100 (72.78%, 83.33%, 70.00%, and 83.89%) and 10/1000 (80.00%, 80.56%, 80.00%, and 71.67%) respectively compared with 48h, inactivated integrate and control treatments. This is because the virus has an incubation period inside the treated larvae, leading to increased infection rates and virus spread between infected larvae. In contrast, the mortality rates increased when the larvae were treated with 200 and 400 μg/kg AgNPs at 72 h after treatment (32.22%, 53.33%, 35.00%, and 56.67% for 200 μg/kg AgNPs; 83.89%, 93.89%, 82.22%, and 89.45% for 400 μg/kg AgNPs). This may be because AgNPs can enter cells by diffusing across the plasma membrane.25 AgNPs affect the plasma membrane permeability, respiration, and molting and development ability of larval cells, leading to toxicity.26
Table (1):
Effect of (CPV1) infection and AgNPs on four-stored product beetles pests
| Treatments | Mortality (%) after 48h. from treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| Drugstore beetle | Saw-toothed grain beetle | Ware house beetle | Cowpea weevil | |||||
| Mean (%) | Mean (I/T) | Mean (%) | Mean (I/T) | Mean (%) | Mean (I/T) | Mean (%) | Mean (I/T) | |
| CPV1 (10/100) | 52.33/60 | 87.22 | 54.33/60 | 90.56 | 52.33/60 | 87.22 | 49.00/60 | 81.67 |
| CPV1 (10/1000) | 48.00/60 | 80.00 | 48.33/60 | 80.56 | 48.00/60 | 80.00 | 43.00/60 | 71.67 |
| AgNPs (200 µg/k) | 18.67/60 | 31.11 | 30.33/60 | 50.56 | 25.00/60 | 41.67 | 18.67/60 | 31.11 |
| AgNPs (400 µg/k) | 39.33/60 | 65.56 | 40.33/60 | 67.22 | 39.33/60 | 65.56 | 29.67/60 | 49.44 |
| CPV1 ( 10/100) + AgNPs (400 µg/k) | 54.67/60 | 91.11 | 55.67/60 | 92.78 | 54.33/60 | 90.56 | 52.00/60 | 86.67 |
| Inactivated integrate | 9.00/60 | 15.00 | 11.00/60 | 18.33 | 9.67/60 | 16.11 | 9.00/60 | 15.00 |
| Control | 5.33/60 | 8.89 | 4.00/60 | 6.67 | 5.67/60 | 9.6 | 4.00/60 | 6.67 |
| Treatments | Mortality (%) after 72h. from treatments | |||||||
| Drugstore beetle | Saw-toothed grain beetle | Ware house beetle | Cowpea weevil | |||||
| Mean (%) | Mean (I/T) | Mean (%) | Mean (I/T) | Mean (%) | Mean (I/T) | Mean (%) | Mean (I/T) | |
| CPV1 (10/100) | 43.67/60 | 72.78 | 50.00/60 | 83.33 | 42.00/60 | 70 | 50.33 | 83.89 |
| CPV1 (10/1000) | 31.00/60 | 51.67 | 42.33/60 | 70.56 | 26.67/60 | 44.44 | 22.33 | 37.22 |
| AgNPs (200 µg/k) | 19.33/60 | 32.22 | 32.00/60 | 53.33 | 21.00/60 | 35.00 | 34.00 | 56.67 |
| AgNPs (400 µg/k) | 50.33/60 | 83.89 | 56.33/60 | 93.89 | 49.33/60 | 82.22 | 53.67 | 89.45 |
| CPV1 ( 10/100) + AgNPs (400 µg/k) | 54.67/60 | 91.11 | 58.33/60 | 96.22 | 57.67/60 | 96.11 | 55.67 | 92.78 |
| Inactivated integrate | 9.00/60 | 15.00 | 11.00/60 | 18.33 | 9.67/60 | 16.11 | 9.00/60 | 15.00 |
| Control | 5.33/60 | 8.89 | 4.00/60 | 6.67 | 5.67/60 | 9.6 | 4.00/60 | 6.67 |
The highest mortality rate was caused by the 10/100 viral component in the larvae of saw-toothed grain beetle (90.56%), drugstore beetle, and warehouse beetle (87.22%) and adult cowpea weevil (81.67%) at 48 after treatment.20-23
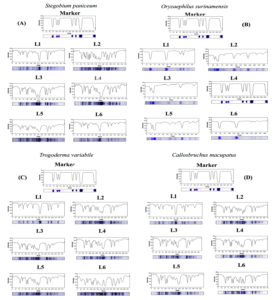
Figure 4. SDS–PAGE profile showing the changes in protein patterns of (A) Stegobium paniceum, (B) Oryzaephilus surinamensis, (C) Trogoderma variabile and (D) Callosobruchus maculates infected with different concentration of (Cypovirus1) and AgNPs. The protein profiling image of the SDS–PAGE electrophoresis M. protein ladder marker, L1. Control healthy stored product beetle, L2. Weevils treated with 10mg/100mg CPV1, L3. Weevils treated with 10mg/1000mg CPV1, L4. Weevils treated with AgNPs (200 µg/k), L5. Weevils treated with AgNPs (400 µg/k)and L6. Weevils treated with CPV1 (10gm/100gm) and AgNPs (400 µg/k)
