Richa Kumari1* and Akash P. Nikoshe2
1Department of Entomology and Agricultural Zoology,
Institute of Agricultural Sciences, BHU, Varanasi, India.
2Division of Entomology, IARI, New Delhi, India.
ABSTRACT
The role of three different hosts viz., eggs of Corcyra cephalonica, brinjal aphids and mango mealy bugs on development, longevity, larval and pupal period of green lacewing (Chrysoperla carnea), was investigated in laboratory conditions (50 ± 1°C, 70 ± 5 % RH and photoperiod of L16: D8). In this study Chrysoperla was fed on Corcyra eggs, brinjal aphids and mango mealy bugs. Significant effects of host on larval duration, total developmental period, pupal duration, per cent larval survival and adult longevity of green lacewing were observed. The average larval period of C. carnea fed on was 14.40, 15.20 and 15.60, respectively. Pupal duration of 9.45, 10.02 and 9.77, respectively was recorded in same order of prey. Also the adult longevity of adults from eggs Corcyra, brinjal aphid and mango mealy bugs treatment was 25.40, 26.20 and 27.80 days for males and 31.40, 32.40 and 36.80 days for females. All the results showed that Total developmental period and per cent larval development suggested that eggs of C. cephalonica as better food for C. carnea.
Keywords: Chrysoperla carnea, biological characteristics, brinjal aphids,mango mealy bugs Corcyra cephalonica.
INTRODUCTION
The prey suitability for generalist predators is an important feature for efficient mass rearing and IPM. Chrysopidae family includes more than 1800 well known species and it has been amongst the useful insects of agricultural ecosystems which are very effective and applicable in biological control programs against agricultural pests (Canard et al., 1984). They are voracious predators of a wide variety of soft bodied arthropods including insect’s i.e. aphids, caterpillar, leafhopper, whiteflies, thrips and insect eggs (Carrillo and Elanov, 2004). C. carnea has got a considerable attention as a biological control agent because of its ability to control a variety of insect pests having higher searching ability and wide adaptability in field (Morrison, 1985). The effects of different prey species were investigated on the pre-imaginal development, survival, adult longevity and fecundity of the green lacewing. According to Kannan (1999), natural enemies encountered preying on aphids were chrysopids, coccinellids and syrphids the first of these being the most important and dominant predators. A number of studies have demonstrated the role of lacewing C. carnea (Stephens) (Neuroptera: Chrysopidae) releases to enhance biological control of aphids (Sarwar et al., 2011; Sarwar, 2013).
The adult is non-predacious, feeds on honeydew and pollen and has a high reproductive potential and long oviposition period (Dean and Satascok, 1983). The adults are not predatory and can be easily cultured on relatively simple diets (Nordlund and Morrison, 1996). Mass rearing of parasites/predators is a pre-requisite for any successful biological control programme, but it is imperative to have suitable host. The C. carnea was mass cultured on the eggs of rice grain moth (Corcyra cephalonica), but it has a good feeding potential on different insect pests of cotton. The feeding potential of C. carnea varied on different host eggs such as cotton bollworms; P. gossypiella, H. armigera, E. vittella and grain moth S. cerealella, and nymphs or adults of aphids (Sarwar et al., 2011).
The studies indicate that larval and pupal durations of the predator C. carnea were significantly affected due to feeding upon different hosts and the total developmental period was significantly shorter when the predator was offered with aphids as host. The fecundity, fertility, pupation, hatchability and longevity of the predator were also higher on aphids followed by pink and spotted bollworms, and Angoumois grain moth eggs. However, sex ratio was not affected due to the feeding upon different hosts. Based on the studies, aphids appeared to be the most promising host for mass rearing of the predator (Sarwar et al., 2011). In several Chrysoperla species (including C. carnea), a high nutritional quality of aphid prey has been shown to result in increased larval development, larval survival, pupal weight, adult longevity, and reproduction rates (Liu & Chen, 2001)
MATERIALS AND METHODS
Lacewings Chrysoperla carnea eggs, larvae, pupae and adults were obtained from Biological control laboratory IIVR, Varanasi. Brinjal aphid (Myzus persicae), Mango mealy bug (Drosicha mangiferae) and Rice moth (Corcyra cephalonica) eggs were utilized for the experiment on predator C. carnea (Stephens). The experiments were conducted under C. R. B. D. with five repetitions. There were five sets in each repetition.
