ISSN: 0973-7510
E-ISSN: 2581-690X
The aim of the present study was to investigate the effect of orally administrated probiotics on the physiological and immunological parameters of Sprague Dawely rats. To improve the efficiency of some local probiotics, dairy products were used as an excellent delivery system for probiotics to the experimental animals. The experimental animals were divided into seven different groups. It was remarkably noticed that the administration of different probiotics results in a significant increase in body weight gain in all feeding rats compared with the control group. Feeding rats with different probiotics led to increase in Hb and PVC in all groups of rats. On the other hand, a significant increase in RBCs in all feeding rats. An increase in the count of both white blood cells and lymphocytes occurred in all feeding rats. A general reduction in ALT, AST and bilirubin was observed in plasma of all feeding rats. A significant decrease was observed in creatinine level of all feeding rats. Concerning urea, a significant reduction in urea level was found in plasma of all feeding rats. Feeding rats with these probiotics showed improvement in lipid metabolism. A significant reduction in cholesterol level was observed in plasma of all feeding rats. An effective decrease in the level of TG, LDL and VLDL and increase in the level of HDL was observed in plasma of all feeding rats compared with the control group. Administration of probiotics showed significant increase in total serum protein especially globulin compared with control group. ELISA analysis showed the presence of marked variation in immunoglobulins level of all feeding rats compared with control group.
Probiotics, Immunological, Biological Systems, Experimental Animals.
Probiotics are living microbial cultures or cultured dairy product which beneficially influences the health and nutrition of the host1. Lactobacillus, Leuconostoc and Pediococcus species have been used extensively in food processing throughout human history, and ingestion of foods containing live bacteria, dead bacteria, and metabolites of these microorganisms has taken place for a long time2. Table 1 listed a few microorganisms which received attention as candidate probiotics3,4. Probiotics are typically provided in products in one of three ways: as a culture added to a food at medium levels (e.g. 106 Colony forming units (CFU) per millimeter) with little or no opportunity for culture growth, inoculated into a milk- based food (or dietary supplements) and allowed to grow to achieve higher levels (> 106CFU/ ml) in a fermented food and as concentrated dried cells packaged as dietary supplements such as powders, capsules or tablets5. Fermented milk products are dairy foods that have been fermented with lactic acid bacteria such as Lactobacillus sp, Lactococcus sp, and Leuconostoc sp or yeasts, are generally considered to be the most suitable method for administration of an adequate amount of probiotic microorganism to the consumer6.
Table (1):
Microorganisms used as probiotics.
Lactobacillus |
Bifidobacterium |
Enterococcus |
Others |
|---|---|---|---|
L. acidophilus |
B. adolescentis |
E. faecium |
Streptococcus thermophilus |
L. plantarum |
B. animalis |
E. faecalis |
Streptococcus salivarius |
L. casei |
B. bifidum |
Escherichia coli |
|
L. rhamnosus |
B. breve |
Bacillus coagulans |
|
L. delbrukeckii spp. Bulgaricus |
B. infantis |
Bacillus clausii |
|
L. fermentum |
B. longum |
Saccharomyces cerevisiae |
|
L. johnsonii |
B. lactis |
||
L. gasseri |
|||
L. salivarius |
|||
L. reuteri |
Milk and dairy product samples used in the present study were freshly collected in sterile jars. Samples were kept under cooling conditions and used in the preparation of probiotics as soon as possible. The organisms used throughout the experiment were two isolates, one bacterial strain namely: Lactobacillus acidophilus and was kindly provided by Microbiology Laboratory, Faculty of Science, Alexandria University, and a yeast strain namely: Saccharomyces cerevisiae y-1347 was kindly provided by MIRCEN (Microbiological Resource Center Ain Shams University). All the prepared reagents used in the present investigation were provided by Fluka, Germany, and all the kits were obtained from Bicon, Germany. Media used, throughout the present study, were supplied by Oxoid Ltd, United Kingdom. Sixty three male Sprague Dawley rats, 3 – months old, weighing 180±10gm were obtained from the animal house, Beirut Arab University, Lebanon. The rats were assigned to seven groups each including nine rats and kept in a cage under conventional conditions of temperature and humidity for 90 days. All groups of rats received the commercial diet and tap water and divided according to the treatment administered daily through a gastric tube needle as follows: Group I: control group; Group II: Goat yogurt group; Group III: Cow yogurt group; Group IV: Saccharomyces cerevisiae goat yogurt group; Group V: Lactobacillus acidophilus goat yogurt group; Group VI: Saccharomyces cerevisiae cow yogurt group and Group VII: Lactobacillus acidophilus cow yogurt group. All the rats were acclimatized to the respective diets for one week before the experiment started. Body weight was measured before, during and after the experiment. The body weight was recorded weekly, using manual balance. At the end of the experiment, rats were anesthetized with chloroform, after an overnight fasting, through inhalation. Blood samples were taken from the abdominal aorta of the rats. Fractions of whole blood samples were collected in heparinized tubes (2U/ml) for determination of total blood count, while the remaining samples were collected into sterile tubes and kept at room temperature for 30 min, then centrifuged at 3000Xg for 15 min to obtain sera which were stored at – 20ºC for further chemical analysis7, which were represented by testing the levels of glucose, total cholesterol, triglyceride, urea, creatinine, SGOT-AST, SGPT-ALT, bilirubin, HDL, total protein albumin and immunoglobulins in the rats’ serum using (Bicon, Germany) kits. Data of the present study were subjected to standard one-way analysis of variance (ANOVA) with probability (P) values < 0.05 considered statistically significant using the COSTAT 2.00 statistical analysis software manufactured by CoHort Software Company8.