Protein pattern of all larva stages infested by Cypovirus1 activated with AgNPs
Figure 4 shows gel images of protein bands detected at 72 h after the treatments with Cypovirus1 (10/100 and 10/1000) and AgNPs (200 and 400 µg/kg) in the four product pest larvae. Table 2 shows information on the distinct protein bands in samples from treated larvae, indicating viral infection. Bands indicating Cypovirus1 infection were absent from healthy larvae and present in infected larvae, including protein band No. 17, 22, 29, 33, and 37 in infected larvae of Stegobium paniceum; 2, 7, 14, 18, and 27 in infected larvae of Oryzaephilus surinamensis; 3, 10, 16, 21 and 26 in infected larvae of Trogoderma variabile; and 11, 23, 28, 31, and 32 in infected adult of Callosobruchus maculates, respectively.16 The presence of these protein bands is considered a means of detecting the virus in infected insect larvae (the presence of the virus was confirmed using TAS-ELIZA previously) and is distinguished from others in that it has high molecular weights, which have not appeared at all in healthy insect larvae. Synthesized protein bundles were present within the larvae treated with AgNPs, which were absent from untreated larvae including the protein band No. 6, 13, 24, 30, and 36 in treated larvae of a S. paniceum; 8, 15, 25, 31, and 38 in treated larvae of O. surinamensis; (1, 5, 12, 20, and 34 in treated larvae of T. variabile; and 4, 9, 19, 30, and 35 in treated adults of C. maculates, respectively. Thus, AgNPs can interfere in vital metabolic processes within larvae. These synthesized proteins influenced by AgNPs strongly impact insect antioxidant and detoxifying enzymes, leading to oxidative stress and cell death.43
The table 2 shows the effect of viral infection on proteins in infected larvae of the four types of pests.16
Table (2):
Relative mobility and molecular weight of protein bands detected in effect of different concentrations treatments from the CPV1 and AgNPs on four stored products pests
| Pands No. | RM | MW | Stegobium paniceum | Oryzaephilus surinamensis | Trogoderma variabile | Callosobruchus maculates | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | L6 | L1 | L2 | L3 | L4 | L5 | L6 | L1 | L2 | L3 | L4 | L5 | L6 | L1 | L2 | L3 | L4 | L5 | L6 | |||||
| 1 | 0.229 | 128 | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | + | + | – | – | – | – | – | + | – | ||
| 2 | 0.252 | 117 | – | + | – | + | – | – | – | + | + | – | – | + | – | – | – | – | – | – | – | + | – | + | – | + | ||
| 3 | 0.256 | 116 | – | – | – | + | – | + | + | – | – | – | – | + | – | + | + | – | – | + | – | – | – | – | – | + | ||
| 4 | 0.266 | 112 | – | + | + | – | – | – | + | + | + | – | – | – | – | – | + | – | – | – | + | + | – | + | + | – | ||
| 5 | 0.304 | 97 | – | – | – | – | – | – | + | – | – | – | – | + | – | – | – | + | + | – | – | – | – | – | – | – | ||
| 6 | 0.309 | 96 | – | – | – | + | + | – | + | – | – | – | – | – | + | – | – | + | – | – | – | – | – | – | – | – | ||
| 7 | 0.312 | 95 | + | + | – | – | – | + | – | + | + | – | – | + | – | + | – | – | – | + | + | + | – | – | – | + | ||
| 8 | 0.314 | 94 | – | – | + | – | – | – | – | – | – | + | + | – | – | – | – | + | – | – | – | – | + | – | – | – | ||
