ISSN: 0973-7510
E-ISSN: 2581-690X
Raw foods contain harmful microorganisms that can infect processed foods and cause them to spoilage. To ensure safety and sustainability, processed foods are categorized depending on the required level of heat treatment and pH levels. This study aimed to assess the effect of different pHs and temperatures on the stability and mode of action of M. paradisiaca L. flower extract. The inhibition zone results after treating extracts with different pHs (3, 6, 7, and 11) for pathogenic bacteria and food spoilage ranged between 6.33 ± 0.47 to 16.67 ± 0.94 mm, and 6.00 ± 0.00 to 10.00 ± 0.00 mm, respectively. In terms of temperatures for foodborne pathogens (30, 50 and 80°C), E. coli showed the highest inhibition zone (11.67 ± 0.47 mm) at 30°C, while B. megaterium (12.00 ± 0.94 mm and 12.33 ± 0.47 mm) at 50 and 80°C. For the food fungi, C. krusei and C. parapsilosis showed the highest inhibition zone (8.33 ± 1.25 mm). The highest cell constituent release was at the concentration of 4×MIC for 4 and 96 h incubation and was found to be at 2.069%, 1.621%, 1.428%, and 1.643% for B. subtilis, E. coli, C.albicans and Asp. niger, respectively. The highest crystal violet uptake for B. subtilis, E. coli, C. albicans, and Asp. niger was 1.881, 2.082, 2.329, and 0.982 at 4׳ MIC after treatment for 4 and 96 h, respectively. In conclusion, M. paradisiaca L. flower extract exhibited antimicrobial activity, which showed stability after being subjected to different pHs and temperatures and can be developed as a natural sanitizing agent for washing raw foodstuffs.
Musa paradisiaca, Antimicrobial Activity, Flower Extract, Cell Constituents Release, Crystal Violet
Raw foods harbor several microorganisms, which may infect processed foodstuffs and commodities, leading to food spoilage. The majority of the microorganisms that cause food spoilage are bacteria and fungi.1 Food spoilage remains a worldwide issue, despite the food science and technological advancement that has been made in recent years. The rate of spoilage is mainly influenced by storage temperature, pH, water accessibility, inappropriate preservation, indigenous microorganisms, processing activities, transportation, and food handling practices.2 Generally, processed foods are categorized into two groups depending on pH and the required degree of heat treatment to ensure food safety and biological sustainability.3
To prevent spoilage and pathogenic microorganisms from growing in foods, a few preservation techniques have been applied in the food industry, such as acidification, salting, heat treatment, sanitizing, and drying.4,5 Sanitizers are often used to inhibit or decrease the volume of undesirable microorganisms in food to a tolerable range without altering the quality and safety of food.6 Thus, many efforts have been conducted to obtain natural alternatives to eliminate harmful microorganisms of growth in foods. Accordingly, plants have been considered the most important and privileged for their natural richness of antimicrobial substances.7 These plant components act as antimicrobials, antioxidants, flavor, and color enhancers. The life span and sensory acceptability of food products have been extended due to the properties of the plant agents. Hence, such compounds perform an indispensable function in prohibiting the growth of foodborne pathogens that leads to reduced chances of illness.8
Musa paradisiaca L. flower extract has been reported to possess antimicrobial activity. The antimicrobial activity of plants’ secondary metabolites is considered an essential source of antimicrobial substances. These substances include but are not limited to essential oils, phenolic compounds, flavonoids, hexanal, hexanol, and alkaloids.9 These secondary metabolites might have direct effects on enzymes and other cell functions, changing the morphology of the microorganisms, aggregating in the cell membrane that leads to destabilization and damage, variation in membrane permeability, and cause cytoplasmic membrane disruption.10-13 While others cause membrane dysfunction and disruption or interact with membrane proteins leading to morphological and functional changes, others interact with protein sulfhydryl and amino groups, causing damage to the microbial membrane cell wall.14-16
In this study, the antimicrobial activities of M. paradisiaca L. flower extracts were evaluated for their stability after treatment with different temperatures and pH on the inhibition zone (mm) to assess their effectiveness as a natural disinfectant and determine the effect of M. paradisiaca L. flower extract on cell constituent release of selected microorganisms through cell constituent’s release and crystal violet analysis and these microorganisms were B. subtilis, E. coli, C. albicans, and Asp. niger.
Samples collection
M. paradisiaca L. flower was purchased from Taman Pertanian University, UPM (University Agricultural Park). Plant taxonomic identification was done by Mohd. Firdaus, Botanist at the Institute of Bioscience (IBS), Universiti Putra Malaysia (UPM) under the voucher specimen number MFI 0207/21. The sample was analyzed in the Natural Medicine and Product Research Laboratory (IBS), UPM. The samples were washed and shade-dried for 10 days at room temperature. The dried flowers were kept at room temperature in sealable plastic bags before further processing.
Preparation of crude extract
M. paradisiaca L. flower extraction has been carried out using the methodology provided by Gil et al.,6 with slight modifications. One hundred grams of dried M. paradisiaca L. flowers were ground using a Panasonic dry blender MK-5087M (Panasonic Corporation, Osaka, Japan). The grounded flowers were then saturated in 400 mL of 99.8% ethanol (R and M Marketing, Essex, UK), and placed in a shaker water bath (Saintifik Maju, Selangor, Malaysia) at 30°C overnight. Afterward, the soaked flower powder was vacuum-filtered using Whatman filter paper No. 2 (Whatman International Ltd., Middlesex, England) by EYELA A-1000S aspirator pump (Tokyo Rikakikai Co., Tokyo, Japan). The filtrate was then concentrated for 3 hours at 40°C and 150 rpm, using a rotating vacuum evaporator Heidolph laborota (Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). The crude ethanolic extract has been kept at 4°C before further use.
