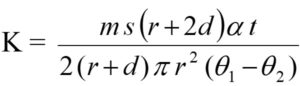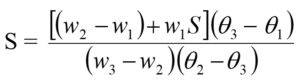ISSN: 0973-7510
E-ISSN: 2581-690X
The study of thermal properties such as conductivity and specific heat of biomaterials is very important as most biological processes, in which biological tissues, cells, and molecules are involved are dependent on body temperature. The main source of body heat is the chemical metabolism of food. Various mechanisms are being adopted by different types of animals to maintain body temperature, such as reducing blood flow through the capillaries nearest the skin surface, body hair can be fluffed up to increase insulation, heat production by shivering, etc. The hard and soft tissues, such as the flesh and bone of animals, play a very important role in keeping the required body temperature. The thermal conductivity and specific heat of the femur, rib, and scapula of two different environment animal ox, the wetland and camel desert dry land are investigated in normal and decalcified conditions. Modified Lee’s apparatus has been used to determine the thermal conductivity, while Renault’s apparatus which is based on the principle of the method of mixtures has been employed for determining the specific heat of samples that were pelletized. A difference in conductivities and specific heat of various bones in both animals was observed due to varied calcium phosphate. The decalcified bone samples of ox and camel show higher thermal conductivity compared to normal bones, while a decrease in specific heat was observed in decalcified bones. The specific heat is affected by the variations in the molecular structure due to changes in temperature. The paper suggests that these techniques are simple, elegant, and inexpensive besides being accurate.
Thermal Conductivity, Specific Heat, Decalcification, Femur, Scapula, Rib
Bones have low density because of their structure and constituent tissue. The outer part of the bone is compact, while the inner layer is spongy. The bones are generally classified as Trabecular cancellous bone and Cortical bone. Cancellous bones are formed at the ends of long bones such as ribs, pelvic region, vertebrates, and skulls. These bones are porous with a honeycomb structure. While cortical bones are compact, very dense, and hence very strong bones generally present at the outer layers of long bones containing a very large amount of tissue. Femoral bones can be considered as consisting of both cortical and cancellous bones. The mechanical properties of these bones are largely dependent on the amount of collagen and minerals present in the bone tissue along with its structure.1,2
The study of thermal properties such as conductivity and specific heat of bones is indispensable and important as most biological processes, in which biological tissues, cells, and molecules involved are temperature dependent. Bones are poor conductors of heat, show very little to moderate thermal conductivity compared to cells and molecules, and are expected to vary inversely with the density of bone. Warm-blooded animals, such as birds and mammals, regulate their own temperature by adjusting the heat loss from their bodies. By contrast, cold-blooded animals are dependent on the environment to maintain their body temperature. Ex-snakes are often found sunning themselves on sun-warmed rocks.3,4 The source of body heat is the chemical metabolism of food. A warm-blooded animal has several mechanisms to use in controlling its temperature. To raise its temperature, the body reduces blood flow through the capillaries nearest the skin surface. The flesh is a poor conductor of heat, so this is effective in reducing heat losses. Also, body hair can be fluffed up to increase insulation. The heat production may be increased by shivering. Studies have been conducted earlier, on the thermal properties of biological macromolecules, tissues, cells, and organs for understanding the thermal conductivity and specific heat of different living systems.
The thermal properties of bone are very important for bone drilling treatments, type of bone cement treatment to treat joint replacements in animal and humans.
Investigations on thermal conductivity and specific gravity of cortical bone were carried out by various research groups and found to have wide variations between them. Vachon et al used the thermal comparator method to investigate the thermal conductivity of bovine cortical bone and found it to be 1.4×10-4 cal/cm/sec/°C, whereas, for in-vivo, it was found to be 5.5×10-4 cal/cm/sec/0C.5 Richard Clattenburg et al.6 reported the thermal conductivity of bovine cancellous bone to be 7×10-4 cal/cm/sec/°C and the specific heat to be 0.73 cal/cm2/°C. The thermal conductivity was found to be very less than the blood, liver, and brain tissue which was around 0.6×10-3 cal/cm/sec/°C.
