ISSN: 0973-7510
E-ISSN: 2581-690X
The study aims to investigate the effectiveness of chicken feather hydrolysate for promoting the growth of Spinacia oleracea L., a commonly consumed leafy green vegetable. An earlier isolated and identified keratinolytic bacterial species Bacillus tropicus was utilized for the preparation of chicken feather hydrolysate through submerged fermentation. Minimal media which was supplemented with chicken feather was used for the preparation of hydrolysate. The bacterial strain degraded chicken feather within 4 days of incubation after which the feather hydrolysate was collected and tested to check plant growth promoting activity through the seed germination trials and greenhouse study. Upon characterization of feather hydrolysate, it was found that the hydrolysate was a cocktail of Nitrogen, Phosphorus and Potassium (NPK) as well as other micro elements needed for plant growth. Four different concentrations of feather hydrolysate were employed for both the seed germination and greenhouse study which ranged from 25% (v/v), 30% (v/v), 35% (v/v) and 40% (v/v) including a control group (CN) which was not supplemented with feather hydrolysate. The hydrolysate supplementation brought about plant growth in all the four test concentrations with 35% (v/v) giving the highest result of 14 cm and 27.6 mg/g for tested parameters like plumule length and total chlorophyll content, respectively. The same concentration supported maximum seed germination and highest radicle extension for the germination studies as well. This study investigates the efficacy of chicken feather hydrolysate in promoting spinach growth, elucidating its potential as a fertilizer.
Chicken feather, Keratin, Biofertilizer, Spinach, Seed germination, Bacillus sp.
Protein hydrolysates, derived from organic sources, are pivotal in sustainable agriculture, enriching soil fertility, stimulating plant growth, and mitigating the need for synthetic fertilizers. Scientific evidence supports the efficacy of protein hydrolysates containing amino acids, dipeptides, and oligopeptides in enhancing plant growth significantly by providing stress defense, improving photosynthesis, increasing biomass and fruit yield and also acting as precursors of hormone and phytochemical synthesis.1 The most commonly exploited sources for the production of bioactive peptides (protein hydrolysates) are plant, animal and fungus. Plant and animal-based protein hydrolysates are procured mostly from waste or from byproducts generated during the growth, harvesting or consumption of plant and animal-based food products.2 Out of the different sources of protein hydrolysates, animal-based protein hydrolysates are more in the focus of research as it is an eco-friendly and sustainable source of protein hydrolysate. Additionally, the number of livestock related waste materials is increasing in the phase of earth and usage of waste from livestock leads to the valorization of waste as well.
Utilization of animal-based protein hydrolysates in agriculture dates to more than 50 years due to its properties like eco-friendliness, sustainability and soil enrichment potential.3 Among these alternatives, feather hydrolysate, derived through the enzymatic breakdown of poultry feathers, has emerged as a promising candidate. The major reason for widespread usage of chicken feather hydrolysate can be attributed to the structural composition of chicken feathers which consist of 80-90% of keratin protein.4 The keratin protein once broken down by keratinolytic or proteolytic enzymes can release bioactive amino acids beneficial for plant growth promotion. Plant roots are capable of absorbing amino acids and smaller molecules and exogenous application of feather hydrolysates have proven to be beneficial for plant growth especially those plants with higher nitrogen demand.5 Plant roots absorb about 5% of protein hydrolysate which is directly used for various anabolic and catabolic processes of the plants. For instance the protein hydrolysate produced by the degradation of chicken feather keratin protein by a newly isolated bacterial strain Bacillus aerius NSMk2 improved the overall growth of Mung bean and also improve beneficial microbes in the soil.1 Amendment of conventionally used organic manures such as vermicompost with feather hydrolysate have proved to increase the growth rate of tomato plants leading to improved growth rate and higher yield.3,4 The rest of the protein hydrolysate which remains in the soil will be exploited by soil microbes which is then made available to the plants via plant microbe interaction.6
Spinach is a very important crop produced and marketed globally. Many parts of the world have a rich cultivation of spinach due to its high nutritional value. Spinach trade of around 30.1 million USD globally.7 The plant contains almost 246.60 mg/100 g of nitrogen, 46.80 mg/100 g of phosphorus, 121.24 mg/100 g of potash, micro elements like calcium, iron, and vitamins like A and K.8 As the economic value of spinach is exponentially increasing, the rate of its farming, harvesting and marketing needs to be expanded. This study centers on the evaluation of feather hydrolysate as a plant growth-promoting factor, emphasizing its potential efficacy.