| 9 | 0.318 | 93 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | + | – | ||
| 10 | 0.32 | 92 | – | – | – | + | – | – | – | – | – | – | – | – | – | + | + | – | – | + | – | – | – | – | – | – | ||
| 11 | 0.394 | 71 | – | – | – | – | – | – | – | + | + | – | – | – | – | – | – | – | – | – | – | + | + | – | – | + | ||
| 12 | 0.395 | 70 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | + | – | – | – | – | – | – | – | ||
| 13 | 0.400 | 69 | – | – | – | + | + | – | – | – | – | – | – | – | – | – | – | + | – | – | + | – | – | – | + | – | ||
| 14 | 0.416 | 65 | + | + | – | – | – | – | – | + | + | – | – | + | – | – | – | – | – | + | – | + | – | + | – | + | ||
| 15 | 0.421 | 64 | – | – | – | – | – | – | – | – | – | + | + | – | + | – | – | + | – | – | – | – | – | – | – | + | ||
| 16 | 0.470 | 54 | + | – | – | – | – | – | – | – | – | – | – | – | – | + | + | – | – | + | – | – | – | – | – | – | ||
| 17 | 0.474 | 53 | – | + | + | – | – | + | – | – | – | – | – | – | – | – | – | – | – | + | – | + | + | + | – | – | ||
| 18 | 0.493 | 49 | – | – | – | + | – | + | – | + | + | – | – | + | – | – | – | – | – | + | – | + | – | – | + | – | ||
| 19 | 0.503 | 48 | – | – | – | – | + | – | + | – | – | – | – | – | + | + | – | – | – | – | – | – | – | + | + | + | ||
| 20 | 0.504 | 47 | + | – | + | – | – | – | + | – | – | – | – | – | – | – | – | + | + | – | + | – | + | – | – | + | ||
| 21 | 0.537 | 42 | – | – | – | – | – | – | – | – | – | – | – | – | – | + | + | – | – | + | – | – | – | – | – | + | ||
| 22 | 0.554 | 40 | – | + | + | – | – | + | + | – | – | + | + | – | – | – | – | – | – | + | – | – | – | – | – | – | ||
| 23 | 0.559 | 39 | + | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | + | + | – | – | + | ||
| 24 | 0.618 | 32 | – | – | – | + | + | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | + | – | – | – | ||
| 25 | 0.622 | 31 | + | – | – | – | – | – | – | – | – | + | + | – | + | – | – | + | – | – | – | – | – | – | – | – | ||
| 26 | 0.631 | 30 | – | + | – | – | – | – | – | + | – | – | – | – | – | + | + | – | – | + | + | + | – | – | – | + | ||
| 27 | 0.644 | 29 | + | – | – | – | – | + | – | + | + | – | – | + | – | – | – | – | – | – | + | – | – | – | – | – | ||
| 28 | 0.647 | 28 | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – | + | + | – | – | + | ||
| 29 | 0.663 | 27 | – | + | + | – | – | + | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | ||
| 30 | 0.673 | 26 | – | – | – | + | + | – | – | – | – | – | – | – | – | – | – | + | + | + | – | – | – | + | + | – | ||
| 31 | 0.691 | 24 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – | + | – | + | + | – | – | + | ||
| 32 | 0.721 | 22 | + | – | – | – | – | – | + | – | – | – | – | – | – | – | + | – | – | – | – | + | + | – | – | + | ||
| 33 | 0.797 | 17 | – | + | + | – | – | + | + | – | – | – | – | – | + | + | – | – | – | – | – | + | – | – | – | – | ||
| 34 | 0.799 | 16 | – | – | – | – | – | – | + | – | – | – | – | – | + | – | – | + | + | – | + | – | + | – | – | + | ||
| 35 | 0.923 | 11 | – | – | – | – | – | + | – | + | + | – | – | + | – | – | – | – | – | – | – | – | – | + | + | – | ||
| 36 | 0.925 | 10 | – | – | – | + | + | – | – | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | + | ||
| 37 | 0.972 | 9 | – | + | + | – | – | + | – | – | – | – | – | + | – | – | – | – | – | + | – | + | – | – | – | + | ||
| 38 | 0.989 | 8 | – | – | – | – | – | + | – | – | – | + | + | – | + | – | – | – | – | – | + | – | – | – | – | – | ||