Liu et al.7 evaluated the effects of various decalcifications on morphological and antigenicity preservation in SD rat femurs. Chukwuneke et al.8 studied the evaluation of the noticeable response of heat of bone cement in hip replacement and simulation was done using the steady state thermal structural analysis. Mei-ling Lau9 investigated the condition of organic constituents of a bovine cortical bone by using thermal gravimetric analysis. Ok et al.4 compared the properties of thermal conductivity of glass ionomer cement in various contents. Poppendiek et al.10 measured thermal conductivity in a large number of normal and frozen samples of biological fluids and tissues using a special unidirectional heat flow apparatus. Babu et al.11 studied a commercially accessible dental Glass Ionomer Cement by adjusting it at room temperature (300 K) for understanding its thermal properties, dielectric, and DC electrical conductivity.
To investigate the thermal properties of bone, decalcification, a histological technique is widely used which removes the hydroxyapatite salts, making the bone soft. The mineral content of the bone is very much dependent on the age, water, and organic content of the bone which also affects its physiological and biophysical properties.12-15 Oikarinen16 prepared decalcified bone matrix from the cortical bones of rats. Rauf and SI Ahmad3 used a uniform bending technique to study the y properties of normal and decalcified rib, femur, and scapula bones of camel and ox. Thermal properties of bone are very much useful in bone drilling and determining the bone necrosis that usually occurs at 50°C. A search of the literature reveals that in spite of extensive investigations on the thermal conductivity of biological macromolecules, cells, tissues, and organs, no detailed information is available on the thermal conductivity of bone. In view of this, in the present investigation studies on these properties have been made on the normal and decalcified femur, rib, and scapula bone of Ox and camel.
Sample preparation
The femur, rib, and scapula of different environmental animals’ Ox and Camel were selected for the study of thermal properties. Fresh samples of bones were collected from the local slaughterhouse. They were boiled for two hours to remove fleshy material and then kept exposed to air for seven days. To determine the thermal conductivity and specific heat of the bones, the samples were cut into circular discs of suitable dimensions. These discs were then ground till the required dimensions, regarding their thickness and diameter, were achieved (Figure 1). The thickness and diameter of the circular disc samples of bones were measured using a screw gauge of least count 0.001 cm.
Determination of thermal conductivity
Thermal conductivity was determined using a modified Lee’s disc apparatus. The samples were placed between two brass discs which were each 1.5 cm in thickness and 1.9 cm in diameter. The upper brass disc has an additional protrusion of 0.5 cm at the center of the top surface into which a hole of 0.7 cm diameter was drilled. An electrically heated soldering rod has been placed in this protrusion, which acts as the source of heat. Minute holes were drilled in the brass discs at 0.4 cm from the sample end into which thermometers can be placed. The entire system was heated by a soldering rod and let reach to steady state as indicated by the steady temperatures of the two thermometers placed in the brass disc, respectively. On reaching a steady state, the two thermometers exhibit a constant steady temperature q1 and q2 of the upper and lower brass discs. Then the sample was eliminated, and the upper brass disc was placed on the lower brass disc until it is heated to about 5°C above q2. Then upper brass disc was eliminated, and the lower brass disc was permitted to cool to about 5°C below its steady temperature. The rate of cooling (a) was determined by plotting a graph between the temperatures at half-minute intervals during cooling versus time. The thermal conductivity (K) of the sample is given by
Where s is the specific heat of the material of the disc, m is of the mass lower brass disc without the attachment of strings, d is the thickness and 2r is the diameter of the lower brass disc. The thickness of the sample (t) was determined by using a micrometer.
Determination of Specific heat
Specific heat (S) of the bone sample under investigation has been determined by the principle of method of mixtures which is based on heat lost by the hot bodies is equal to the heat gained by the cold bodies when they are brought into contact inside a calorimeter. The sample pieces were heated to a steady temperature (q2) in Renault’s apparatus. The copper calorimeter’s weight with a stirrer (w1) was obtained by employing a single pan balance. Water is filled in the calorimeter just enough for submerging the samples of bone. We determine the weights of the calorimeter, stirrer, and water (w2). The initial temperature (q1) of the calorimeter and water kept at room temperature is seen. The sample pieces of bone, at temperature q2, are dumped into the calorimeter having water. The mixture is stirred up thoroughly and the resultant temperature (q3) is measured. The weight (w3) of the calorimeter with a stirrer, water, and samples was determined. Then the Specific heat of the sample is given by
where s be the specific heat of the material of the calorimeter.