Figure 1 shows the effect of different probiotic treatments on body weight gain of rats within 90 days. There was a significant increase (P ≤ 0.05) in the body weight gain in all of the experimental animals. Figure 2 shows the effect of different probiotics treatments on hematological parameters of Sprague Dawley rats, there was a significant (P≤0.05) increase in hemoglobin (Hb) and hematocrit (PVC) in all experimental rats fed with different probiotics compared with the control group. A significant (P≤0.05) increase in the RBCs, WBCs and lymphocyte count was recorded in all probiotics fed experimental rats.
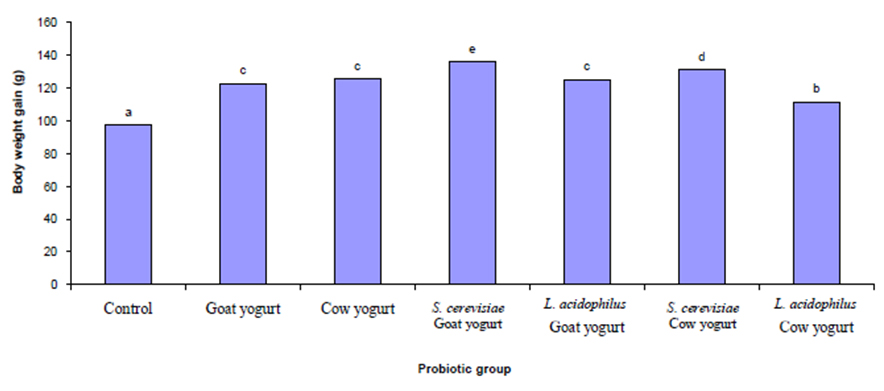
Fig.1. Effect of different probiotic treatments on body weight gain of Sprague Dawley rats
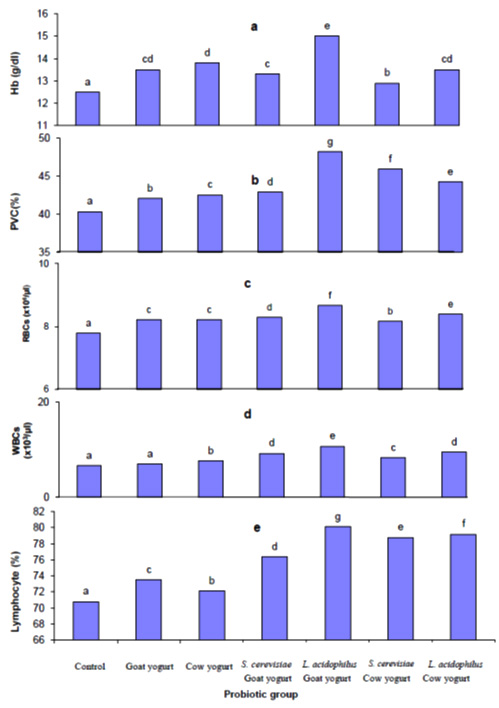 Fig. 2. Effect of different probiotic treatments on hematological parameters: a, hemoglobin; b, Hematocrit; c, Red blood cells; d, White blood cells and e, Lymphocytes
Fig. 2. Effect of different probiotic treatments on hematological parameters: a, hemoglobin; b, Hematocrit; c, Red blood cells; d, White blood cells and e, LymphocytesParameters as bilirubin, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in the plasma of rats in order to assess the liver function. Figure 3 shows the effect of different probiotic treatments on the liver function of rats. A significant reduction (P≤0.05) in alanine aminotransferase (ALT) activity in plasma levels of rats fed with goat yogurt, cow yogurt, Saccharomyces cerevisiae goat yogurt, Lactobacillus acidophilus goat yogurt, Saccharomyces cerevisiae cow yogurt and Lactobacillus acidophilus cow yogurt. Also, a significant reduction (P≤0.05) in aspartate amino transferase (AST) activity was noticed in the plasma levels of rats in relation to the control. Otherwise, the lowest activity of ALT and AST was found in plasma of rats fed with Lactobacillus acidophilus cow yogurt. A significant decrease (P≤0.05) in bilirubin concentration was noticed in plasma of all experimental rats. Figure 4 shows a significant decrease (P≤0.05) in creatinine levels in plasma of all probiotics fed rats in relation to the control group. Concerning the urea level, results showed a significant reduction (P≤0.05) in plasma of rats fed with Saccharomyces cerevisiae cow yogurt and Lactobacillus acidophilus goat yogurt (59% and 83.2% respectively). The highest reduction of urea level was noticed in the plasma level of rats fed on Lactobacillus acidophilus goat yogurt. The present study showed a great effect of different probiotic treatments on the lipid profile in the experimental rats. Results obtained in figure 5 shows a significant decrease (P≤0.05) in the cholesterol level, triglyceride level, LDL and VLDL plasma level. On the other hand, a significant increase (P≤0.05) in high-density lipoprotein (HDL) level was revealed in plasma of rats fed on different probiotics in relation to the control.