| Total number of bands | 16 | 19 | 20 | 13 | 14 | 21 | 18 | 20 | 21 | 14 | 15 | 22 | 16 | 21 | 20 | 15 | 13 | 21 | 15 | 20 | 21 | 15 | 14 | 21 | ||||
(+): Present; (-): Absent; Rm: relative mobility; L1. Control healthy stored product beetle, Weevils treated with 10mg/100mg CPV1, L3. Weevils treated with 10mg/1000mg CPV1, L4. Weevils treated with AuNPs (200 µg/k), L5. Weevils treated with AgNPs (400 µg/k)and L6. Weevils treated with CPV1 (10gm/100gm) and AgNPs (400 µg/k).
From four types of pests of stored products (S. paniceum, O. surinamensis, T. variabile, and C. maculates) from seven different locations in the Mecca region. We found that 561/756 samples (74.21%) collected from the stored products were infested with at least one pest. Drugstore beetle showed the higher average infestation rate of stored products (64.29%). The second most prevalent pest in stored products was saw-toothed grain beetle (54.89%), with the highest infestation rate in Jeddah (66.67%) and lowest infestation rate in Rabigh (38.89%). The morphological characteristics of these insects were identified. The presence of distinctive occlusion bodies of the virus inside infected cells is considered conclusive evidence of a viral infection. In addition, Treating stored grain pest larvae under study with the viral compound mixture consisting of 10/100 viral and 400 µg/kg AgNPs led to the highest average mortality rates of the four insect larvae at 72 h after treatment, that attack stored grain materials and others in the warehouse. Therefore, we recommend in this study the use of this treatment to combat these pests, as it is considered a safe and environmentally friendly method that is not harmful to human health.
The effects of the results of electrophoresis of protein bands of larvae treated with the viral compound and silver nanoparticles show that viral infection of larvae in the presence of silver nanoparticles leads to the creation of protein bands indicating the presence of the viral infection and the concealment of other protein bands compared to untreated larvae.
The study also shows the effective combined effect of each of them in interfering with the vital processes inside the cells of the treated larvae.
ACKNOWLEDGMENTS
The authors would like to thank the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant number no. G: 1324-130-1440.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Lee KW, Powers NR, Walker TW. A preliminary survey of insects and mites associated with stored food products in Korea. Korean J Entomol. 1992;22(1):5-12.
- Kumar KR, Reddy CN. Detection of Hidden Infestation of Cigarette Beetle Lasioderma serricorne F. in Turmeric Rhizomes by X-Ray Radiography. Indian Journal of Entomology, 2022;84(3), 631-633.
Crossref - Yu C, Shuai L, Giovanni B, et al. Olfactory responses of Stegobium paniceum to different Chinese medicinal plant materials and component analysis of volatiles. J Stored Prod Res. 2018;76:122-128.
Crossref - Astuti L, Mario MB, Widjayanti T. Preference, growth and development of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) on red, white and black rice in whole grain and flour form. J Entomol Res. 2018;42(4):461-468.
Crossref - Eliopoulos PA. Life table parameters of the parasitoid Cephalonomia tarsalis (Hymenoptera: Bethylidae) and its host the saw-toothed grain beetle Oryzaephilus surinamensis (Coleoptera: Silvanidae). J Stored Prod Res. 2019;59(4): 544-551.