Microbial strains and maintenance
In this study, six strains of foodborne bacteria and food spoilage fungi including Escherichia coli ATCC 43895, Klebsiella pneumoniae ATCC 13773, Bacillus subtilis ATCC6633, Bacillus pumilus ATCC14884, Proteus mirabilis ATCC 21100, Bacillus megaterium ATCC14581, Aspergillus niger ATCC9029, Rhizopus oligosporus ATCC22959, Rhizopus oryzae ATCC22580, Candida albicans ATCC10231, Candida krusei ATCC32196, and Candida parapsilosis ATCC22019. The bacterial and fungi strains utilized in the current study were retained by culturing on MHA and PDA, incubated for 1 day (bacteria), 2 days (Candida spp.), and 3-7 days (filamentous fungi), and stored at 4°C.
Exposed to different pHs and temperatures
The antimicrobial stability of M. paradisiaca L. flower extract was evaluated at different pHs following the method reported by Durairaj et al.,17 with few changes. In brief, the antimicrobial activity (disc diffusion assay) of M. paradisiaca L. flower extract has been exposed to different pHs ranging from pH 3, pH 6, pH 7, to pH 11 by adding 0.1 M of hydrochloric acid (HCl), (Merck, Darmstadt, Germany) or 0.1 M of sodium hydroxide (NaOH, Sigma Aldrich, United States), also the extracts was exposed to different temperatures (30°C, 50°C, and 80°C) for 15 min. The analysis was done in three times with three replications each (n = 3 × 3). The original extract (untreated extract) pH, with a pH of 8.86, was used as a control. A clear zone surrounding the filter discs provides evidence of microbial growth suppression, and their diameter was calculated in mm.
Cell constituent’s release analysis
The method described by Tao et al.,13 has been employed with slight modification, to measure the release of cell components into the suspension. Concisely, cells of B. subtilis, E. coli, C. albicans, and Asp. niger were obtained by centrifugation at 3000 × g for 20 min, followed by three times rinsing and suspension in 0.1% PBS (pH 7.0). Then, the suspensions were maintained at 35°C (200 rpm) in an incubator shaker for 0.5, 1, 2, and 4 h for B. subtilis, E. coli, and C. albicans, while Asp. niger was incubated for 24, 48, 72, and 96 h in the presence of M. paradisiaca L. flower extraction at various concentrations of 0 × MIC, 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC. Samples were then centrifuged at 13400 × g for 15 min. UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan) was used to measure the absorption of the supernatant at 260 nm.
Crystal violet assay
The permeability of modified microbial cell membranes was determined using a crystal violet assay (CV) as described by Halder et al.18 Briefly, cells of B. subtilis, E. coli, C. albicans, and Asp. niger were obtained through a centrifuge at 3000 × g for 20 min, rinsed thrice, and resuspended in 0.1% PBS (pH 7.0). Subsequently, the suspensions were incubated in an incubator shaker (200 rpm) at 35°C for 0.5, 1, 2, and 4 h for B. subtilis, E. coli, and C. albicans, where as Asp. niger incubated for 24, 48, 72, and 96 h in the presence of M. paradisiaca L. flower extraction at different concentrations of 0 × MIC, 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC. The suspensions were centrifuged at 9300 × g for 5 min. The harvested cells were then suspended with 1 mL of 0.1% PBS solution (pH 7.2) comprising 10 µg of crystal violet (Sigma-Aldrich, St. Louis, MO, USA). Next, the suspensions were directly centrifuged at 13400 × g for 15 min after being incubated in an incubator shaker (200 rpm) for 10 minutes. The optical density (OD) was used to measure the absorbance of the supernatants at a wavelength of 590 nm. The OD of the crystal violet solution was determined to be 100%. The analysis was conducted employing the following formula:
Crystal violet uptake (%) = (OD value of sample/OD value of CV solution) × 100
Statistical analysis
All experiments have been conducted three times, each of which has three replicates (n = 3 × 3). MINITAB software was used to analyze the collected data by analysis of variance (ANOVA). Turkey’s test was used to determine the significance of the difference (P < 0.05) between the treatments. The outcomes of the replicate analysis have been reported as means ± standard deviation (SD).
Yield of Extract
The sample of M. paradisiaca L. flower has been extracted via the maceration method using 99.9% ethanol. 100 g of dried M. paradisiaca L. flower has been immersed into 400 mL ethanol solvent and yielded in semi-viscous crude that appears brownish with 8.023 g (v/w) for the first attempt and 9.531 g (v/w) for the second attempt, resulting in a total yield value of 8.777 ±1.045%.