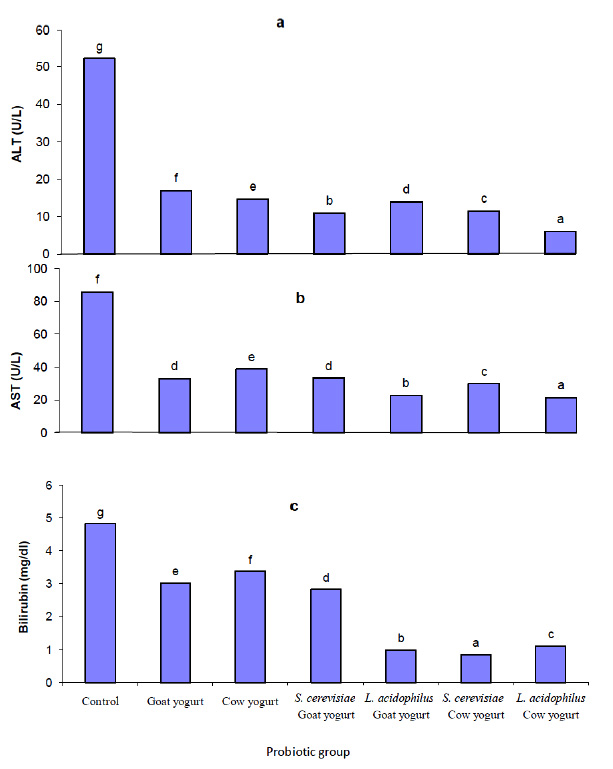 Fig. 3. Effect of different probiotic treatments on liver function: a, ALT; b, AST and c, Bilirubin
Fig. 3. Effect of different probiotic treatments on liver function: a, ALT; b, AST and c, Bilirubin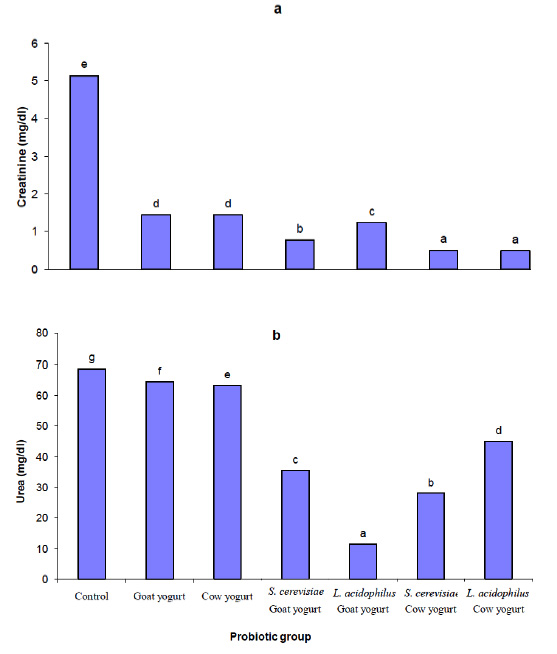 Fig. 4. Effect of different probiotic treatments on kidney function: a, Creatinine and b, Urea
Fig. 4. Effect of different probiotic treatments on kidney function: a, Creatinine and b, Urea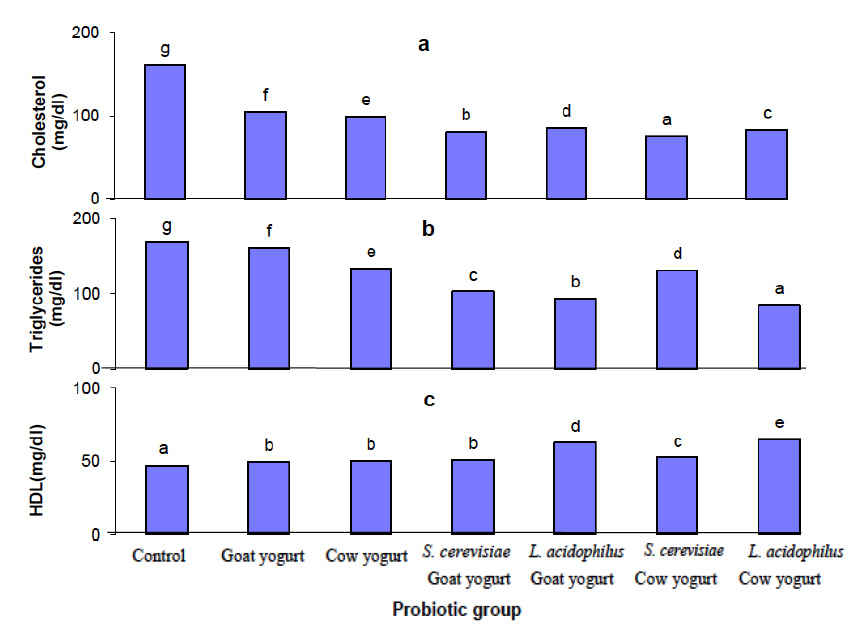
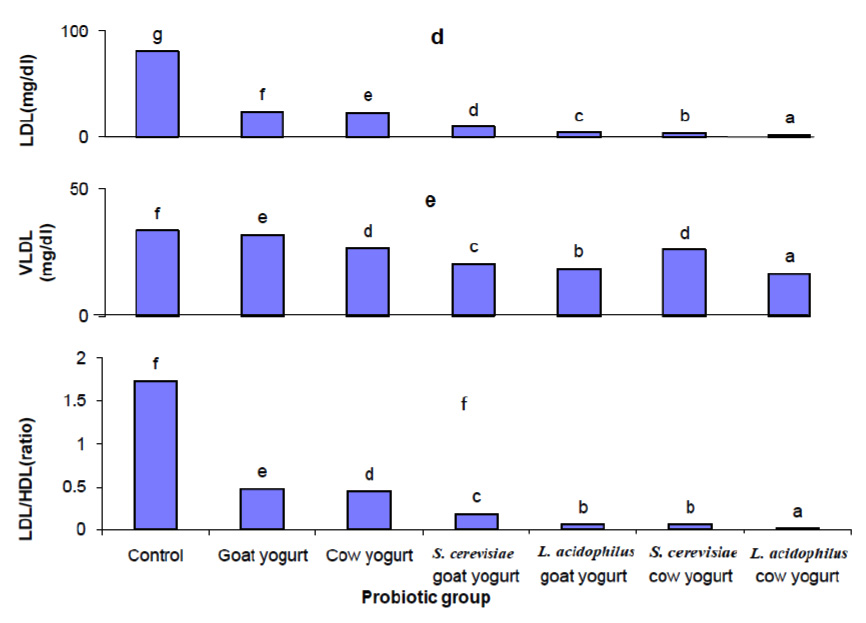 Fig. 5. Effect of different probiotic treatments on Plasma Lipids: a, cholesterol; b, triglycerides; c, HDL; d, LDL; e, VLDL and f, LDL/HDL.
Fig. 5. Effect of different probiotic treatments on Plasma Lipids: a, cholesterol; b, triglycerides; c, HDL; d, LDL; e, VLDL and f, LDL/HDL.Figure 6 shows the effect of different probiotic treatments on blood glucose level. It has been remarkably noticed that there is no significant (P≤0.05) change in the blood glucose level of the experimental animals fed with different probiotics in relation to the control group.
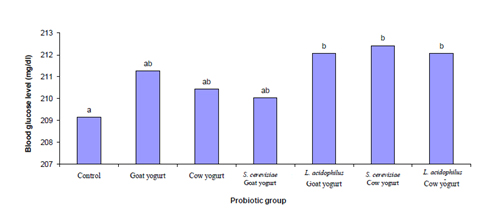 Fig. 6. Effect of different probiotic treatments on blood glucose level of Sprague Dawley rats
Fig. 6. Effect of different probiotic treatments on blood glucose level of Sprague Dawley ratsFigure 7 shows the effect of different probiotic treatments on serum proteins. A significant increase (P≤0.05) in protein and globulin concentrations were observed in serum of rats fed on goat yogurt, cow yogurt, Saccharomyces cerevisiae goat yogurt, Lactobacillus acidophilus goat yogurt, Saccharomyces cerevisiae cow yogurt and Lactobacillus acidophilus cow yogurt in comparison with the control group. The highest decrease (P≤0.05) in the albumin concentration was revealed by rats fed on Lactobacillus acidophilus cow yogurt.
