ISSN: 0973-7510
E-ISSN: 2581-690X
Streptococcus pyogenes is a gram positive pathogen causing pharyngitis, mild infections to chronic complications (Rheumatic Heart Disease, RHD). In this study, echocardiographic and clinical profile in pharyngitis, rheumatic fever patients were compared with virulent genes emm, spe A, spe B and sof. Nearly 107 subjects were classified into Group I – Pharyngitis (n=30), Group II – Rheumatic Fever (n=30) and Group III – healthy controls (n=47). The isolated S.pyogenes from Group I and Group II patient’s throat swab were subjected to 16S rRNA gene sequence. Multiplex PCR was done for identification of virulent genes. Electrocardiogram and Echocardiography was done for all the groups. For statistical analysis ANOVA and t-test were used. Comparison between groups were done by Tukey’s Multiple Comparison test. Among 107 isolates, 16.7% emm gene were detected in Group I and 23.3% in Group II, 56.67 % of spe B in Group I and 73.33 % in Group II, 36.67% of sof gene in Group I and 40% in Group II. Mitral Regurgitation was most commonly encountered in rheumatic fever. Hemoglobin (<0.001) and RDW (<0.001) was significantly lower in Rheumatic Fever whereas Platelet count (<0.001) and Neutrophil (<0.001) was significantly higher when compared with control subjects by Tukey’s Multiple Comparison test. When we compared the genetic relationship with the Echocardiographic findings, presence of one, two or three genes showed moderate to severe regurgitation in Rheumatic Fever subjects.
Pharyngitis, Rheumatic Fever, Virulent genes (emm, spe A, spe B, sof), 16S rRNA sequence, carditis
Streptococcus pyogenes, a β-hemolytic group A Streptococcus (GAS) is a pathogen primarily being transmitted through droplet inhalation, skin contact and common cause of morbidity worldwide.1,2 The spectrum of diseases caused by GAS may be mild sore throat or life threatening invasive infections, flesh-eating diseases (necrotizing fasciitis)3,4,5 with chronic complications like glomerulonephritis and an immune mediated, rheumatic fever (RF).2 Fever, nodules under the skin, rash, arthritis and carditis are the commonest symptoms of rheumatic fever.6 M protein is a virulence, a major antigenic epitope is being encoded by the emm gene which is cell wall associated and involved in the pathogenesis of S. pyogenes(Streptococcus pyogenes) infections.2,7
S. pyogenes produces a number of super-antigens, including erythrogenic toxins and antigenically distinct streptococcal pyrogenic exotoxins (spe), presence of these toxins is thought to be linked to pyrogenicity and myocardial necrosis. The pyrogenic exotoxin A (SpeA) is a causal factor in the pathogenesis of toxin-like-shock syndrome.8 Another virulence factor is streptococcal pyrogenic exotoxin B (speB) which is chromosomally encoded for pyrogenicity and cardiotoxicity.6,9 SOF encodes serum opacity factor, an unique protein with multiple functions, including an ability to bind to different proteins, including fibronectin, fibulin-1, and fibrinogen, thus enabling bacterial adhesion.10 In our study, echocardiographic and clinical profile in pharyngitis, rheumatic fever patients were compared with virulent genes like emm, spe A, spe B and sof.
Study Population
The individuals presenting with the history of pharyngitis, rheumatic fever were recruited from the Chettinad Super Specialty Hospital between January 2020 to January 2021. Institutional Human ethical committee (619/IHEC/12-19) approval was obtained.
The study population consisted of 107 subjects which were classified into Group I as Pharyngitis (n=30), Group II as Rheumatic Fever (n=30) and Group III as healthy controls (n=47). Throat swabs and blood samples were collected from all the subjects with the history of throat infection. All the patients in group II met the modified Duckett Jone’s Criteria.11 A few patients presented with the reactivation of RF were also enrolled in this group.
Patients having secondary infections or any other diseases, suffering from other autoimmune diseases, features of heart failure (Left Ventricular Ejection Fraction, LVEF<50%) were excluded after analyzing their relevant investigations and careful clinical examination. Patients with suspected or proven throat infection/pharyngitis, Positive GAS culture, Recurrent/high fever with tonsillitis were included in this study.
Group III includes age and sex matched individuals with the negative family history, risk factors, past history of RF as well as not undergoing any treatment. These individuals were negative for throat swab culture and other parameters were normal.