Effect of different pHs of extracts on disc diffusion assay (DDA)
To find the sensitivity and stability of the bioactive compounds present in the M. paradisiaca L. flower extract, it was undergone and tested at different pHs. The pH is considered one of the most important parameters influencing the efficacy of the antimicrobial activities of the plant extract in food sanitizers.19,20 In the food industry, especially for freshly produced food, chlorine is the most used sanitizer because of its ease of use, low cost, complete dissolution in water, and high antimicrobial activity.21,22 The chlorine-based wash water systems pH at a pH range of 6.0 – 7.5 was found to have a lethal effect.23 For the application of a sanitizer, there are a few factors required to be considered, which are: the temperature at which the sanitizer will be used, the time duration at which the sanitizer will be in contact with the surface to be sterilized as well as the pH of the sanitizer.24
Furthermore, some factors affect sanitizer effectiveness, and these factors are physical factors (surface features, time of exposure, temperature, and concentration), chemical elements (pH, water properties, and inactivators), and biological parameters (microbiological load).25 The value of original pH of the M. paradisiaca L. flower extract was 8.86. In general, the inhibition zones (mm) of all tested foodborne pathogens remained the same or increased following exposure to the treated extract (pH 3, pH 6, pH 7, and pH 11), as illustrated in Table 1.
Table (1):
Antimicrobial stability of M. paradisiaca L. flower extract at different pHs on the zone of inhibition of foodborne pathogenic bacteria.
Strains |
pH 3 |
pH 6 |
pH 7 |
pH 8.86* |
pH 11 |
|---|---|---|---|---|---|
K. pneumoniae ATCC13773 |
Dc7.33 ± 1.25 |
Bc10.00 ± 0.00 |
Ab11.00 ± 0.82 |
Cc8.83 ± 0.29 |
Bb10.33 ± 0.47 |
P. mirabilis ATCC21100 |
Bb9.67 ± 0.47 |
Ab12.33 ± 2.49 |
Aa12.67 ± 0.94 |
Bb9.50 ± 0.50 |
Aa12.33 ± 0.47 |
E. coli ATCC43895 |
Dd6.33 ± 0.47 |
Aa16.67 ± 0.94 |
Bc10.33 ± 0.47 |
Cb9.17 ± 0.76 |
Cc9.00 ± 0.81 |
B. pumilus ATCC14884 |
Ba10.00 ± 0.0 |
Bc10.33 ± 0.47 |
Aa12.00 ± 0.00 |
Cb9.67 ± 0.29 |
Bb10.33 ± 0.47 |
B. megaterium ATCC14581 |
Cb9.33 ± 0.94 |
Ab12.67 ± 0.94 |
Aa12.67 ± 0.94 |
Cb9.67 ± 0.58 |
Bb10.33 ± 0.47 |
B. subtilis ATCC6633 |
Cb9.67 ± 0.47 |
Bc10.67 ± 0.47 |
Ab11.33 ± 0.47 |
Aa10.67 ± 0.29 |
Ab10.00 ± 0.00 |
* Original pH of the M. paradisiaca L. flower extract
**: Mean values ± standard deviation with different lowercase letters in the same column are significantly different (P<0.05). Mean values ± standard deviation with different uppercase letters in the same row are significantly different (P<0.05).
Overall, the antimicrobial activity of the M. paradisiaca L. flower extract after being treated with different pH showed stability values compared to the original pH of the untreated extract. Food spoilage microorganisms also showed a slight increase in the inhibition zone for all microorganisms after being exposed to the treated extract (Table 2). In general, all food spoilage showed the same or slight increase in the inhibition zone with increased and decreased pH values, while the filamentous fungi showed a decrease with increasing and decreasing pH values.
Table (2):
Antimicrobial stability of M. paradisiaca L. flower extract at different pHs on the zone of inhibition of food spoilage fungi.
Strains |
pH 3 |
pH 6 |
pH 7 |
pH 8.86* |
pH 11 |
|---|---|---|---|---|---|
C. albicans ATCC10231 |
Ab7.00 ± 0.82 |
Ba6.33 ± 0.47 |
Bc6.67 ± 0.47 |
Bc6.43 ± 0.06 |
Ab7.00 ± 0.00 |
C. krusei ATCC32196 |
Ca8.00 ± 0.00 |
Da6.33 ± 0.47 |
Ba9.33 ± 0.47 |
Dc6.57 ± 0.06 |
Aa10.00 ± 0.00 |
C. parapsilosis ATCC22019 |
Bc6.33 ± 0.47 |
Ba6.67 ± 0.47 |
Ab7.67 ± 0.47 |
Bc6.13 ± 0.06 |
Ab7.33 ± 0.47 |
Rh. oryzae ATCC22580 |
Bc6.00 ± 0.00 |
Ba6.00 ± 0.00 |
Bc6.33 ± 0.47 |
Aa9.67 ± 0.62 |
Bc6.00 ± 0.00 |
Rh. oligosporus ATCC22959 |
Bc6.33 ± 0.47 |
Ba6.33 ± 0.47 |
Bc6.00 ± 0.00 |
Ab7.83 ± 0.62 |
Bc6.00 ± 0.00 |
Asp. niger ATCC9029 |
Bc6.00 ± 0.00 |
Ba6.33 ± 0.47 |
Ab7.00 ± 0.82 |
Ab7.00 ± 0.82 |
Bc6.33 ± 0.47 |
* Original pH of the M. paradisiaca L. flower extract
**: Mean values ± standard deviation with different lowercase letters in the same column are significantly different (P<0.05). Mean values ± standard deviation with different uppercase letters in the same row are significantly different (P<0.05).