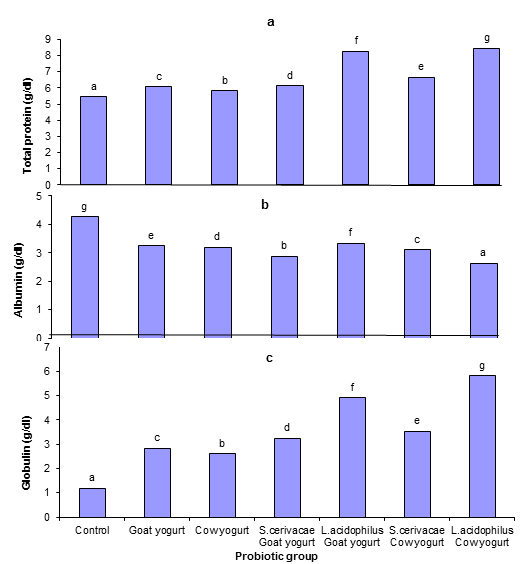
Fig. 7. Effect of different probiotic treatments on Plasma proteins: a, Total protein; b, Albumin and c, Globulin
Figure 8 shows the highest increase in immunoglobulin A (IgA) concentration was recorded in serum of rats fed on Lactobacillus acidophilus cow yogurt, followed by rats fed on Lactobacillus acidophilus goat yogurt. Concerning the immunoglobulin G (IgG) concentration, a significant increase (P≤0.05) was achieved in rats fed on Lactobacillus acidophilus goat yogurt and Lactobacillus acidophilus cow yogurt respectively.
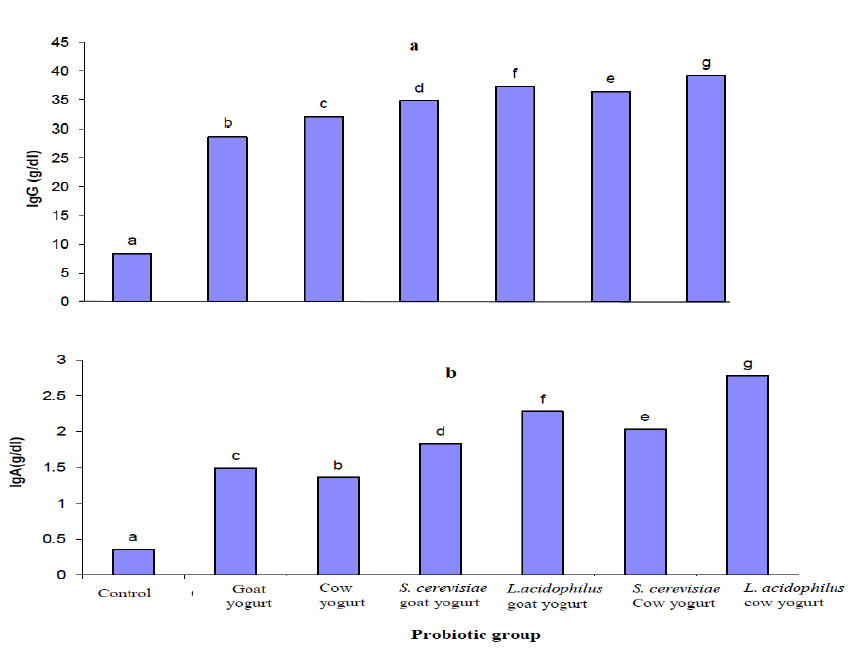 Fig. 8. Effect of different probiotic treatments on the immunoglobulin concentration of Sprague Dawley rats: a, IgG and b, IgA
Fig. 8. Effect of different probiotic treatments on the immunoglobulin concentration of Sprague Dawley rats: a, IgG and b, IgAResults demonstrated the highest body weight gain was noticed in Sprague Dawley rats fed with Saccharomyces cerevisiae goat yogurt and Saccharomyces cerevisiae cow yogurt. These results coincide with those reported by9 who mentioned that Lactobacillus plantarum isolated from fermented corn slurry is safe and can improve the performance of rats in terms of weight gain. Probiotics also produce many important enzymes and increase the availability of vitamin B, vitamin K, fatty acids and calcium as well as the enhancement of mineral availability such as iron10. Bifidobacterium longum SPM 1205 have no adverse effect on the general health status, such as behavior and activity, of Sprague Dawley rats11.