Chemicals and media
All the chemicals, reagents, and media used for the study were obtained from Nice Chemicals Pvt. Ltd., Kochi.
Collection of samples
Seeds of Spinacia oleracea L. required for the experiment were acquired from Indian Institute of Horticultural Studies (IIHR), Bangalore. The seeds variety procured were of ARKA anupama which is a fast-growing seed variety developed in IIHR. Chicken feathers required for the experiment were obtained from a local slaughterhouse, S.G. Palya, Bangalore. Chicken feathers were collected, washed, dried and stored for future use.
Isolation of keratinolytic bacteria
The keratinolytic bacterial strain employed for current study was isolated from poultry waste dumping soil, Russell Market, Shivajinagar, Bangalore (12.9850° N, 77.6059° E). The isolated bacterial strain was characterized through Gram staining and biochemical methods. Based on 16srRNA sequencing, the strain was identified as Bacillus tropicus LS27. The sequence data has already been submitted to GenBank (accession number OM108144).9
Preparation of protein hydrolysate
Protein hydrolysate was prepared by supplementing minimal media with chicken feathers as the only source of carbon and nitrogen. The media used for the preparation of feather hydrolysate was earlier optimized in the work of Liya et al. 2023 which was followed in the present study to prepare a fresh batch of protein hydrolysate.9 The minimal media (MM) consisted of (g/L) of K2HPO4 (0.3), KH2PO4 (0.4), NaCl (0.5) and chicken feathers (10 g) pH 7, the media was inoculated with 5% (v/v) of overnight bacterial culture and incubated for 4 days. After 4 days of incubation, the feather hydrolysate was obtained by filtering out the undigested feather residues and collecting the liquid. The obtained liquid was labeled as feather hydrolysate which is stored at 4°C until further use.
Effectiveness of feather hydrolysate as a liquid fertilizer
Feather hydrolysate prepared from bacterial degradation of chicken feathers was tested to evaluate the effectiveness of it as a liquid fertilizer. The tests were performed according to the protocol mentioned in the work of Gurav et al. 2020 with some modifications.10 Several tests were performed to determine the properties of feather hydrolysate which included examination of its color, odour, pH, electrical conductivity, presence of pathogens and estimation of total NPK, Iron (Fe), Calcium (Ca), Sulphur (S), Manganese (Mn), Copper (Cu), Lead (Pb), Cadmium (Cd), Chromium (Cr), Nickel (Ni).
In vitro toxicity assessment of feather hydrolysate
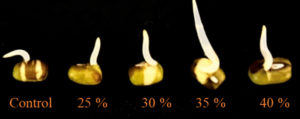
The toxicity test, adapted from Walter et al.11 with some modifications, was followed for toxicity assessment of feather hydrolysate. Absorbent cotton was impregnated with 15 mL of different concentration of feather hydrolysate namely 25% (v/v), 30% (v/v), 35% (v/v) and 40% (v/v) and placed in Petri dishes. Twenty green gram seeds were added per dish and incubated for 24 hours at 25°C in darkness. Green gram seeds are quick growing seeds which give out radicles within 24 hours and are suitable to check for in vitro toxicity studies. Post-incubation, parameters such as germination potential (GP), relative seed germination (RSG), relative root growth (RRG), and germination index (GI) were determined as follows:
GP (%) = (germinated seeds/total seeds) x 100.
RSG (%) = (germinated seeds in treatment/germinated seeds in control) x 100.