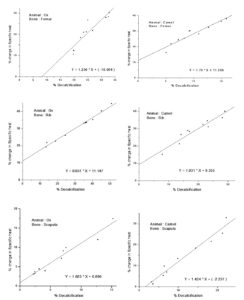
The bone is highly resistivity to flow of temperature flow. The knowledge of thermal conductivity will help surgeons to ease tumor demolition while reducing the related effect on bone loss and damage to surrounding structures. The study will help to verify whether an animal bone can be a proxy for human bone. The information on the thermal properties of bone is also essential because bones are susceptible to mortification at low temperatures which may be higher than that required to kill the tumors.17,18 The thermal conductivity of normal and decalcified femur, rib, and scapula of the animals’ ox and camel, was determined by using a modified Lee’s apparatus and tabulated in Table 1. The specific heat determined using the principle of method of mixture is tabulated in Table 2. where each bone specimen is coded in the (xymn) format, where x represents the animal (O: ox, C: camel) y represents the name of bone (f: femur, R: rib, S: scapula) mn represent the two-digit serial number of the specimen. A change of percentage in thermal conductivity (K%) and specific heat as a function of percentage decalcification is determined, where the percentage of calcium phosphate removed from the bone specimen is noticeable for the samples of femur, rib, and scapula of ox and camel. To observe the effect of decalcification, plots are drawn between the percentage change in thermal conductivity and specific heat versus the percentage change in decalcification (Figure 2 and Figure 3) and observed that these parameters increase with increasing decalcification.
Table (1):
Thermal conductivity, K (×10-4 cal/sec/cm2/unit temp grad) of normal and decalcified bones of Ox femur, rib, and scapula.
| Ox Femur | Ox Rib | Ox Scapula | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample code | K Normal | K Decalcified | Sample code | K Normal | K Decalcified | Sample code | K Normal | K Decalcified |
| OF25 | 3.87 | 4.62 | OR25 | 2.48 | 3.33 | OS25 | 2.21 | 2.83 |
| OF26 | 4.23 | 4.80 | OR26 | 3.40 | 4.74 | OS26 | 3.75 | 4.402 |
| OF27 | 3.76 | 4.37 | OR27 | 2.74 | 4.69 | OS27 | 2.32 | 3.72 |
| OF28 | 3.804 | 4.45 | OR28 | 2.46 | 3.49 | OS28 | 2.22 | 3.39 |
| OF29 | 4.76 | 5.48 | OR29 | 1.84 | 2.76 | OS29 | 3.45 | 4.56 |
| OF30 | 4.36 | 4.92 | OR30 | 4.78 | 5.67 | OS30 | 2.08 | 3.23 |
| OF31 | 4.12 | 4.79 | OR31 | 2.98 | 4.16 | OS31 | 3.33 | 4.81 |
| OF32 | 4.02 | 4.89 | OR32 | 3.64 | 4.40 | OS32 | 2.16 | 3.28 |
| OF33 | 4.74 | 5.41 | OR33 | 4.25 | 5.10 | OS33 | 3.05 | 4.38 |
| OF34 | 4.11 | 4.92 | OR34 | 3.93 | 5.71 | OS34 | 2.29 | 3.82 |
Table (2):
Thermal conductivity, K (×10-4 cal/sec/cm2/unit temp grad) of normal and decalcified bones of Camel femur, rib, and scapula.
| Camel Femur | Camel Rib | Camel Scapula | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample code | K Normal | K Decalcified | Sample code | K Normal | K Decalcified | Sample code | K Normal | K Decalcified |
| CF25 | 4.025 | 4.59 | CR25 | 3.94 | 4.73 | CS25 | 4.2000 | 5.6300 |
| CF26 | 5.540 | 6.55 | CR26 | 2.07 | 3.93 | CS26 | 3.9900 | 5.8600 |
| CF27 | 3.760 | 4.96 | CR27 | 2.34 | 3.34 | CS27 | 3.6700 | 4.7500 |
| CF28 | 4.090 | 4.42 | CR28 | 2.61 | 3.12 | CS28 | 3.1500 | 5.0900 |
| CF29 | 3.850 | 4.30 | CR29 | 2.00 | 2.92 | CS29 | 4.6200 | 5.3810 |
| CF30 | 4.400 | 5.72 | CR30 | 1.74 | 2.60 | CS30 | 4.3900 | 6.2600 |
| CF31 | 3.780 | 4.87 | CR31 | 2.92 | 4.55 | CS31 | 3.5400 | 5.1600 |
| CF32 | 4.930 | 6.26 | CR32 | 2.47 | 3.28 | CS32 | 2.0817 | 4.4002 |
| CF33 | 3.850 | 4.67 | CR33 | 2.47 | 4.67 | CS33 | 2.7560 | 4.8200 |
| CF34 | 4.520 | 5.69 | CR34 | 2.93 | 4.64 | CS34 | 3.8200 | 5.3400 |
Figure 3. % Variation of Specific heat with % Decalcification of femur, rib and scapula of ox and camel
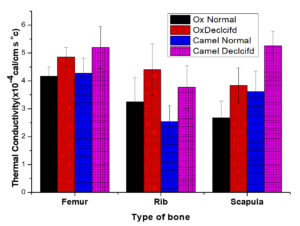
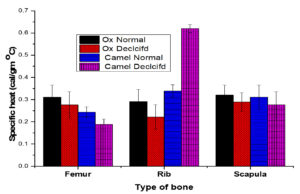