Mass Production of Corcyra cephalonica
Maize grain free from any infestation was coarse grind and heat sterilized in hot air oven at 100°C for 30 minutes. About 2.5 kg of the maize grains were mixed with 100 g of the groundnut powder and 5 g powdered yeast were kept in plastic tray (45x30x10cm). A spray of streptomycin sulphate 0.05% was given @ 10-20 ml per tray to prevent bacterial infection. Sulphur 80 WP was added @ 5 g per tray to prevent mite infection. Corcyra eggs 0.5cc (8000-9000 eggs) per tray were sprinkled uniformly in grain medium of each tray. This tray was covered with muslin cloth. The hatched larvae feed on the grain by webbing. Full grown larvae pupate inside the webbed grains mass for 5-7 days and adult moths emerged after 35-40 days from date of inoculation. The emerged Corcyra adults were collected daily and they were transferred in to a specially designed oviposition cage. The collected eggs were rolled over for cleaning. 1.00 cc of Corcyra eggs approximately contains 18000-20000 eggs. About 100 pairs of Corcyra moths (50 per cent females) produce 1.5cc of eggs during its eggs laying period of 4 days. Then these sieved eggs were sterilized under 365 nm UV lamp for 45 min, these eggs were used as food for rearing of C. carnea.
Production of Chrysoperla carnea
For mass production of C. carnea 50 pairs of adults were kept in ovipositional cage, measuring 65 x 30 x 25cm. The sides of the cage were lined with smooth nylon wire mesh with the sliding top cover with black cloth (inner side) for facilitating the egg laying by females. The sliding top cover was replaced every day starting from 4th day onwards. The ovipositional cage was kept for 30 days and the dead adults were removed every alternate day. The adult in oviposition cage were fed daily with equal quantity of protinex + fructose + castor/maize pollen + yeast and drinking water in the ratio of (1:1:1:1:4). One day old eggs were dislodged from the black top cover of the oviposition cage by rubbing gently with a piece of sponge. Eggs thus collected were utilized for further multiplication. The larval rearing of C. carnea was performed in multi cavity trays with 12 cells/tray of 2 x 1.5cm. Three days old C. carnea eggs were mixed with Corcyra eggs. After hatching larva were transferred singly into each cell with the help of moist camel hair brush. The inactivated Corcyra eggs were provided in the entire cells by sprinkling @ 0.5cc Corcyra eggs for 100 larvae of C. carnea. The cocoons were collected after 24 hours of formation and housed in oviposition cage for emergence of adult.
Total larval period of C. carnea
The newly hatched larvae of C. carnea were individually transferred to Petri dishes (5.5cm x 1.5cm), with the help of a fine hair brush. A leaf disk (6cm diameter) of cotton on a filter paper (5cm diameter) was placed in the Petri dish and a few drops of water were added to moisten the leaf disc and filter paper. The larva was supplied with fresh nymphs and eggs until it pupated. Time between hatching and pupal initiation was recorded as the total larval period.
Pupal period of C. carnea
The pupas were kept inside the 9cm diameter Petri dish for emergence of the adults. Pupal period was recorded by constant inspection of the larva for the exact timing of pupal initiation till the emergence of the adult.
Adult longevity of C. carnea
Two days old virgin adults were paired in the rearing glass vials (4 x 7.5cm), provided with standardized adults diet on hard paper card and wet cotton was placed in glass vials. The period of survival of each male and female was observed regularly in order to record longevity (days). Care was taken not to injure predator when transferring them to fresh food or while cleaning the container.
Total developmental period of C. carnea
The newly hatched larvae of C. carnea were transferred singly into Petri dishes (9cm diameter and 1.5cm height). There will be 5 repetitions for each treatment each repetition having 5 sets. At the beginning a wet filter paper placed at the bottom of every Petri dish. At least 60 aphid nymphs and equal number of crawlers of mealy bugs counted and placed in these dishes. The developmental periods (larval instars, cocoon period etc.) of the predator were recorded on each of the hosts separately.