The finding from this study was similar to that of Molan26, who stated the activity of bioactive compounds as an antimicrobial agent in the plant extract could increase in acidic conditions. Alternatively, the results from the current study were contradicted by Ammer et al.20 and Doughari27 study results. They found that the antimicrobial activities of methanolic extracts of Eucalyptus tereticornis and ethanolic extracts of Moringa oleifera and Balanites aegyptiaca descended once the pH has been shifted from acid to alkaline.17 reported a similar tendency of antimicrobial activity of Allium sativum extraction when the pH was altered. On the other hand, Mahfuzul et al.28 demonstrated that pH fluctuations extract a negligible impact on the antimicrobial activity of Guava and Neem extractions towards L. monocytogenes and S. aureus. Adeshina et al.29 made the same observation, finding that the antimicrobial activity of ethanolic extraction of Ficussycomorus as well as Ficus platyphylla towards the organisms observed at diverse pH levels. Generally, the antimicrobial activity of the M. paradisiaca L. flower extract was found to be significantly stable for Candida spp. In contrast, it decreased for filamentous fungi with increasing or decreasing pH values.
Effect of different temperatures on extracts in disc diffusion assay (DDA)
In this study, the effect of different temperatures on M. paradisiaca L. flower extract has been investigated. The obtained results showed that the antimicrobial effect of the ethanolic extract of M. paradisiaca L. flower that was exposed to different temperatures (25 ± 2°C, 30 ± 2°C, 50 ± 2°C, and 80 ± 2°C) against all studied foodborne bacteria was affected increasingly except for B. subtilis ATCC6633 where it found to be stable at different temperatures (Table 3). The zones of inhibition ranged between 8.83 ± 0.29 to 12.33 ± 0.47mm. B. megaterium showed the highest inhibition zone of 12.00 ± 0.94 and 12.33 ± 0.47 mm at 50°C and 80°C, respectively, among the studied bacteria. On the other hand, the treated extracts with different temperatures were stable against B. subtilis with an inhibition zone ranging between 9.67 ± 0.47 mm and 10.67 ± 0.29 mm. The antimicrobial activity of M. paradisiaca L. flower extract against food spoilage fungi is illustrated in Table 4. The results showed a similar or a slight increase in the inhibition zones at different temperatures where the inhibition zones were between 6.00 ± 0.00 to 10.00 ± 0.00 mm. Rh. oryzae showed decreases in the zone of inhibition at 30°C, 50°C, and 80°C, whereas Rh. oligosporus and Asp. niger showed almost similar patterns at different temperatures.
Table (3):
Antimicrobial stability of M. paradisiaca L. flower extract at different temperatures on the zone of inhibition of pathogenic bacteria.
Bacterial strains |
25 ± 2 ºC |
30 ± 2ºC |
50 ± 2ºC |
80 ± 2ºC |
|---|---|---|---|---|
K. pneumoniae ATCC13773 |
Bc8.83 ± 0.29 |
Aa11.00 ± 0.82 |
Ab11.00 ± 0.81 |
Ab11.67 ± 0.47 |
P. mirabilis ATCC21100 |
Cb9.50 ± 0.50 |
Bb10.33 ± 0.47 |
Ab11.00 ± 0.00 |
Ab11.33 ± 0.47 |
E. coli ATCC43895 |
Cb9.17 ± 0.76 |
Aa11.67 ± 0.47 |
Bc10.67 ± 0.47 |
Ab11.67 ± 0.47 |
B. pumilus ATCC14884 |
Cb9.67 ±0.29 |
Bb10.00 ± 0.82 |
Ab11.67 ± 0.47 |
Bc10.33 ± 0.47 |
B. megaterium ATCC14581 |
Cb9.67 ± 0.58 |
Ba11.33 ± 0.47 |
Aa12.00 ± 0.94 |
Aa12.33 ± 0.47 |
B. subtilis ATCC6633 |
Aa10.67 ± 0.29 |
Ab10.33 ± 0.94 |
Ac10.33 ± 0.47 |
Bd9.667 ± 0.47 |
Mean values ± standard deviation with different lowercase letters in the same column are significantly different (P<0.05). Mean values ± standard deviation with different uppercase letters in the same row are significantly different (P<0.05).
Table (4):
Antimicrobial stability of M. paradisiaca L. flower extract at different temperatures on the zone of inhibition of food spoilage fungi.
Fungal strains |
25 ± 2 ºC |
30 ± 2ºC |
50 ± 2ºC |
80 ± 2ºC |
|---|---|---|---|---|
C. albicans ATCC10231 |
Dc6.43 ± 0.06 |
Cb7.67 ± 0.47 |
Aa9.00 ± 0.00 |
Bc8.33 ± 0.47 |
C. krusei ATCC32196 |
Cc6.56 ± 0.06 |
Ba8.33 ± 1.25 |
Bb8.66 ± 0.47 |
Ab9.33 ± 0.47 |
C.parapsilosis ATCC22019 |
Dc6.13 ± 0.06 |
Ba8.33 ± 1.25 |
Cc7.33 ± 1.24 |
Aa10.00 ± 0.00 |
Rh. oryzae ATCC22580 |
Aa9.67 ± 0.62 |
Bc6.00 ± 0.00 |
Bd6.00 ± 0.00 |
Be6.33 ± 0.47 |
Rh. oligosporus ATCC22959 |
Bc6.13 ± 0.06 |
Ab7.00 ± 0.00 |
Bd6.00 ± 0.00 |
Be6.33 ± 0.47 |
Asp. niger ATCC9029 |
Ab7.00 ± 0.82 |
Bc6.33 ± 0.47 |
Bd6.33 ± 0.47 |
Ad7.00 ± 1.41 |
Mean values ± standard deviation with different lowercase letters in the same column are significantly different (P<0.05). Mean values ± standard deviation with different uppercase letters in the same row are significantly different (P<0.05).