Sprague Dawley rats fed with Lactobacillus acidophilus goat yogurt showed significant improvement in the hematological parameters where significant increase in the packed value cells (PVC), hemoglobin (Hb) and RBCs count was recorded in treated rats. These results were similar to those obtained by9 who revealed that Rattus norvegicus rats fed with Lactobacillus plantarum showed signs of better health based on their hematological status. Four weeks feeding with Lactobacillus rhamnosus HN001 (DR20), Lactobacillus acidophilus HN017, and Bifidobacterium lactis HN019 (DR10) strains had no adverse effects on mice general health status, hematology, blood biochemistry, gut mucosa histology or the incidence of bacterial translocation12. Oral ingestion of lactic acid bacteria by Sprague Dawley rats increases the proliferation of lymphocytes13. Leucocyte is important in defense mechanism against infection14. The primary role of lymphocyte is in humoral antibody formation and cellular immunity14,15. Feeding with Bifidobacterium lactis HN019 resulted in an increase of peripheral blood leucocytes and natural killer cells were active in tumor killing and viral destruction16,17. Such results coincide with that reported by18 who found a significant reduction in AST and ALT serum level in rats fed with fermented soybean as probiotics. Serum AST, ALT and total bilirubin concentration showed slight improvement in rats fed with lactulose and probiotics19.
The obtained results coincide with20 who observed lower AST and ALT activities in Wistar albino rats fed with Lactobacillus casei. Also, significant reduction in bilirubin plasma level was noticed in Sprague Dawley rats fed with Lactobacillus plantarum21.
Sprague Dawley rats fed on Lactobacillus acidophilus goat yogurt recorded the highest reduction in urea plasma level, however the highest reduction in creatinine level was noticed with Saccharomyces cerevisiae cow yogurt and Lactobacillus acidophilus cow yogurt fed rats respectively. These results were similar to those obtained by22 who found that dietary probiotics decrease urease activity and subsequently reduce ammonia production in the small intestine of Sprague Dawley rats. It was also reported that the use of probiotic Lactobacillus delbrueckii reduced plasma urea concentration23. The use of probiotic bacteria is reported to decrease blood urea and serum creatinine levels in rats24,25. Probiotics showed an excellent effect on lowering the level of ammonemia and endotoxemia of rats26. In the present investigation, it was revealed that probiotic administration has modulated the lipid metabolism of all fed rats. These data were similar to those presented by27 who stated that probiotic intervention lead to changes in serum global lipid profile. A significant reduction in the plasma cholesterol concentration was observed in rats treated with Lactobacillus plantarum I-UL4. The results indicated that the metabolite produced by Lactobacillus plantarum I-UL4 had lowering effect on plasma cholesterol in rats28. Sprague Dawley rats fed on Lactobacillus acidophilus cow yogurt revealed a significant reduction in serum LDL and VLDL. Similar results were obtained by29 who recognized low LDL level in rats fed on fermented soybean. Fermented milk produced from Lactobacillus gasseri significantly reduced LDL of rats30. In the present study, Sprague Dawley rats fed on Lactobacillus acidophilus cow yogurt showed a significant elevation in HDL level. Remarkable increase in HDL level was noticed in rats fed on yogurt supplemented with Bifidobacteria31. Lactobacillus sporogenes improved HDL level in blood32. Upon investigating the influence of probiotics on glucose blood level it has been found that there is no significant change in the blood glucose level of all fed Sprague Dawley rats. These findings were inconsistent with that obtained by33 who reported that probiotics had no effect on healthy rats but reduced the blood glucose level in diabetic rats. Probiotics can obviously improve the glucose absorption in craniocerebral injury rats where results showed reduction in glucose serum level as well as glucose urine level34. The use of Saccharomyces cerevisiae goat yogurt and Saccharomyces cerevisiae cow yogurt was considered the best treatment for the stimulation of the immune system; these results were in agreement with that obtained by35 who revealed that the use of probiotics was associated with higher levels of total serum protein due to increased globulin levels. Probiotic bacteria have a possible competition for nutrients with pathogens in the digestive tract36 or the hypothetical stimulation of the immune system, as the activation of macrophage37.
The lowest albumin concentration was achieved by rats fed on Lactobacillus acidophilus cow yogurt. These data coincide with that obtained by38 who found Wistar albino rats’ serum albumin was significantly reduced with Lactobacillus acidophilus, Bifidobacterium bifidum and Enterococcus faecium. Similar findings were observed by39 who found that probiotic treatments activated protein metabolism in animal, redistributed protein fractions for raising globulin content and decrease albumin concentration in serum.