RRG (%) = (average radicle length in treatment/average radicle length in control) x 100.
GI (%) = RSG x RRG / 100.
Preparation and compositional analysis of potting mixture
The testing of feather hydrolysate as a plant growth promoting factor was assessed in a greenhouse plant growth trial experiment. For the preparation of potting soil a potting mixture was prepared by adding red soil, cocopeat and sand in the ratio 2:2:1. The potting mixture was tested for Nitrogen, Phosphorus and Potassium (NPK) content according to the standard protocols by Food and Agricultural organization (FAO) 2018.12 Apart from NPK, the pH of the soil as well as organic carbon content was also tested.
For the estimation of organic content of the potting mixture, weight loss method was used. About 5-10 g of potting mixture was weighed and dried in a hot air oven at 105°C for 4 hours. After drying in a hot air oven, 0.01g of dried sample was weighed and transferred to a silica crucible to incinerate it in a muffle furnace at 400°C for 4 hours. The weight of the sample (0.01 g) after drying in a hot air oven was labeled as W1. The weight of the sample after incinerating in the muffle furnace was labeled as W2.13 The percentage loss of weight in the sample was calculated and is determined as the amount of organic carbon in the sample.
Available organic nitrogen (N) in the potting mixture was calculated using the Kjeldahl method with some modification.14 Available phosphorus (P) in the potting mixture sample was calculated using Oslens’ method.15 Organic phosphorus in the sample was extracted using Ammonium Fluoride (NH4F) and Hydrochloric acid (HCl). The blue-coloured compound formed during the addition of Stannous Chloride (SnCl2) once it reacts with extracted organic phosphorus was estimated spectrophotometrically at 660 nm and the result was expressed as Kg/ha of sample. The total available potassium (K) in the sample was determined with the help of a Flame photometer.16 From 25 g of sample, organic potassium was extracted using Ammonium Acetate (NH4CH3CO2). The potash in the filtrate was determined with a flame photometer and the result was expressed as Kilogram/hectare (Kg/ha) of sample.17
Greenhouse study
Plant growth promoting activity of feather hydrolysate in promoting Spinacia oleracea L. growth was assessed. The experiment was laid out in randomized block design.18 Autoclaved soil-filled pots, each measuring 25 cm in width and 20 cm deep, were utilized for the experiment. A total of 20 treatment groups, including 4 concentrations (25%, 30%, 35% and 40% (v/v)) of feather hydrolysate, and 1 control group were established. Each treatment and control group consisted of triplicate pots, ensuring experimental reliability. Over a four-week period, the experiment was conducted in the greenhouse facilities of CHRIST (Deemed to be University) in Bangalore, under natural light conditions. Prior to planting, 1 kg of sterilized soil was added to each pot. The treatment groups received the designated concentration of feather hydrolysate, while the control groups received no supplementation. Throughout the study, plant growth parameters such as plumule length and chlorophyll content which are directly related to the vegetative growth and productivity of spinach were monitored and recorded each week for 4 weeks. The results were represented as mean ± standard deviation.
Preparation of feather hydrolysate
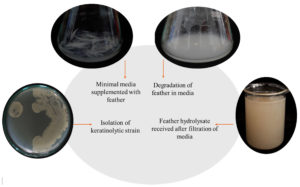
The feather hydrolysate required for the experiment was prepared using chicken feathers. The optimum incubation time for the degradation of chicken feathers was 4 days. After the incubation, a considerable amount of chicken feathers were degraded which was examined visually by the disintegration of the intact structure of chicken feathers. The transparent media turned to be opaque after the degradation of chicken feathers due to the breakage of protein leading to the dissolution of amino acids, oligopeptides and dipeptides. The process of feather degradation and the final feather hydrolysate obtained after degradation is depicted in Figure 1. A keratinolytic bacterial strain named as Kocuria rhizophila p3-3 was found to degrade 52% of chicken feathers with 4 days of incubation time leading to the generation of feather hydrolysate.19 Bacterial isolate Bacillus aerius NSMk2 showed hydrolysis of chicken feather in minimal salt medium with 5 days of incubation producing nutrient rich feather hydrolysate.20 The results of feather degradation align with the earlier reports of feather hydrolysate preparation indicating the effective production of feather hydrolysate by the incorporated keratinolytic bacterium Bacillus tropicus utilized for the present study.