The findings were in line with those of Ammer et al.20 who demonstrated that the antimicrobial activity of Eucalyptus tereticornis extract remained relatively unaffected at different temperatures of 35, 37.5, 40, and 42.5°C. They suggested that the antimicrobial components of the extracts remained thermostable, highlighting the ability to employ the plant extracts at elevated temperatures without compromising the antimicrobial efficacy. Doughari27 reported that the antimicrobial activities of Balanites aegyptiaca as well as Moringa oleifera were stable after being treated with different temperatures. Similarly, Mahfuzul et al.28 noticed that the antimicrobial activities of Neem and Guava were not affected by changes in temperature values. On the other hand, these findings were inconsistent with Arabshahi et al.30 study results, which found that the antimicrobial activities of drumstick leaf extraction after being treated under heat processing were significantly decreased.
Cell constituent release analysis
The cell constituent’s release of the selected strains was obtained by measuring the absorption of the supernatants of each strain at 260 nm. The results of cell constituent release for B. subtilis, E. coli, C. albicans, and Asp. niger were treated with M. paradisiaca L. flower extract for 4 h and 96 h, respectively, depicted in Figure 1 (a, b, c, and d). The highest cell release indicated the highest absorbance. The cell constituent release for B. subtilis and E. coli was found at 2.069 and 1.621, respectively, after being treated with 4 × MIC for 4 h incubation.
Figure 1. Cell constituent release analysis of (a) B. subtilis ATCC6633, (b) E. coli ATCC43895, (c) C. albicans ATCC10231, and (d) Asp. niger ATCC9029 after being treated with M. paradisiaca L. flower extract at a concentration of 0× MIC, 0.5× MIC, 1× MIC, 2× MIC, and 4× MIC, respectively
On the other hand, the highest absorbance of cell constituent release for C. albicans was 1.428, which was observed after being subjected to extraction at 4 × MIC for 4 h incubation, while it was 1.643 for Asp. niger after exposure to the extract at 4 × MIC for 96 h incubation. The release of cell components means irreversible damage happens to the cytoplasmic membrane. Therefore, the obtained results indicated that the treated microorganisms with M. paradisiaca L. flower extract increased their cell constituents with increased extract concentration and longer incubation time compared to the control group (untreated stains).
Research performed by Ramli et al.31 demonstrated that the treated microorganisms (K. pneumoniae, S. aureus, C. albicans, and Asp. niger) with 4 × MIC of S. polyanthum leaves extract for 4 h and 72 h, respectively, found to indicated that the highest absorbance of cell constituent releases was ranged between 0.371 to 0.642. Compared with this study, M. paradisiaca L. flower extract revealed a more potent antimicrobial effect against the tested microorganisms, with constituents’ releases ranging between 1.428 to 2.069 at the same concentration and incubation time. On the other hand,32 discovered that when a Gram-positive foodborne bacterium, B. subtilis ATCC6633, was treated with Trachyspermum ammi (L.) fruit essential oil, it induced a drastic reduction of 260 nm-absorbing material and freed potassium ions. However, such observations led to the hypothesis that the buildup of essential oil molecules inside cytoplasmic membranes causes an immediate loss of membrane stability and a cumulative increase in permeability for ions, which might be the basis for antibacterial action. A noticeable discharge of cytoplasmic components is often associated with severe and permanent distortion of cytoplasmic and plasma membranes.33
Furthermore, the monitoring of the magnitude loss of 260-nm-absorbing substances was as vast as the emission of potassium particles, which might be indicated that the membrane constitutional distortion caused by B. subtilis ATCC6633 cells resulted in the release of cytosolic macromolecular molecules.34 Consequently, even inconsiderable alterations to the structural stability of cell membranes can adversely disturb cell metabolism, comprising the composition of molecules as an extrinsic toxin.32 According to Zhang et al.,35 the viability of cellular membranes can be assessed by measuring the intracellular component leakage and the absorption at 260 nm of microorganisms’ supernatants. Likewise, the nucleic acid and protein concentrations in treated suspensions rose dramatically in a dose-dependent method. Such findings suggested that microbial cells subjected to plant extracts suffered irreversible damage, and the integrity of the cellular membranes was drastically compromised.