In the present study, it was revealed that Sprague Dawley rats fed on Lactobacillus acidophilus cow yogurt recorded the highest increase in immunoglobulin A (IgA) concentration as well as immunoglobulin G (IgG) concentration. Probiotics also enhance humoral immune responses by increasing Immunoglobulin A (IgA) producing cells and stimulating antibody responses to some specific antigens40. Rats of AGUS strain fed with Lactobacillus plantarum showed increased IgG levels41. Probiotic-supplemented milk formula (milk supplemented with Bifidobacteria) were not significantly affecting IgG antibody titers42.
- Salminen, S. Uniqueness of probiotic strains. IDF Nutr News Lett., 1996; 5:16-18.
- Mäyrä-Mäkien, A. and Bigret, M. Industrial use and production of lactic acid bacteria. In: Salminen S, von Wright A, eds. Lactic acid bacteria. Marcel Dekker: New York, 1993; 65-95.
- Goldin, B. Health benefits of probiotics. Br. J. Nutr., 1998; 80: 203-207.
- Holzapfel, W. and Schillinger, U. Introduction to pre- and probiotics. Food Res. Int., 2002; 35: 109-116.
- Azcarate-Peril, M.; Altermann, E.; Hoover-Fitzula, R.; Cano, R.; Klaenhammer, T. Identification of inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance. Appl. Env. Microbiol., 2004; 70: 4001-4009.
- Casteele, S.; Vanheuverzwijn, T.; Ruyssen, T.; Assche, P.; Swings, J. and Huys, G. Evaluation of culture media for selective enumeration of probiotic strains of Lactobacilli and Bifidobacteria in combination with yoghurt or cheese starters. Int. Dairy J., 2006; 16: 1470-1476.
- Oser, B. Preparation of serum. In: Hawk’s Physiological Chemistry, Oser, B. (Ed.), TATA Mc Graw-Hill Publishing Co., New Delhi, 1965; 357-358.
- Zar, K. Anova Statistical Analysis of Biological data. App. Microbiol., 1984; 122: 345-352.
- Aboderin, F. and Oyetayo, V. Haematological studies of rats fed different doses of probiotic, Lactobacillus plantarum, isolated from fermented corn slurry. Pakistan. J. Nutr., 2006; 5: 102-105.
- Christopher, M.; Padmanablna, R. and Venkateswarlu, K. Effect of whey protein concentrate supplementation on the viability of Bifidobacterium bifidum in probiotic yogurt. J. Food Sci. Technol., 2006; 43: 552-554.
- Choi, S.; Kang, B.; Chung, M.; Kim, S.; Park, S.; Kim, J.; Kang, C. and Ha, N. Safety Assessment of potential Lactic Acid Bacteria Bifidobacterium longum SPM1205 isolated from healthy Koreans. J. Microbiol., 2005; 43: 493-498.
- Zhou, J.; Shu, Q.; Rutherfurd, K.; Prasad, J.; Birtles, M.; Gopal, P. and Gill, H. Safety assessment of potential probiotic lactic acid bacterial strains Lactobacillus rhamnosus HN001, L. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int. J. Food Microbiol., 2000; 56: 87-96.
- Aattouri, N.; Bouras, M.; Tome, D.; Marcos A. and Lemonnier, D. Oral ingestion of lactic acid bacteria by rats increases lymphocyte proliferation and interferon production. Br. J., 2001; 87: 367-373.
- Schalm, O.; Jain, N. and Carrol, E. Veterinary Haematology 3rd Edition. Lea and Febiger, Philadephia, 1975; 421-538.
- Baker, F. and Silver, R. Introduction to Medical Laboratory Technology, Macmillan Press 6th Edition, 1985; 320-328.
- Arunachalam, K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr., 2000; 54: 263-267.
- Gill, H. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J. Clin. Immunol., 2001; 21: 264-271.
- Ali, A.; Velasquez, M.; Hansen, C.; Mohamed, A. and Bhathena, S. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem., 2005; 16: 693-699.
- Lin, J. and Zhang, M. Comparison of probiotics and lactulose in the treatment of minimal hepatic encephalopathy in rats. World J. Gastrolenterol., 2005; 1: 908-911.
- Oyetayo, V.; Adetuyi, F. and Akinyosoye, F. Safety and protective effect of Lactobacillus acidophilus and Lactobacillus casei used as probiotic agent invivo. African J. Biotechnol., 2003; 2: 448-452.
- Osman, N.; Adawi, D.; Ahrne, S.; Jeppddon, B. and Molin, G. Probiotic strains of Lactobacillus and Bifidobacterium affect the translocation and intestinal load of Enterobacteriacaea differently after D- galactosamine-induced liver injury in rats. Microbial Ecology in Health and Disease, 2005; 17: 40-46.
- Kim, T. and Kim, K. Effects of feeding diets containing probiotics or antimicrobial agent on urease activity and ammonia production in the intestinal contents of rats. Korean J. Anim. Sci., 1992; 34: 167-173.