Characterisation of feather hydrolysate
The filtrate received after separating undegraded chicken feather from the media is termed as feather hydrolysate which consist of amino acids, oligopeptides and dipeptides along with the cultured bacterial cells. Amino acids such as cysteine, valine, methionine, phenylalanine, isoleucine, lysine and proline are present in the feather hydrolysate which was estimated and quantified in the previous work of Liya and Umesh 2023.17 This feather hydrolysate will come under the category of liquid biofertilizer whose characteristics properties were tested to know the nature and composition of nutritional content of the liquid fertilizer. The results of the analysis have been consolidated in Table 1. The results revealed that the pH of the hydrolysate is suitable for application in soil as it comes under the neutral range which will not alter the natural pH of the soil/potting mix. The fertilizer was free of pathogens and heavy metals and was also odourless and colourless. Total NPK content of the hydrolysate was found to be 0.658% (v/v). Trace amounts of copper, iron, manganese, calcium and sulfur were also revealed in the elemental analysis of feather hydrolysate. Liquid fertilizer exhibits superiority over other forms of fertilizer due to its high potential to improve crop yield, improving overall growth of the plant, storage and ease of application.21 Application of chicken feather hydrolysate alone and chicken feather hydrolysate along with the fermentative bacterial culture showed the highest growth rate for Zea mays and Corchorus olitorius, respectively.22 Chicken feather hydrolysate produced by fermentation activity of Pseudomonas aeruginosa C1M strain, supported maximum growth of spinach as was reported by the works of Saba et al.23 In the current study, the nutritional composition analysis of feather hydrolysate revealed the presence of NPK needed for plant growth as well as trace amounts of other beneficial nutrients suggesting the applicability of hydrolysate as a liquid fertilizer.
Table (1):
Overview of compositional analysis of chicken feather hydrolysate
Parameters |
Values |
|---|---|
pH |
7.12 |
Colour |
Colourless |
Odour |
Odourless |
Electrical conductivity |
0.44 dms-1 |
Total Nitrogen |
0.65% (v/v) |
Total Phosphorus |
0.002% (v/v) |
Total potash |
0.006% (v/v) |
Copper |
1.70 ppm |
Iron |
23.26 ppm |
manganese |
1.49 ppm |
Calcium |
0.005 % (v/v) |
Sulphur |
0.001% (v/v) |
Lead, Cadmium, Chromium, Nickel |
Absent |
Pathogens |
Absent |
Germination Study
Green gram seeds were used for assessing the toxicity of chicken feather hydrolysate. The seeds put in 35% (v/v) concentration of chicken feather hydrolysate showed maximum growth of radicle upto 1 cm within 24 hours of incubation followed by 40% (v/v) which showed radicle growth around 0.8 cm. The concentration of feather hydrolysate upto 40% was found to be non-toxic to seeds. Radicle growth upto 0.5 cm and 0.6 cm was observed for 25% (v/v) and 30% (v/v), respectively. Control group of seeds which was put in distilled water instead of feather hydrolysate showed equal radicle growth length up 0.5 cm with the 25% (v/v) concentration. Figure 2 provides a visual representation of radicle growth of green gram soaked in above mentioned individual concentrations of feather hydrolysate in comparison to the control. Using the measurement of radicle length from each of the test and control group, germination index (GP%), relative seed germination (RSG%), relative root growth (RRG) and germination index (GI) was calculated which is summarized in Table 2. Germination percentage was equal for 35%, 40% as well as the control group which was followed by 30%. Seeds in 20% concentration and control seeds showed least seed germination percentage and all other calculated parameters when compared to test