A comparison on average values of thermal conductivity of normal and decalcified animal bone of ox and camel bone is shown in Table 3. The present investigation on the thermal properties of animal bones suggests that the thermal conductivity of the femur, rib, and scapula of the animal- ox, and camel are constant irrespective of the animal due to their identical composition of organic and inorganic materials. But when types of bones like the femur, rib, and scapula are considered, a significant difference exists in thermal conductivity. Thermal conductivity is more in the femur than rib and scapula irrespective of the animal. The significant differences in these thermal parameters may be due to calcium phosphate deposition and their structure. The variation in thermal conductivity of the femur, rib, and scapula is much expected since their functions are entirely different in nature (Figure 4). Depending upon their function the bones have different structures and compositions. The rib and scapula, known as spongy bones, are composed of two thin layers of compact tissue enclosing between them a variable quantity of cancellous tissue. While femur is a compact bone that consists of dense, compact tissue of considerable thickness and is tough in nature.18,19
Table (3):
A Comparison on average values of thermal properties, the thermal conductivity (x 10-4 cal/s/cm/°c) and Specific heat (cal/gm °C) of normal & decalcified animal bone.
| Animal | Bone | Thermal conductivity | Specific heat | ||
|---|---|---|---|---|---|
| Normal | Decalcified | Normal | Decalcified | ||
| OX
|
Femur | 4.17 ± 0.33 | 4.86 ± 0.34 | 0.311 ± 0.055 | 0.276 ± 0.060 |
| Rib | 3.25 ± 0.86 | 4.4 ± 0.93 | 0.291 ± 0.055 | 0.222 ± 0.035 | |
| Scapula | 2.68 ± 0.6 | 3.84 ± 0.63 | 0.312 ± 0.045 | 0.290 ± 0.042 | |
| Camel | Femur | 4.27 ± 0.55 | 5.2 ± 0.75 | 0.243 ± 0.240 | 0.189 ± 0.023 |
| Rib | 2.54 ± 0.58 | 3.77 ± 0.77 | 0.339 ± 0.028 | 0.620 ± 0.018 | |
| Scapula | 3.62 ± 0.73 | 5.26 ± 0.52 | 0.311 ± 0.055 | 0.276 ± 0.060 | |
Figure 4. Average values of Thermal conductivity of Normal and Decalcification femur, rib, and scapula of ox and camel
Calcium phosphate plays a vital role in thermal behavior as is evident from the data on decalcified bones of ox and camel. It is evident from Figures 4 and 5 that, the graphs are linear, but not passing through the origin. This is because it is not possible to perform experiments same specimen with different percent of decalcification, the properties may be greatly affected by the rearrangement of collagen molecules in bone.20 Observations are made on different specimens of the same type of bone having different quantities of calcium phosphate. This is the limitation of experimentation. However, the main aim is to find how calcium phosphate influences thermal properties like the thermal conductivity of animal bone.
Figure 5. Average values of Specific heat Normal and Decalcification of femur, rib, and scapula of ox and camel
The present investigation on the thermal properties of decalcified animal bones suggests that the thermal conductivity of bone after decalcification increases. The difference in thermal conductivity of calcified and decalcified bone of ox and camel are more or less constant irrespective of animals. The data shows that the thermal conductivity increases with the variation in the percentage of decalcification.21,22 Sean R.H. Davidson and David F James have observed thermal conductivity of 0.56 W/mK for a bovine cortical bone which is roughly equal to 13.48×10-4 cal/s/cm/0C and argued that this bone may be treated as thermally isotropic. The thermal conductivity of decalcified camel scapula was found to be 5.25 ± 0.52 which is equal to 0.22 W/m/K which is almost 30% less than bovine cortical bone (0.56 W/m/K). The higher values of thermal conductivity in cortical bone could be due to the dense structure of bone.23,24
The variations in the molecular structure that take place due to change in temperature affects the specific heat of the bone. The variation is observed among the specimens of the same bone of different animal species. It is observed that the specific heat of the femur is less than that of the rib and scapula. The data on the percentage change in the specific heat of bone specimens with respect to the percentage change in decalcification is presented in Figure 5, the plots show a linear trend. Furthermore, a decrease in the specific heat was observed with dryness of bone due to decalcification for all samples. Suleyman Biyikli et al. observed a reverse trend where the specific heat has increased with dryness in the human femora.25 The dense and compact human cortical bone shows a little higher specific heat compared to the bovine bone. The rib of camel showed higher specific heat among all the bones could be due to its inhomogeneous character of camel rib.