The membrane expansion often leads to destabilization and, consequently, leakage of ions.36 Furthermore, the release of 260 nm-absorbing compounds is an indicator of cell breakdown and the formation of non-selective pores.37 The leakage of intracellular constituents indicates that the crucial effect of antimicrobial agents on the tested strains could cause some alteration in the plasma membrane and lead to pore formation.38
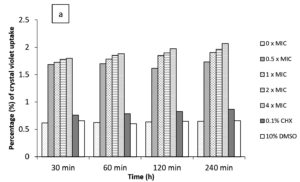
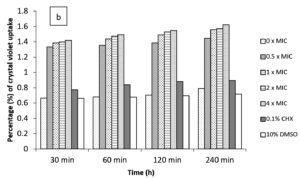
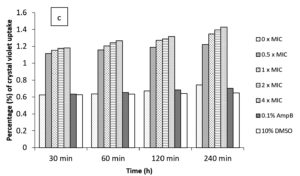
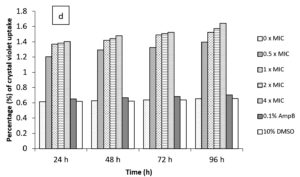
Crystal violet assay
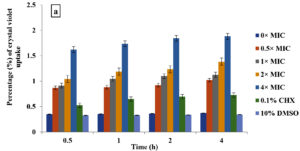
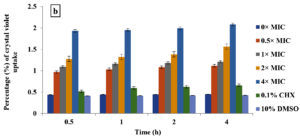
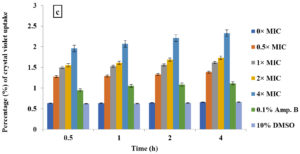
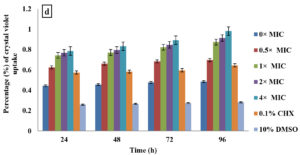
In the current study, M. paradisiaca L. flower extract was noticed to show a concentration- and time-dependent increase of crystal violet uptake as depicted in Figure 2 (a, b, c, and d) for B. subtilis, E. coli, C. albicans, and Asp. niger, respectively. The highest uptake of crystal violet in B. subtilis, E. coli, C. albicans, and Asp. niger were incubated at 4h and 96 h with 1.881, 2.082, 2.329, and 0.982, respectively. Crystal violet hydrophobic property is well recognized for its poor ability to enter the exterior membranes of microorganisms. In contrast, it was discovered to permeate the microbial cells (in the presence of the extract that caused damage to the cell membrane). Subsequently, the crystal violet assay could be used to discover the cellular membrane concussion.
Figure 2. Crystal violet assay of (a) B. subtilis ATCC6633, (b) E. coli ATCC43895, (c) C. albicans ATCC10231 and (d) Asp. niger ATCC9029 after being treated with M. paradisiaca L. flower extract at a concentration of 0× MIC, 0.5× MIC, 1× MIC, 2× MIC, and 4× MIC, respectively
Moreover, such a study with crystal violet might offer helpful data on changes in membrane permeability.18 In this study, M. paradisiaca L. flower extract was found to show a concentration and time-dependent increase of crystal violet uptake in which exposure to a higher extract concentration and more prolonged incubation will lead to higher uptake of crystal violet. Some researchers proposed that the antimicrobial constituents of the plant’s extraction, such as phenolics, alkaloids as well as terpenoids engage with proteins and enzymes of cellular membranes of microorganims, causing its disruption and dispersing protons toward the cellular boundary, thereby inducing apoptosis or inhibiting essential enzymes for the biosynthesis of amino acids.39 Other researchers suggested that the antimicrobial activity of plant extractions was attributed to the hydrophobic nature that capacitates them to interact with proteins of cellular and mitochondrial membranes, hence disrupting their cellular membranes and porosity.40,41 Generally, although crystal violet exhibits weak penetration, it will be easy to enter the microbial cell when the membranes are damaged.38
Also, Sutrisna et al.42 demonstrated that flavonoids were found in the plant leaf extract, which mainly contained flavanols. Flavanols are hydrophobic and consist of a hydroxyl group and phenolic rings. These flavanols easily aggregate in the cell membrane, thus increasing cells’ interaction with the protein and microbial cell wall to form complexes, penetrate the cellular system, and thus cause membrane disruption and death.43 The observed damage to the treated B. subtilis, E. coli, C. albicans, and Asp. niger might suggest this phenomenon and prove that the effect of phenolic compounds in M. paradisiaca L. flower extracts acted on the cellular walls and plasma membranes, subsequently causing leakage of the cytoplasmic organelles.
Besides, Mujeeb et al.,44 mentioned that essential oils, flavonoids, alkaloids, and terpenoids had an antimicrobial activity of phytochemical compounds such as phenolic compounds. Different phytochemical compounds may act on various sites of action with multiple modes of action. Among these compounds, flavonoid compounds have been widely reported to possess antimicrobial activity by disrupting membrane function, causing loss of cellular membranes’ integrity, and resulting in cellular death.45 The plasma membrane of macroorganisms regulates osmotic pressure, respiration, transportation, production and cross-linking of glycoproteins, and synthesis of lipids. The integrity of the membrane is required for all of these processes to be carried out, and its damage may directly or indirectly induce metabolic malfunction and the ultimate death of the bacterial cell.46 In addition, flavonoid compounds with hydrophobic character are also important because they enable them to attach and accumulate in cell membranes, increase cell permeability, and lead to leakage of intracellular constituents, including genetic material and other organelles, therefore causing death to the cells. According to Tsuchiya,47 the two processes regulate the interaction between flavonoids and lipid bilayers. The first process is linked to the separation of non-polar molecules in the hydrophobic core of membranes.