groups as well as control. The major reason for this is because of the least concentration of feather hydrolysate when compared to the other test groups while in the control group the natural germination environment favored the seed germination. Even though the germination percentage was equal for 35%, 40% and control, the presence of growth promoting factor in the test groups elevated the growth of radicles leading to more length gain of radicle length in test groups than in control groups. Similar results were observed in the work of Kaur et al. where higher concentration of feather hydrolysate supported maximum mung bean seeds germination.16 Relative seed germination rate has reached upto 193.3% for poultry based manure which is a representation that feather hydrolysate can contribute to seed germination regardless of plant growth.24 In line to the findings of present study, wheat seed germination and seedling growth were facilitated by chicken feather hydrolysate.25
Table (2):
Estimation of germination of green gram seeds under the influence of feather hydrolysate (FH)
| Parameters Estimated | Concentration of FH (% (v/v)) | ||||
|---|---|---|---|---|---|
| 25 | 30 | 35 | 40 | control | |
| Germinated seeds (no.s) | 16/20 | 19/20 | 20/20 | 20/20 | 20/20 |
| Average radicle length (cm) | 0.5 | 0.6 | 1.00 | 0.8 | 0.5 |
| GP % | 80 | 95 | 100 | 100 | 100 |
| RSG % | 80 | 95 | 100 | 100 | 100 |
| RRG % | 100 | 120 | 200 | 320 | 100 |
| GI % | 80 | 114 | 200 | 320 | 100 |
Determination of NPK content of potting mixture
The effect of feather hydrolysate on the growth of spinach was tested by adding feather hydrolysate to soil in which the spinach seeds have been sown and germinated. The potting mixture consisted of red soil, cocopeat and sand mixed in a specific ratio which was tested for its NPK content. The pH of the potting mixture after its preparation was 7.5, available nitrogen in the potting mixture was estimated to be 113 Kg/ha, available phosphorus content was found to be 8.4 Kg/ha and available potassium content was estimated to be 1792 Kg/ha. Apart from NPK content the amount of organic carbon in the potting mixture was also estimated and it was found to be 4.8% (w/w).
Spinach plant is a quick growing plant which gets adapted to soil conditions especially sandy soil. The pH of the potting mixture is well enough to support plant growth as previous reports suggest that the optimum pH for the healthy growth of spinach ranges between 6 and 7.5.26 The potting mixture prepared in the current study consists of an estimated nitrogen content of 113 Kg/ha which is beneficial for the growth of spinach. This result is supported by the experimental results from the work of Ekinci et al. 2019 which reports higher leaf area, leaf number, Stem diameter, leaf and root fresh and dry weight with an addition of 150-200 kg/ha of nitrogen.27 Phosphorus content of the potting mixture was estimated to be 8.4 Kg/ha. Earlier studies on the yield and seed setting potential of spinach on the influence of phosphorus reports that a maximum of 31.68 Kg/ha of phosphorus is enough to produce good yield of spinach and further seed setting.28 In the current study, the amount of phosphorus present in the potting mixture is efficient enough to support spinach growth which could also be elevated by the addition of feather hydrolysate supplement. Significant amounts of NPK in the potting mixture is advantageous for growth of spinach as the interactive effect of NPK improved total phenolic content, total antioxidant activity, total flavonoid content and total vitamin c content of spinach.29 Biochars which consist mainly of carbon have been found to support the growth of spinach even in heavy metal contaminated soil.30 In addition to NPK, the availability of carbon in the potting mixture is a desirable factor for plant growth.