Bone can be considered as an amalgamation of its various constituent compounds which can be divided into organic and inorganic. The major components about 70% are the inorganic mineral which is in the form of crystals of hydroxyapatite having formula Ca10(PO4)6(OH)2, 25% collagen, a protein which is an organic compound having triple-helix structure, and the rest 5% other proteins (5%). Depending on the amount of the triple-helix molecule, the collagen can be broadly classified into various types from Type-I to type XII, containing collagen triple-helix from 96% to 10%. Type-I collagen is very much abundant in Bone, cornea, skin, blood vessels dentin, etc. the bone material also has type V cornea.26-28 The composition like collagen which makes the difference in density plays an important role in specific heat. However, apart from the calcification, the age and physiological conditions, and anatomic sites of sample collections could affect the data.6,26,27,29,30
Animal bones have a complex structure consisting of hydroxyapatite with salts of Ca and P and collagen. These minerals and proteins play an important role in the physiological and biophysical properties of porous bone matrix. The decalcification of bone removes the hydroxyapatite salts and collagen affecting the properties of bone which is carried out in the natural environment by exposing the bone samples to sunlight fortnightly in the present investigation. Animal bone shows poor thermal conductivity compared to other biological tissues due to its porous structure. Thermal conductivity and specific heat of the femur, rib, and scapula of ox and camel have been studied. It has been observed that the thermal conductivity of the femur, rib, and scapula of the animals- ox and camel are more or less constant irrespective of the animal. Thermal conductivity is more in the femur than rib and scapula in both animals. The significant variations in thermal parameters may be due to calcium phosphate deposition and its structure. It has been observed that after the decalcification of bone the thermal conductivity increases and specific heat decreases, the properties may be greatly affected by the rearrangement of collagen molecules in bone. It is obvious from Figure 2 that the graphs are linear, but not passing through the origin. This is because it is not possible to perform experiments on the same specimen with different percentages of decalcification. Different specimens of the same type of bone with different quantities of calcium phosphate have been taken for investigation. The decalcified rib of camel shows a very high specific heat compared to other bone camel and ox and can be considered. Decalcified bone shows higher thermal conductivity and low specific heat at all temperature ranges compared to normal bone. However, more insight with the in-depth investigation is required to understand the thermal behavior of animal bone based on various types of decalcification techniques; concomitantly, the method of investigation of thermal properties is needed.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Adeel Ahmad, Professor of Physics at Nizam College, Osmania University Hyderabad, India, for his guidance and mentorship throughout the research project.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng. 2015;137(1):0108021-01080215.
Crossref - Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM. Comparison of the Elastic and Yield Properties of Human Femoral Trabecular and Cortical Bone Tissue. J Biomech. 2004;37(1):27-35.
Crossref - Rauf A, Ahmad SI. Study of mechanical energy dissipation by normal and decalcified animals bones. Biosci Biotech Res Asia. 2019;16(1):113-119.
Crossref - Ok E, Kamalak H, Canbay CA, Altin S. The evaluation of thermal conductivity of different restorative glass ionomer cements. Intl J Appl Dental Sci. 2018;4(3):352-354.
- Vachon R, Walker F, Walker D, Nix G. In Vivo Determination of Thermal Conductivity of Bone Using the Thermal Comparator Technique, Digest Seventh Int Conf Med Biol Eng. 502, 1967.
- Clattenburg R, Cohen J, Conner S, Cook N. Thermal Properties of Cancellous bone. J Biomed Mater Res. 1975;9(2):169-82.
Crossref - Liu H, Zhu R, Liu C, et al. Evaluation of Decalcification Techniques for Rat Femurs Using HE and Immunohistochemical Staining. Biomed Res Int. 2017:9050754.