In contrast, the second one involves the development of hydrogen bonding at the membrane interface between polar head groups of lipids and hydrophilic flavonoids. Additionally, the nonspecific relations of flavonoids with phospholipids may cause structural modifications in membrane properties such as thickness and fluctuations, regulate the function of membrane proteins indirectly, and impact the therapeutic activities of flavonoids themselves.46 Moreover, this hydrophobicity character allows the extract to cross the cellular membrane and interact with cell components, disturbing membranes and intracellular enzymes.48
Moreover, Donadio et al.49 described three principal mechanisms that could be responsible for the connection of flavonoid compounds with microbial membranes’ proteome. These mechanisms are; (1) interaction and deactivation of efflux pump transporters, specifically in bacteria; (2) physical membrane disruption and depolarization induced by direct contact with lipidic membranes, leading to the transformation of membrane permeability and (3) suppression of ATP-synthase and instability of cellular energy, by energy transfer.
In conclusion, M. paradisiaca L. flower extract has consistent antimicrobial activity at different pHs and temperatures and modes of action against a wide range of foodborne pathogens and food spoilage indicating that the extracts could be further assess its evaluation in a variety of foods as a sanitizer or preservative.
ACKNOWLEDGMENTS
The author would like to thank genuine appreciation to University Putra Malaysia for granting permission to conduct the study and experiments. Furthermore, we would like to offer our thanks to all those who provided assistance for this research, whether directly or indirectly.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Srividya N, Ghoora MD, Padmanabh PR. Antimicrobial nanotechnology: Research implications and prospects in food safety. Food Preservation. 2017:125-165.
Crossref - Odeyemi OA, Alegbeleye OO, Strateva M, Stratev D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr Rev Food Sci Food Saf. 2020;19(2):311-331.
Crossref - Featherstone S. A complete course in canning and related processes: Volume 3 Processing Procedures for Canned Food Products. 14th Ed. Woodhead Publishing. 2016.
- Farkas J. Physical methods of food preservation. In Doyle MP, Beuchat LR (eds.), Food Microbiology: Fundamentals and Frontiers, 3rd Ed. American Society of Microbiology Press, Washington, D.C. 2007:685-712.
Crossref - Davidson, PM, Taylor TM, Schmidt SE. Chemical preservatives and natural antimicrobial compounds. Food Microbiology: Fundamentals and Frontiers. 2012;1:765-801.
Crossref - Gil MI, Selma MV, Lopez-Galvez F, Allende A. Fresh-cut product sanitation and wash water disinfection: problems and solutions. Int J Food Microbiol. 2009;134(1-2):37-45.
Crossref - Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21(9):1199-1218.
Crossref - Rohani SM, Moradi M, Mehdizadeh T, Saei-Dehkordi SS, Griffiths MW. The effect of nisin and garlic (Allium sativum L.) essential oil separately and in combination on the growth of Listeria monocytogenes. LWT-Food Sci Technol. 2011;44(10):2260-2265.
Crossref - Ciocan ID, Bara I. Plant products as antimicrobial agents. Universitatii Ale Stiintifice Analele Al I Cuza. 2007;8:151-156.
Crossref - Soylu EM, Soylu S, Kurt S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia. 2006;161(2):119-128.
Crossref - Rao A, Zhang Y, Muend S, Rao R. The mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob Agents Chemother. 2010;54(12):5062-5069.
Crossref - Da Cruz-Cabral L, Pinto VF, Patriarca A. Application of plant-derived compounds to control fungal spoilage and mycotoxin production in foods. Int J Food Microbiol. 2013;166(1):1-14.
Crossref - Tao N, OuYang Q, Jia L. Citral inhibits the mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control. 2014;41:116-121.
Crossref - Fallik E, Archbold DD, Hamilton-Kemp TR, Clements AM, Collins RW, Barth MM. (E)-2-hexenal can stimulate Botrytis cinerea growth in vitro and on strawberries in vivo during storage. Journal of the American Society for Horticultural Science. 1998;123(5):875-881.
Crossref - Da Rocha Neto AC, Maraschin M, Di Piero RM. Antifungal activity of salicylic acid against Penicillium expansum and its possible mechanisms of action. Int J Food Microbiol. 2015;215:64-70.
Crossref - Rukayadi Y, Shim JS, Hwang JK. Screening of Thai medicinal plants for anticandidal activity. Mycoses. 2008;51(4):308-312.
Crossref - Durairaj S, Srinivasan S, Lakshmanaperumalsamy P. In vitro antibacterial activity and stability of garlic extract at different pH and temperature. Electron J Biol. 2009;5(1):5-10.
- Halder S, Yadav KK, Sarkar R, et al. Alteration of Zeta potential and membrane permeability in bacteria: a study with cationic agents. Springer Plus. 2015;4(1):1-14.
Crossref - Velez Rivera E. A review of chemical disinfection methods for minimally processed leafy vegetables (Doctoral dissertation), Kansas State University. 2005. 2022. https://tinyurl.com/fh9svvnz
- Ammer MR, Zaman S, Khalid M, et al. Optimization of antibacterial activity of Eucalyptus tereticornis leaf extracts against Escherichia coli through response surface methodology. Journal of Radiation Research and Applied Sciences. 2016;9(4):376-385.
Crossref - Allende A, Selma MV, Lopez-Galvez F, Villaescusa R, Gil MI. Impact of wash water quality on sensory and microbial quality, including Escherichia coli cross-contamination, of fresh-cut escarole. J Food Prot. 2008;71(12):2514-2518.