Sample Collection and Laboratory Testing
Two throat swabs were collected from the inflamed areas with the help of a tongue depressor, one swab for the smear and the other for culture. The beta haemolytic colonies on blood agar after incubation at 37˚C, 5% CO2 were processed.12
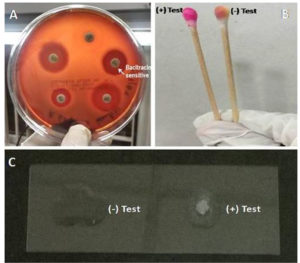
Isolates showing Gram positive cocci arranged in short chains, negative slide catalase test and positive PYR (Pyrrolidonyl arylamidase) test with cherry red colour were confirmed as genus Streptococci (Fig. 1) and group identification was done by Latex agglutination test (LK06 – HiStrep™ Latex Test Kit)
Fig. 1. Confirmation of Streptococcus pyogenes.
Note: A) Antibacterial Susceptibility Test. B) Pyrrolidonyl Arylamidase (PYR) Test. C) Catalase Test.
Kirby Bauer disk diffusion method was used for Antibiotic Susceptibility Testing with Muller Hinton agar (5% sheep blood).13 The antibiotic discs used were penicillin (10 units), cefotaxime (30μg), ampicillin (10μg), vancomycin (30μg), tetracycline (13μg), erythromycin (15μg), azithromycin (15μg), linezolid (30μg), ofloxacin (5μg), clindamycin (2μg), and bacitracin (0.04 units) (Fig. 1).
Nucleotide Sequencing
The characterization and recognition of 10 S. pyogenes strains were done by 16S rRNA sequences. The individual sequences were subjected to similarity search and comparison in the NCBI database using the similarity tool BLAST.14 The identification of strains was based on the maximum similarity hits obtained from BLAST. The 16S rRNA sequence strains were submitted to the GenBank nucleotide database of NCBI, the sequence was accepted and the accession number for each sequence was obtained separately.
PCR amplification
For DNA extraction from the isolates, a commercial extraction kit (Qiagen) was used as per manufacturer’s instructions. Virulent genes such as emm, speA, speB, sof were screened using PCR specific primers:emm15F:5’-TATTCGTTAGAAAATTAA-3′, R: 5’-GCAAGTTCTTCAGCTTGTTT-3’, speA16F: 5′-TAA GAA CCA AGA GAT GG-3′, R:5′-ATT CTT GAG CAG TTA CC -3′, speB17F: 5′-CAA CCA GTT GTT AAA TCT CT-3′, R: 5′-CTA AGG TTT GAT GCC TAC AA-3′, sof18F: 5′-TAGCCCCGACAGTT TTAGGA-3′, R: 5′-AGGCTGGAGTAGTGCCTGAA-3′. The primer concentration used here is 200nM per reaction and the concentration of DNA is 20ng/ml. spe A gene was not detected in uniplex PCR. Therefore, multiplex PCR was carried out for emm, spe B and sof genes. Reactions were run in a thermal cycler (Eppendorf, Applied Biosystem) with following conditions: 25 µl mixture containing 0.5μl each of forward and reverse primers, DNA Template- 5μl, Master mix- 12.5μl, Milliqwater- 4.5 μl under the following thermocycling conditions: Initial denaturation at 96°C for 5 min; 35 cycles of Denaturation 96°C, 50 s, Annealing at 50°C, 65 s and Extension at 72°C, 1 min and one final extension cycle at 72°C for 5 min. The size of each PCR product is variable which was analyzed by electrophoresis in 0.8% agarose gel and visualized under an ultraviolet spotlight, after staining with ethidium bromide.
Antistreptolysin O (ASO)
ASO titers were analyzed, > 200 IU/ml were taken as positive (Beacon Latex agglutination kit). Other biochemical and hematological profile like Hemoglobin (Hb), C – Reactive protein (CRP), Red Blood cell Distribution Width (RDW), Platelets, Neutrophil, were taken from patients records.
Electrocardiogram and Echocardiography
A 12-Lead ECG was performed by using BPL CARDIART 6208 VIEW PLUS. Echocardiograms were performed for all the subjects at rest. It included the M-mode and two-dimensional mode, besides a doppler examination with the color-flow mapping by using Vivid S5, Esaote [MyLab 25Gold] machines. From the parasternal short axis view; by using M-mode, systolic and diastolic diameters were measured. Systolic function and dimensions of the left ventricle were obtained and the ejection fraction was obtained by the Simpson’s method, and values above 55% were considered normal.