- Chow, K.; Liu, Z.; Prakash, S. and Chang, T. Free and microencapsulated Lactobacillus and effects on metabolic induction on urea removal. Artificial cells, Blood substitutes and Biotechnol., 2003; 31: 425-434.
- Ranganathan, N.; Patel, G.; Ranganathan, P.; Marczely, J.; Dheer, R.; Chordia, T.; Dunn, R.; Friedman, A. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci. World J., 2005; 5: 652-660.
- Ranganathan, N.; Patel, G.; Ranganathan, P.; Marczely, J.; Dheer, R.; Pechenyak, B.; Dunn, R.; Vestraete, W.; Decroos, K.; Mehta, R. and Friedman, A. In Vitro and In Vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J., 2006; 52: 70-79.
- Chiofalo, V.; Liotta, L. and Chiofalo, B. Effects of the administration of lactobacilli on body growth and metabolic profile in growing maltose goat kids. Reprod. Nutr. Dev., 2004; 44: 449-457.
- Kekkon, R.; Sysi-Aho, M.; Seppanen-Laakso, T.; Julkunen, I.; Vapaatola, H.; Oresic, M. and Korpela, R. Effect of probiotic strain Lactobacillus rhamnosus GG intervention on global serum lipidomic profile in healthy adults World J. Gastroenterol., 2008; 14: 3188-3194.
- Loh, T.; Chong, S.; Foo, H. and Law, F. Effects on growth performance, faecal microflora and plasma cholesterol after supplementation of spray-dried metabolite to postweaning rats. Czech J. Anim. Sci., 2009; 54: 10-16.
- Ali, A.; Velasquez, M.; Hansen, C.; Mohamed, A. and Bhathena, S. Effects of Soybean isoflavones, Probiotics, and their interactions on lipid metabolism and endocrine system in an animal model of obesity and diabetes. J. Nutr. Biochem., 2004; 15: 583-590.
- Usman, A. Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. J. Dairy Sci., 2000; 83: 1705-1711.
- Beena, A. and Prasad, V. Effect of yogurt and Bifidus yogurt fortified with skim milk powder condensed whey and lactose-hydrolysed condenced whey on serum cholesterol and triglyceride level in rats. J. Dairy Res., 1997; 64: 453-457.
- Pulusani, S. and Rao, D. Whole body, liver and plasma cholesterol levels in rats fed thermophilus, bulgaricus, and acidophilus milks. J. Food Sci., 1983; 48: 220-281.
- AL-Salami, H.; Butt, G.; Fawcett, J.; Tucker, Ian, G.; Golocorbin-Kon, S. and Mikov, M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur. J. of drug metabolism and pharmacokinetics, 2008; 33: 101-106.
- Lesniewska, V.; Rowland, I. and Laerke, H. Relationship between dietary induced changes in intestinal commensalmicroflora and duodenojejunal myoelectric activity monitored by radioelemetry in rat in vivo. Exp. Physiol., 2006; 91: 229-233.
- Dock, D.; Aguilar-Nascimento, E. and Latorraca, M. Enhanced immunological response influenced by probiotics during the recovery of experimental malnutrition. Rev. Bras. Nutr. Clin., 2003; 18: 157-162.
- Gatesovpe, F. Siderophor production and probiotic effect of Vibrio sp. Associated with turboe larvae, Scophthalmus maximus. Aquatic Living Resour., 1997; 10: 239-246.
- Perdigon, G.; Alvarez, S., Nader de Macias, M.; Roux, M. and Ruiz Holgado, A. The oral administration of lactic acid bacteria increase the mucosal intestinal immunity in response of enterpathogens. J. Food Prot., 1990; 53: 404-410.
- Souza, M.; Aguilar-Nascimento, J. and Dock-Nascimento, D. Effects of budesonide and probiotics enemas on the systemic inflammatory response of rats with experimental colitis, 2007; 1: 40-145.
- Wang, Y.; Tian, Z.; Yao, J. and Li, W. Effect of probiotics, Enterococcus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture, 2008; 277: 203-207.
- Sheryl, H.; Petra, E.; Daesong, Y.; Gary, W. and Cynthia, A. Da ily ingestion of a nutritional probiotics supplement enhances innate immune function in healthy adults. Nutr. Res., 2006; 26: 454-459.
- Herias, M.; Hessle, C.; Telemo, E.; Midtvedt, T.; Hanson, L. and Wold, E. Immunomodulatory effect of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin. Exp. Immunol., 1999; 116: 283-290.
- Batac, M.; Charina, R. and Guno, V. Effects of a probiotic formula on measles, mumps and rubella IgG production and on anthropometric measurements of infants aged 11-15 months in a tertiary hospital. J. Med., 2005; 52:124-130.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