RESULTS
Larval period
The data collected on the effect of different hosts on total larval duration, pupal period, adult longevity and total developmental period were summarized in Table 1 and Figure 1 clearly suggested that larval duration was highest in case of C. cephalonica (15.60 days), minimum larval duration was recorded in case of mango mealy bugs (14.40 days) and treatment involving the food of brinjal aphid was found at par with the Corcyra eggs.
Pupal period
It was observed that C. carnea when fed with eggs of C. cephalonica recorded shortest pupal period of 9.45 days followed by mango mealy bug 9.77 days. Pupal period of C. carnea when fed with brinjal aphid during their larval period showed a significantly longer pupal period of 10.02 days when compared to other treatments (Table 1, Fig. 1).
Longevity of adults
Data presented in Table 1 and Figure 1 for the longevity of the adult showed maximum male and female longevity in case of treatment involving the eggs of C. cephalonica (27.80 and 36.80 days). Treatment with mango mealy bug showed the lowest adult male and female longevity of the C. carnea i.e. 25.40 and 31.40, respectively. Whereas the mealy bug treatment showed no significant difference in the treatment involving brinjal aphid.
| Table 1: Comparison of larval, pupal, and total life duration and longevity of C. carnea, when fed on different hosts | ||||||
| Hosts | Total larval duration | Pupal duration | Adult longevity | Total development period | ||
| Male | Female | Male | Female | |||
| Eggs of C. cephalonica | 15.60 | 9.45 | 25.40 | 31.40 | 50.45 | 56.40 |
| Brinjal aphids | 15.20 | 10.02 | 26.20 | 32.40 | 51.52 | 57.60 |
| Mango mealy bugs | 14.40 | 9.77 | 27.80 | 36.80 | 51.97 | 61.00 |
| C. D. @ 1% | 0.318 | 0.235 | 0.463 | 0.856 | 0.318 | 0.450 |
| S. Em.± | 0.103 | 0.075 | 0.150 | 0.278 | 0.103 | 0.146 |
| C.V. | 3.42 | 1.73 | 2.84 | 4.14 | 1.00 | 1.25 |
| * Average of 5 sets in each replication | ||||||
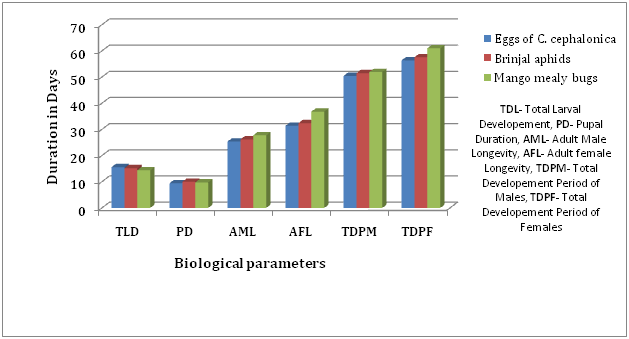
Fig. 1. Comparison of larval, pupal, and total life duration and longevity of C. carnea, when fed on different hosts
Total developmental period
Developmental period of both male and female showed the longest time (51.97 and 51.97 days, respectively) in case of mango mealy bugs as presented in Table 1 and Figure 1. Whereas, the treatment involving the eggs of C. cephalonica showed the shortest developmental period, 50.45 and 56.60 of male and female of C. carnea.
Per cent larval survival
Data presented in Table 2 represents the per cent larval survival of C. carnea. It suggested that maximum larval survival was obtained with eggs of Corcyra during all three instars with average of 95.03 per cent.