- Erifili PN, Kavajlierates NG, Papanikolaoy NE. Development and reproductive biology of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) on seven commodities. J Stored Prod Res. 2020;87:10612.
Crossref - Awadalla HS, Guedes RNC, Hashem AS. Feeding and egg-laying preferences of the sawtoothed grain beetle Oryzaephilus surinamensis: Beyond cereals and cereal products. J Stored Prod Res. 2021;93(1):1-8.
Crossref - Olson RLO, Farris RE, Barr NB, Cognato AI. Molecular identification of Trogoderma granarium (Coleoptera: Dermestidae) using the 16s gene. Journal of Pest Science. 2014;87(4):701-710.
Crossref - Castane C, Agusti N, del Estal P, Riudavets J. Survey of Trogoderma spp. in Spanish mills and warehouses. J Stored Prod Res. 2020;88:101661.
Crossref - Athanassiou CG, Phillips TW, Wakil W. Biology and control of the khapra beetle, Trogoderma granarium, a major quarantine threat for global food security. Ann Rev Entomol. 2019;64:131-148.
Crossref - Bidar F, Razmjou J, Golizadeh A, Fathi SAA, Ebadollahi A, Naseri B. Effect of different legume seeds on life table parameters of cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae). J Stored Prod Res. 2021;90:101755.
Crossref - Naseri B, Hamzavi F, Ebadollahi A, Sheikh F. Physicochemical traits of Vicia faba L. seed cultivars affect oviposition preference and demographic parameters of Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae). J Stored Prod Res. 2022;95:101924.
Crossref - Upama Adhikari (2022). Insect Pest Management: Mechanical and Physical Techniques. Reviews In Food and Agriculture, 2022;3(1):48-53.
Crossref - Mertens PPC, Crook NE, Rubinstein R, Pedley S, Payne CC. Cytoplasmic polyhedrosis virus classification by electropherotype: validation by serological analyses and agarose gel electrophoresis. J Gen Virol. 1989;70:173-185.
Crossref - Mertens PPC, Pedley S, Crook NE, Rubinstein R, Payne CC. A comparison of the genomic dsRNA segments of six cypovirus isolates by cross-hybridisation of their dsRNA genome segments. Arch Virol. 1999;144(3):561-566.
Crossref - Hagiwara K, Rao S, Scott W, Carner GR. Nucleotide sequences of segments 1, 3, and 4 of the genome of Bombyx mori cypovirus1 encoding putative capsid proteins VP1, VP3, and VP4, respectively. J GenVirol. 2002;83(6):1477-1482.
Crossref - International Committee on Taxonomy of Viruses Executive Committee. The new scope of virus taxonomy. Partitioning the virosphere into 15 hierarchical ranks. Nat Microbiol. 2020; 5(5):668–674.
Crossref - Shapiro AM, Becnel JJ, White SE. A nucleopolyhedrovirus from Uranotaenia sapphirina (Diptera: Culicidae). J Invertebr Pathol. 2004;86(3):96-103.
Crossref - Takatsuka J. A new cypovirus from the Japanese peppered moth, Biston robustus. J Invertebr Pathol. 2020;174:107417.
Crossref - Arella M, Lavalle C, Belloncik S, Furuichi Y. Molecular cloning and characterisation of cytoplasmic polyhedrosis virus polyhedron and a viable deletion mutant gene. J Virol. 1988;62(1):211-217.
Crossref - Zhang H, Zhang J, Yu X, et al. Visualisation of protein-RNA interactions in cytoplasmic polyhedrosis virus. J Virol. 1999;73(2):1624-1629.
Crossref - Vavra J, Tomas B, Jana N, Brian F. Occurrence, pathology, and ultrastructure of iridovirus and cytoplasmic polyhedrosis viruses in daphnids from the Czech Republic. J Invertebr Pathol. 2016;140:35-38.
Crossref - Kelland K. Cold virus hitches a ride to kill cancer: study. Reuters. 2012. Retrieved 17 June 2012.
- Paur HR, Cassee FR, Teeguarden J, et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung-A dialog between aerosol science and biology. J Aerosol Sci. 2011;42(10):668- 692.