Crossref - Chukwuneke JL, Ikekwem JU, Okokpujie IP, Ongbali SO. Evaluation of Heat Transfer on Bone Cemented Hip Replacement. J Phys: Conf Ser. 2019;1378(2):022035. 2019.
Crossref - Lau M-l, Lau K-t, Ku H, Cardona F, Leec J-H. Analysis of heat-treated bovine cortical bone by thermal gravimetric and nanoindentation. Composites Part B: Eng. 2013;55:447-452.
Crossref - Poppendiek HF, Randall R, Breeden JA, Chambers JE, Murphy JR. Thermal conductivity measurements and predictions for biological fluids and tissues. Cryobiol. 1967;3(4):318-327.
Crossref - Babu TA, Ramesh KV, Sastry DL. Studies on electrical and thermal properties of dental glass ionomer cement. J Biomed Sci Engg. 2012;05(11):634-638.
Crossref - Bonicelli A, Kranioti EF, Xhemali B, Arnold E, Zioupos P. Assessing bone maturity: Compositional and mechanical properties of rib cortical bone at different ages, Bone. 2022;155:116265.
Crossref - Fajardo JE, Carlevaro CM, Vericat F, Berjano E, Irastorza RM. Effect of the trabecular bone microstructure on measuring its thermal conductivity: A computer modeling-based study. J Therm Biol. 2018;77:131-136.
Crossref - Wang H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers. 2021;13(22):3868.
Crossref - Miszuk J, Liang Z, Hu J, et al. An Elastic Mineralized 3D Electrospun PCL Nanofibrous Scaffold for Drug Release and Bone Tissue Engineering. ACS Appl Bio Mater. 2021;4(4):3639-3648.
Crossref - Oikarinen J. The Bone Inductive Capacity of Decalcified Bone Matrix Modified by Diphenylhydantoin. Acta Orthop Scand. 1981;52(5):505-511.
Crossref - Brazda IJ, Reeves J, Langohr GDG, Crookshank MC, Schemitsch EH, Zdero R. Biomechanical properties and thermal characteristics of frozen versus thawed whole bone. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2020;234(8):874-883.
Crossref - Walker KE, Baldini T, Bennie LG. Thermal Conductivity of Human Bone in Cryoprobe Freezing as Related to Density. Orthopedics. 2017;40(2):90-94.
Crossref - Rauf A. A Review on Mechanical Properties of Animal Bone. Intl J Sci Env Tech. 2014;3(4):1419-1435.
- Ranu HS. The thermal properties of human cortical bone: an in vitro study. Engg. Med. 1987;16:175-176.
Crossref - Abdul Rauf. Thermal Properties of Animal Bone – A Brief Review. Intl J Phy Chem Math Sci. 2014;3(1).
- Karmani S. The thermal properties of bone and the effects of surgical intervention. Current Orthopaedics. 2006;20(1):52-58.
Crossref - Sean RH. Davidson, David F. James. Measurement of thermal conductivity of bovine cortical bone. Med Engg Phys. 2000;22(10):741-747.
Crossref - Moses WM, Witthaus FW, Hogan HA, Laster WR. Measurement of the thermal conductivity of cortical bone by an inverse technique. Exp Thermal Fluid Sci. 1995;11(1):34-39.
Crossref - Biyikli S, Modest MF, Tarr R. Measurements of thermal properties for human femora. J Biomed Mater Res. 1986;20:1335-1345.
Crossref - Leon-Lopez A, Morales-Penaloza A, Martinez-Juarez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Alvarez G. Hydrolyzed Collagen-Sources and Applications. Molecules. 2019;24(22):4031.
Crossref - BT, Kondareddy KM, A R, N R, E SRR, Prakash R. Efficacy of Bovine Hydroxyapatite and Collagen Along With Platelet-Rich Fibrin as a Scaffold and Human Chorion as a Membrane for Ridge Preservation: A Case-Control Study. Cureus. 2022;14(1):e21362.
Crossref - Cornette P, Jaabar IL, Dupres V, et al. Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules-Effect on Structural and Mechanical Properties. Int J Mol Sci. 2022;23(4):2297.
Crossref - Chen HL, Gundjian AA. Specific heat of bone. Med Biol Engg. 1976;14:548-550.
Crossref - Feldmann A, Wili P, Maquer G, Zysset P. The thermal conductivity of cortical and cancellous bone. European Cells Mater. 2018;35:25-33.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.