Growth enhancement of spinach by feather hydrolysate
Spinach plants require a considerable amount of nitrogen and other bioactive compounds for its healthy growth. Feather hydrolysate being a mixture of amino acids and bioactive compounds is sufficient to support plant growth. Application of chicken feather hydrolysate has proved to increase the growth of spinach in all the test groups starting from the lowest concentration till the highest concentration in consideration. The test groups showed better growth performance than the control group with 35% (v/v) of the feather hydrolysate supplemented group being the highest promoter of spinach growth. Under this concentration, the plant acquired a plumule length of 14 cm at the end of 4 weeks and a total chlorophyll content of 27.6 mg/g. From 20% concentration, plumule length and total chlorophyll content increased steadily till 35% concentration after which the values declined for 40%. This is majorly because of the presence of excessive amounts of amino acids around the roots of the plants which caused toxicity to the plant leading to lesser nutrient absorption than the lower concentrations. Amino acids contain nitrogen. Excess of nitrogen in around the plant root causes inhibition of amino acid uptake via active transport causing building up of nitrogen and burning of roots.30 Thus, the current study provided an insight into the effective concentrations of feather hydrolysate for plant growth promotion with 35% (v/v) selected as the optimum concentration to support growth of spinach. The graphical representation of plumule growth and total chlorophyll content for the plant over the 4 weeks of experimentation is given in Figure 3.
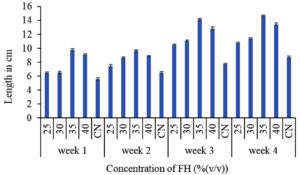 (a) Effect of feather hydrolysate on plumule length of spinach
(a) Effect of feather hydrolysate on plumule length of spinach
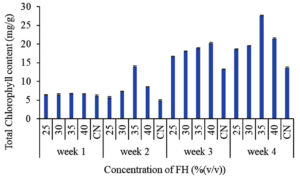 (b) Effect of feather hydrolysate on total chlorophyll content of spinach
(b) Effect of feather hydrolysate on total chlorophyll content of spinach
Figure 3. Graphical representation of influence of feather hydrolysate (a) plumule length and (b) total chlorophyll content
Feather protein hydrolysate has been found to complement chemical fertilizers leading to improved plant growth. For instance, use of feather protein hydrolysate along with chemical fertilizer have shown to improve leaf area, dry weight, and chlorophyll content in mung beans compared to the control plants.31 Greener plants with increased leaf biomass, leaf number and plant length were observed when treated poultry feather waste was used as an agent for plant growth promotion.32,33 In a study conducted by Gurav et al. 2020, feather hydrolysate concentration 20% (v/v) provided to crops like Brinjal and Chilli through root drenching displayed better plant growth with increased plant height and early flower setting suggesting that higher concentrations of feather hydrolysate supports plants in diverse means.10 Higher concentrations of chicken feather hydrolysate have been found to be beneficial for plants like tea plants but found to be phytotoxic to some fruiting plants after repeated application of hydrolysate.34,35
Spinach is a cash crop whose production can be increased by advanced fertilization and agricultural techniques. Chicken feather hydrolysate which are usually left out as waste material could be valorised so that plant growth promoting factors like amino acids, oligopeptides and dipeptides could be generated from the waste which could serve as a nutritional supplement for the growth of plants. The potential of an earlier isolated keratinolytic bacterial strain Bacillus tropicus to degrade chicken feathers and produce feather hydrolysate have been experimented in this study; to test out the effectiveness of protein hydrolysate to support spinach growth. It was found that feather hydrolysates at different concentrations can promote plant growth by increasing total plumule length and chlorophyll content leading to increased productivity. The current study gives a preliminary insight into preparation and application of feather hydrolysate. Further study on changes in phytochemical content as well as biochemical stress response upon feather hydrolysate addition can bring about more addition to the existing knowledge.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Life Sciences, CHRIST (Deemed to be University) for providing the necessary facilities and support for carrying out the research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
LMS performed methodology and investigated the study. MU supervised the work, reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
The project was funded by a scholarship provided to the author on behalf of Savitribai Jyotirao Phule Fellowship for Single Girl Child (SJSGC).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Shahrajabian MH, Cheng Q, Sun W. The effects of amino acids, phenols and protein hydrolysates as biostimulants on sustainable crop production and alleviated stress. Recent Pat Biotechnol. 2022;16(4):319-328.