By using two-dimensional echocardiography, morphological features of the valves were evaluated. From the color-flow Doppler examination, the severity of regurgitations and stenosis were determined and these were reported according to the recommendations of ASE (American Society of Echocardiography). Depending on the severity of valve pathology, grading were given as mild, moderate, or severe. Stenosis of the valve was assessed based on the valve area, mean gradient, peak gradient and velocity. In parasternal long axis and short axis views, valve leaflet morphology was analyzed.
Statistical Analysis
GraphPad PRISM 8 software was used for Data analysis. Continuous variables were represented as the mean ± standard deviation (SD). Categorical variables were expressed as numbers with proportions, n (%). Comparison of Blood profile for all the three groups, One way ANOVA was used to find the statistical significance and the statistical comparison between the groups were seen by using Tukey’s Multiple comparison test. For comparing two groups, unpaired t-test was used. Box whisker plot were used for identifying the distribution of data through their quartiles. p value <0.05 was taken as statistical significance.
During a period of one year, throat swabs of patients with pharyngitis and rheumatic fever were correlated for genetic profiling with clinical and echocardiographic findings. Of the 107 throat swabs, 45 were positive for GAS. Of which, 30 were of Group I and 15 were of Group II.
Antibiotic susceptibility test showed that most of the isolates were highly sensitive to the antibiotics penicillin (100%), Bacitracin (100%), Ampicillin (93.3%), Cefotaxime (91.1%), Azithromycin (91.1%), Ofloxacin (88.8%), Linezolid (84.4%), Clindamycin (84.4%), Tetracycline (84.4%), Vancomycin (80%) and Erythromycin (73.3%) Table 1.
Table (1):
Antibiotic susceptibility pattern of S.pyogenes.
| Antibiotic | Total Isolates | Sensitive | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|---|
|
45 | 45 | 100% | – | – | – | – |
| 2. Cefotaxime | 41 | 91.1% | 4 | 8.8% | – | – | |
| 3. Ampicillin | 42 | 93.3% | 3 | 6.6% | – | – | |
| 4. Vancomycin | 36 | 80% | 6 | 13.3% | 3 | 6.6% | |
| 5. Tetracycline | 38 | 84.4% | 3 | 6.6% | 4 | 8.8% | |
| 6. Erythromycin | 33 | 73.3% | 7 | 15.5% | 5 | 11.1% | |
| 7. Azithromycin | 41 | 91.1% | 1 | 2.2% | 3 | 6.6% | |
| 8. Linezolid | 38 | 84.4% | 5 | 11.1% | 2 | 4.4% | |
| 9. Ofloxacin | 40 | 88.8% | 1 | 2.2% | 4 | 8.8% | |
| 10. Clindamycin | 38 | 84.4% | 5 | 11.1% | 2 | 4.4% | |
| 11. Bacitracin | 45 | 100% | – | – | – | – | |
16s rRNA sequence analysis
In the current study, the pure isolated Streptococcus pyogenes from Group I and Group II patients are exposed to 16S rRNA sequences. The different lengths of the nucleotide bases created by this study have been exposed to a BLAST analysis. In Table 2, BLAST results of 16S rRNA gene sequence showed all the isolates were greater than 99.10% similarity (99.10 – 100%) is among the objective series.
Table (2):
BLAST analysis, similarity and NCBI Genbank accession numbers of S.pyogenes.
No |
Clinical Sample |
Bacteria |
Similarity (%) |
Length |
Blast Result |
Accession No |
|---|---|---|---|---|---|---|
1 |
Throat Swab |
S.pyogenes strain S1 |
100 |
220 |
S.pyogenes |
OK632107 |
2 |
Throat Swab |
S.pyogenesstrain S2 |
99.10 |
226 |
S.pyogenes |
OK632065 |
3 |
Throat Swab |
S.pyogenesstrain S3 |
99.11 |
238 |
S.pyogenes |
OK632066 |
4 |
Throat Swab |
S.pyogenesstrain S4 |
100 |
215 |
S.pyogenes |
OK632282 |
5 |
Throat Swab |
S.pyogenesstrain S5 |
100 |
234 |
S.pyogenes |
OK632273 |
6 |
Throat Swab |
S.pyogenesstrain S6 |
99.57 |
236 |
S.pyogenes |
OK632365 |
7 |
Throat Swab |
S.pyogenesstrain S7 |
100 |
185 |
S.pyogenes |
OK632376 |
8 |
Throat Swab |
S.pyogenesstrain S8 |
100 |
228 |
S.pyogenes |
OK632681 |
8 |
Throat Swab |
S.pyogenesstrain S9 |
100 |
233 |
S.pyogenes |
OK632463 |
10 |
Throat Swab |
S.pyogenesstrain S10 |
100 |
221 |
S.pyogenes |
OK632677 |
Group I
Throat swab culture were positive for all the patients. In blood profile, CRP and ASO titres were elevated among these individuals with the mean of 27.7 ±15.4 and 683.3±322.6 respectively. Other parameters like Hb, Platelets, Neutrophil and RDW and their mean ± SD are listed in Table 3. Pharyngitis cases were predominantly seen in male patients (Fig. 2).