Crossref - Geiser M, Rothen-Rutishauser B, Kapp N, et al. Ultrafine Particles Cross Cellular Membranes Nonphagocytic mechanism in lungs and in Cultured Cells. Environ Health Perspect. 2005;113(11):1555-1560.
Crossref - Unfried K, Albrecht C, Klotz L, Mikecz AV, Grether-Beck S, Schins RPF. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1(1):52-71.
Crossref - Cock MJW, Beseh PK, Buddie AG, Cafá G, Crozier J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Scientific Reports, 2017;7: 4103.
Crossref - Cherry AJ, Jenkins NE, Heviefo G, Bateman RG, Lomer CJ. Operational and economic analysis of a West African pilot scale production plant for aerial conidia of Metarhizium spp. for use as a mycoinsecticide against locusts and grasshoppers. Biocontrol Sci Technol. 1999;9:35-51.
Crossref - Larsen RC, Duffus JE. A simplified procedure for the purification of curly top virus and the isolation of its monomer and dimer particles. Phytopathology. 1984;74:114-118.
Crossref - Dollet M, Accoto GP, Lisa V, Menissier J, and Boccardo G. A geminivirus , serologically related to maise streak virus, from Digitaria sanguinalis from Vanuatu . J Gen Virol. 1986;67(5):933-937.
Crossref - Lacey L, Grzywacz D, Shapiro-Ilan D, Frutos R, Brownbridge M, Goettel M. Insect pathogens as biological control agents: back to the future. Journal of Invertebrate Pathology, 2015;132:1-41.
Crossref - Yerragopu PS, Hiregoudar S, Nidoni U, Ramappa KT, Sreenivas AG, Doddagoudar SR. Chemical Synthesis of Silver Nanoparticles Using Tri-sodium Citrate, Stability Study and Their Characterization International Research. J Pure Appl Chem. 2020;21(3):37-50.
Crossref - Suriati G, Mariatti M, Azizan A. Synthesis of Silver Nanoparticles by Chemical Reduction Method: Effect of Reducing Agent and Surfactant Concentration. Int J Automot Mech Eng (IJAME). 2014;10:1920-1927.
Crossref - Dadosh T. Synthesis of uniform silver nanoparticles with a controllable size. Mater Lett. 2009;63(26):2236-2238.
Crossref - Halder S, Aninda NA, Gafur A, Seong G, Hossain MZ. Size-Controlled Facile Synthesis of Silver Nanoparticles by Chemical Reduction Method and Analysis of Their Antibacterial Performance. Chemistry Select. 2021;6(36):9714-9720.
Crossref - Hames BD, Rickwood D. Gel Electrophoresis of Proteins, IRL Press, Ox. 1981.
- An X, Gu Q, Wang J, et al. Insect-specific RNA virus affects the stylet penetration activity of brown citrus aphid (Aphis citricidus) to facilitate its transmission. Insect Science. 2023.
Crossref - Pai A, Bennett L, Yan G. Female multiple mating for fertility assurance in red flour beetles (Tribolium castaneum). Can J Zool. 2005;83(7):913-919.
Crossref - Sallam MN. Insect damage: damage on post-harvest (PDF). In compendium on post-harvest operations. 2008.
- Ince IA, Ismail D, Zihni D, AND Remziye N. A Cytoplasmic Polyhedrosis Virus Isolated from the Pine Processionary Caterpillar, Thaumetopoea pityocampa. J Microbiol Biotechnol. 2007;17(4):632-637.
- Marzban R, He Q, Liu XX, Zhang QW. Effects of Bacillus thuringiensis toxin Cry1Ac and Cytoplasmic polyhedrosis virus of Helicoverpa armigera (Hubner) (HaCPV) on Cotton bollworm (Lepidoptera: Noctuidae). J Invertebr Pathol. 2009;101(1):71-76.
Crossref - Marzban R. Midgut pH Profile and Energy Differences in Lipid, Protein and Glycogen Metabolism of Bacillus thuringiensis Cry1Ac Toxin and Cypovirus-infected Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J Entomol Res Soc. 2012;14(2):45-53.
- Benelli G. Mode of action of nanoparticles against insects. Environ Sci Pollut Res. 2018;25(13):12329-12341.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.