Crossref - Czelej M, Garbacz K, Czernecki T, Wawrzykowski J, Wasko A. Protein Hydrolysates Derived from Animals and Plants-A Review of Production Methods and Antioxidant Activity. Foods. 2022;11(13):1953.
Crossref - Rouphael Y, Carillo P, Cristofano F, Cardarelli M, Colla G. Effects of vegetal- versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci Hortic . 2021;284:110123.
Crossref - Ramalingum N, Bhagwat P, Permaul K, Pillai S. Production, characterization, and application of Pseudomonas aeruginosa S-04 keratinase for feather utilization. Biomass Conv. Bior. 2024;14(10):11683-11695.
Crossref - Khan S, Yu H, Li Q, et al. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy. 2019;9(5):266.
Crossref - Corte L, Dell’abate MT, Magini A, et al. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates—a laboratory multidisciplinary approach. J Sci Food Agric. 2014;94(2):235-245.
Crossref - Bhattarai G, Shi A. Research advances and prospects of spinach breeding, genetics, and genomics. Vegetable Research. 2021;1(1):1-18.
Crossref - Manisha V, David AA, Thomas T, Swaroop N, Hasan A. Effect of integrated nutrient management practices on soil health, quality and yield of spinach (Beta vulgaris L.) grown on alluvial soil. Pharma Innovation. 2021;10(10):2068-2071.
- Liya SM, Umesh M, Nag A, et al. Optimized production of keratinolytic proteases from Bacillus tropicus LS27 and its application as a sustainable alternative for dehairing, destaining and metal recovery. Environ Res. 2023;221:115283.
Crossref - Gurav R, Nalavade V, Aware C, et al. Microbial degradation of poultry feather biomass in a constructed bioreactor and application of hydrolysate as bioenhancer to vegetable crops. Environ Sci Pollut Res Int. 2020;27(2):2027-2035.
Crossref - Walter I, Martinez F, Cala V. Heavy metal speciation and phytotoxic effects of three representative sewage sludges for agricultural uses. Environ Pollut. 2006;139(3):507-514.
Crossref - Rodriguez-Espinosa T, Papamichael I, Voukkali I, et al. Nitrogen management in farming systems under the use of agricultural wastes and circular economy. Sci Total Environ. 2023;876:162666.
Crossref - Nyabami P, Weinrich E, Maltais-Landry G, Lin Y. Three years of cover crops management increased soil organic matter and labile carbon pools in a subtropical vegetable agroecosystem. Agrosyst Geosci Environ. 2024;7(1):e20454.
Crossref - Keskinen R, Suojala-Ahlfors T, Sarvi M, et al. Granulated broiler manure based organic fertilizers as sources of plant available nitrogen. Environ Technol Innovat. 2020;18:100734.
Crossref - Akter J, Islam A, Kibria KQ, Limon SH, Romic M, Islam A. Effects of chicken feather hydrochar on soil amelioration and plant growth in an alkaline soil. Arabian J Geosci. 2022;16(1):9.
Crossref - Kaur M, Bhari R, Singh RS. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia. 2021;76(6):1807-1815.
Crossref - Liya SM, Umesh M. Bioconversion of chicken feather waste into feather hydrolysate by multifaceted keratinolytic Bacillus tropicus LS27 and new insights into its antioxidant and plant growth-promoting properties. Biomass Conv. Bioref. 2023.
Crossref - Patel VK, Vikram B, Sikarwar PS, Sengupta J. Effect of different levels of nitrogen and phosphorus on growth and yield of spinach (Spinacea oleracea L.) cv. all green. J Pharmacogn Phytochem. 2021;10(1):2229-2231.
- Laba W, Zarowska B, Chorazyk D, et al. New keratinolytic bacteria in valorization of chicken feather waste. AMB Expr. 2018;8(1):9.
Crossref - Bhari R, Kaur M, Singh RS, Pandey A, Larroche C. Bioconversion of chicken feathers by Bacillus aerius NSMk2: A potential approach in poultry waste management. Bioresour Technol Repo. 2018;3:224-230.