DISCUSSION
The shortest larval period was recorded on eggs of C. cephalonica, while longest was on mango mealy bug, similar results were reported by Balasubramani and Swamiappan (1994), where they recorded longest larval period (8.20 days). The survival of larvae of C. carnea feeding on C. cephalonica was 86.7% same treatment was reported best in present study with 95.03% larval survival. Significantly minimum (5.88 days) duration for pupae was registered when fed with eggs of C. cephalonica (Nandan et al., 2014), which was in accordance with the present study where C. cephalonica eggs recorded the least pupal period of 9.45 days. Same study also reported male longevity (27.57 days) with eggs of C. cephalonica which was also found least (25.40 days) in our study. Adult female longevity was reported least with the C. cephalonica eggs. Total developmental period of male and female was recorded least with Corcyra eggs i.e. 50.45 and 56.40 days, respectively no related results were found from the cited literature.
| Table 2: Per cent larval survival of C. carnea on different hosts | |||||
| Hosts | *Average per cent larvae complete their development | ||||
| 1st instar | 2nd instar | 3rd instar | Mean | ||
| Eggs of C. cephalonica | 96.30 | 95.80 | 93.00 | 95.03 | |
| Brinjal aphids | 93.52 | 91.50 | 87.50 | 90.84 | |
| Mango mealy bugs | 91.60 | 89.40 | 86.40 | 89.13 | |
| C. D. at 1% | 1.929 | 2.601 | 3.248 | – | |
| S.Em.± | 0.619 | 0.835 | 1.042 | – | |
| C.V. | 1.476 | 2.021 | 2.620 | – | |
| * Average of 5 sets in each replication | |||||
CONCLUSION
From the present study it can be concluded that the eggs of C. cephalonica as the most suitable for laboratory host of C. carnea. Larval food significantly affected the length of larval period where Corcyra eggs showed longest. Similarly, minimum pupal period, male and female longevity was also recorded in this study by the larva fed on C. cephalonica eggs. Per cent larval survival of the C. carnea was recorded maximum in case of the eggs of C. cephalonica.
ACKNOWLEDGEMENTS
Sincere thanks to Dr. A. B. Rai from IIVR, Varanasi for providing the Corcyra culture. Special thanks to Professor N. N. Singh for his guidelines during the course of this research.
REFERENCES
- Balasubramani, V. and Swamiappan, M. Development and feeding potential of the green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) on different insect pests of cotton. Anz. Schardlingsk. Pflaanzensch. (Germany), 1994; 67: 165-167.
- Canard, M. and Principi, M. M. Life histories and behavior. In Biology of Chrysopidae (M. Canard, Y. Semeriaand T. R. New (eds.). Dr W. Junk Publishers, The Hague, 1984; pp: 57–149.
- Carrillo, M. and Elanov, P. The potential of Chrysoperla carnea as a biological control agent of Myzus persicae in glass houses. Annl. Appl. Biol. 2004; 32: 433-439.
- Dean, G. J. and Satasook, C. Response of Chrysoperla carnea Stephens (Neuroptera: Chrysopidae) to some potential attractants. Bull. Ento. Res., 1983; 73: 619-624.
- Kannan, H. O. Population dynamics of the wheat aphid, Schizaphis graminum, (Rondani) (Homoptera, Aphididae) and its natural enemies in the field. Sudan J. Agril. Res., 1999; 2: 65- 68.
- Liu, T. X. and Chen. T. Y. Effects of three aphid species (Hom. Aphididae) on development, survival and predation of Chrysoperla carnea (Neu. Chrysopidae). Appl. Ento. Zoo., 2001; 36(3): 361-366.
- Morrison, R. K. Handbook of insect rearing, Elsevier, Amsterdam the Netherlands. 1985;
pp. 419-426. - Nordlund, D. A. and Morrison, R. K. Mass rearing of Chrysoperla spp. In: Anderson, T. E. and Leppla, N. C. (eds). Advances in insect rearing for research and pest management. Boulder, Colorado Westview press. 1992;
pp. 427. - Sarwar, M. Studies on Incidence of Insect Pests (Aphids) and Their Natural Enemies in Canola Brassica napus L. (Brassicaceae) Crop Ecosystem. Inter. J. Scint. Res. Envi. Sci., 2013; 1(5), 78-84.
- Sarwar, M., Ahmad, N., Tofique, M. and Salam, A. Efficacy of some natural hosts on the development of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). A laboratory investigation. The Nucleus. 2011; 48(2), 169-173.
- Nandan, N. Korat, D. M. and Dabhi, M. R. Influence of different host insects (prey) on biological parameters of Chrysoperla zastrowi sillemi (Esben-Peterson). Insect Environment. 2014; 20(2), 40-44.