Crossref - Alvaro JE, Moreno S, Dianez F, Santos M, Carrasco G, Urrestarazu M. Effects of peracetic acid disinfectant on the postharvest of some fresh vegetables. J Food Eng. 2009;95(1):11-15.
Crossref - Sapers GM. Washing and sanitizing raw materials for minimally processed fruit and vegetable products. In Juneja VK, Novak JS, Sapers GM (eds), Microbial Safety of Minimally Processed Foods, 1st Ed. CRC Press: Boca Raton. 2003:221-253.
Crossref - Schuler GA, Nolan MP, Reynolds AE, Hurst WC. Cleaning, sanitizing, and pest control in food processing, storage, and service areas. University of Georgia College of Agricultural and Environmental Sciences Cooperative Extension Service. 1999:1-16. https://vdocuments.mx/cleaning-sanitizing-and-pest-control-in-food-processing-storage-.html?page=1, Accessed 18 October 2021.
- Schmidt RH. Basic elements of equipment cleaning and sanitizing in food processing and handling operations. University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS. 2009:1-11. https://edis.ifas.ufl.edu/publication/FS077, Accessed 24 July 2021.
- Molan PC. The antibacterial activity of honey 1. The nature of the antibacterial activity. Bee World. 1992;73(1):5-28.
Crossref - Doughari JH. Antimicrobial activity of Tamarindusindica Linn. Trop J Pharm Res. 2006;5(2):597-603.
Crossref - Mahfuzul HMD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S. Antibacterial activity of guava (Psidiumguajava L.) and neem (Azadirachtaindica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathog Dis. 2007;4(4):481-488.
Crossref - Adeshina G, Okeke CL, Onwuegbuchulam N, Ehinmidu J. Preliminary studies on antimicrobial activities of ethanolic extracts of Ficus sycomorus Linn. and Ficusplatyphylla Del. (Moraceae). Int J Biol Chem Sci. 2009;3(5):1013-1020.
Crossref - Arabshahi DS, Devi DV, Urooj A. Evaluation of the antioxidant activity of some plant extracts and their heat, pH, and storage stability. Food Chem. 2007;100(3):1100-1105.
Crossref - Ramli S, Radu S, Shaari K, Rukayadi Y. Antibacterial activity of ethanolic extract of Syzygium polyanthum L. (Salam) leaves against foodborne pathogens and application as food sanitizer. BioMed Research International. 2017;217:9024246.
Crossref - Paul S, Dubey RC, Maheswari DK, Kang SC. Trachyspermum ammi (L.) fruit essential oil influences membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22(5):725-731.
Crossref - Cox SD, Gustafson JE, Mann CM, et al. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett Appl Microbiol. 1998;26(5):355-358.
Crossref - Cox SD, Mann CM, Markham JL, Gustafson JE, Warmington JR, Wyllie SG. Determining the antimicrobial actions of tea tree oil. Molecules. 2001:6(2):87-91.
Crossref - Zhang LL, Zhang LF, Xu JG. Chemical composition, antibacterial activity, and action mechanism of different extracts from hawthorn (Crataegus pinnatifida Bge.). Sci Rep. 2020;10(1):1-13.
Crossref - Ultee A, Bennik MHJ, Moezelaar RJAEM. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68(4):1561-1568.
Crossref - Zhou K, Zhou W, Li P, Liu G, Zhang J, Dai Y. Mode of action of pentocin 31-1: an antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control. 2008;19(8):817-822.
Crossref - Devi KP, Nisha SA, Sakthivel R, Pandian SK. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol. 2010;130(1):107-115.
Crossref - Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018;25(2):361-366.
Crossref - Friedman M, Henika PR, Levin CE, Mandrell RE. Antibacterial activities of plant essential oils and their components against Escherichia coli O157: H7 and Salmonella enterica in apple juice. J Agric Food Chem. 2004;52(19):6042-6048.
Crossref - Tiwari BK, Valdramidis VP, O’Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. Application of natural antimicrobials for food preservation. J Agric Food Chem. 2009;57(14):5987-6000.
Crossref - Sutrisna E, Tanti Azizah S, Lutfiana Dewi I. Antidiabetic activity of ethanolic extract of Eugenia polyantha wight leaf from Indonesia in diabetic rat Wistar strain induced by alloxan. Asian J Pharm Clin Res. 2016;9(1):374-376.
- Zongo C, Savadogo A, Somda KM, Koudou J, Traore AS. In vitro evaluation of the antimicrobial and antioxidant properties of extracts from the whole plant of Alternanthera pungens and leaves of Combretum sericeum. Int J Phytomed. 2011;3:182-191.
- Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res Int. 2014;497606.
Crossref - Yun J, Lee H, Ko HJ, Woo ER, Lee DG. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2015;1848(2):695-701.
Crossref - Gorniak I, Bartoszewski R, Kroliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019;18(1);241-272.
Crossref - Tsuchiya H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules. 2015;20(10):18923-18966.
Crossref - Lambert PA. Cellular impermeability and uptake of biocides and antibiotics in Gram positive bacteria and mycobacteria. J Appl Microbiol. 2002;92:46S-54S.
Crossref - Donadio G, Mensitieri F, Santoro V, et al. Interactions with microbial proteins driving the antibacterial activity of flavonoids. Pharmaceutics. 2021;13(5):660.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.