Crossref - Allouzi MMA, Allouzi SMA, Keng ZX, Supramaniam CV, Singh A, Chong S. Liquid biofertilizers as a sustainable solution for agriculture. Heliyon. 2022;8(12):e12609.
Crossref - Gupta S, Sharma S, Aich A, et al. Chicken Feather Waste Hydrolysate as a Potential Biofertilizer for Environmental Sustainability in Organic Agriculture Management. Waste and Biomass Valorization. 2023;14(9):2783-2799.
Crossref - Saba M, Akhter A, Ahmed H, et al. Sustainable Valorization of Chicken Feathers and Grocery Waste as Organic Fertilizer and its Impact on Yield and Quality of Spinach (Spinacia oleracea) Plant. Commun Soil Sci Plant Anal. 2023;54(21):2995-3005.
Crossref - Haruna SG, Mahmud BA, Dawakiji AY. Evaluation of vermicomposts from three selected agricultural wastes on seed germination and seedling growth of tomato (Solanum lycopersicum l). ADAN Journal of Agriculture. 2020;1(1):55-64.
Crossref - Sun Z, Li X, Liu K, Chi X, Liu L. Optimization for Production of a Plant Growth Promoting Agent from the Degradation of Chicken Feather Using Keratinase Producing Novel Isolate Bacillus pumilus JYL. Waste Biomass Valorization. 2021;12(4):1943-1954.
Crossref - Parwada C, Chigiya V, Ngezimana W, Chipomho J. Growth and Performance of Baby Spinach (Spinacia oleracea L.) Grown under Different Organic Fertilizers. International Journal of Agronomy. 2020;2020.
Crossref - Ekinci M, Atamanalp M, Turan M, et al. Integrated Use of Nitrogen Fertilizer and Fish Manure: Effects on the Growth and Chemical Composition of Spinach. Commun Soil Sci Plant Anal. 2019;50(13):1580-1590.
Crossref - Islam MR, Khatun K, Mostarin T, et al. Effect of nitrogen and phosphorus on the growth and seed yield of spinach. Asian Plant Res J. 2019;3(1):1-9.
Crossref - Zikalala BO, Nkomo M, Araya H, Ngezimana W, Mudau FN. Nutritional quality of baby spinach (Spinacia oleracea L.) as affected by nitrogen, phosphorus and potassium fertilisation. S Afr J Plant Soil/S-Afr Tydskr Plant Grond. 2017;34(2):79-86.
Crossref - Boostani HR, Najafi-Ghiri M, Mirsoleimani A. The effect of biochars application on reducing the toxic effects of nickel and growth indices of spinach (Spinacia oleracea L.) in a calcareous soil. Environ Sci Pollut Res Int. 2019;26(2):1751-1760.
Crossref - Nurdiawati A, Suherman C, Maxiselly Y, et al. Liquid feather protein hydrolysate as a potential fertilizer to increase growth and yield of patchouli (Pogostemon cablin Benth) and mung bean (Vigna radiata). Int J Recycl Org Waste Agricult. 2019;8(3):221-232.
Crossref - Joardar JC, Rahman MM. Poultry feather waste management and effects on plant growth. International Int J Recycl Org Waste Agricult. 2018;7(3):183-188.
Crossref - Raguraj S, Kasim S, Md Jaafar N, Nazli MH. Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers. Agronomy. 2022;12(2):299.
Crossref - Biswas I, Mitra D, Senapati A, et al. Valorization of Vermicompost with Bacterial Fermented Chicken Feather Hydrolysate for the Yield Improvement of Tomato Plant: A Novel Organic Combination. International Journal of Recycling of Organic Waste in Agriculture (IJROWA). 2023;10 (1): 29-42.
Crossref - Jones DL, Darrah PR. Amino-acid Influx at the Soil-root Interface of Zea Mays L. and Its Implications in the Rhizosphere. Plant Soil. 1994;163(1):1-12.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.