Table (3):
Blood Profile of the Groups.
Blood Profile |
Group I (Mean ± SD) |
Group II (Mean ± SD) |
Group III (Mean ± SD) |
p-value |
|---|---|---|---|---|
CRP |
27.7 ± 15.4 |
54.4 ± 41.6 |
0.0017a |
|
ASO |
683.3 ± 322.6 |
896.6 ± 349.0 |
0.0262 a |
|
Hb |
13.2 ± 1.7 |
11.6 ± 1.6 |
13.7 ± 1.3 |
< 0.0001 b |
Platelets |
398267 ± 111983 |
485500 ± 102250 |
294617 ± 91243 |
< 0.0001b |
Neutrophil |
57.4 ± 4.3 |
69.6 ± 4.9 |
53.4 ± 7.0 |
< 0.0001 b |
RDW |
12.7 ± 0.7 |
11.8 ± 1.2 |
13.2 ± 1.0 |
< 0.0001 b |
Note: SD-standard deviation, a t-test, b ANOVA
In ECG, Sinus tachycardia was the commonest finding (n=21). In all these pharyngitis cases, echocardiography was normal. Genotypic screening confirmed the presence of three genes (emm, spe B, sof) in one patient, two genes (emm, spe B) in four patients and (spe B, sof)in five patients. In single gene presentation, seven patients were positive for spe B gene and five were positive for sof gene.
Group II- Rheumatic Fever
In rheumatic fever (n=30), all the patients met the modified Duckett Jone’s criteria. Among RF, reactivation noted in eight patients, four patients with two major criteria, three patients with two major + two minor criteria, one patient with one major + three minor criteria. In newly diagnosed RF, twelve patients with one major and two minor criteria, seven patients with two major + three minor criteria and three patients with two major + one minor criteria were noted.
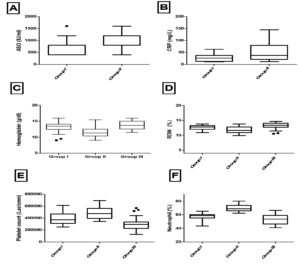
Blood Profile like Hb, RDW, Platelets, Neutrophil, ASO, CRP for these RF cases are given in Table 3. One patient in this group was negative for ASO titre and CRP, but was positive for anti-DNAse B titre (751 IU/ml) and he had severe mitral and aortic regurgitation in Trans-esophageal echocardiography. Distribution of blood profile data through their quartiles are given in Fig. 3 and significance of these profile for group comparison are given in Table 4.
Table (4):
Tukey’s Multiple comparison Test.
| Blood Profile | Groups | Mean Diff. | Q | Significant P < 0.05 |
Summary | 95% CI |
|---|---|---|---|---|---|---|
| Hemoglobin | Group I vs Group II | 1.58 | 5.77 | Yes | *** | 0.66 to 2.50 |
| Group I vs Group III | -0.54 | 2.19 | No | Ns | -1.38 to 0.29 | |
| Group II vs Group III | -2.12 | 8.56 | Yes | *** | -2.96 to -1.29 | |
| Platelets | Group I vs Group II | -87233 | 4.76 | Yes | ** | -149004 to -25462 |
| Group I vs Group III | 103650 | 6.24 | Yes | *** | 47743 to 159557 | |
| Group II vs Group III | 190883 | 11.5 | Yes | *** | 134976 to 246790 | |
| RDW | Group I vs Group II | 0.86 | 4.86 | Yes | ** | 0.26 to 1.46 |
| Group I vs Group III | -0.52 | 3.22 | No | Ns | -1.06 to 0.024 | |
| Group II vs Group III | -1.38 | 8.59 | Yes | *** | -1.92 to -0.84 | |
| Neutrophil | Group I vs Group II | -12.12 | 11.49 | Yes | *** | -15.68 to -8.57 |
| Group I vs Group III | 4.08 | 4.27 | Yes | ** | 0.87 to 7.30 | |
| Group II vs Group III | 16.20 | 16.96 | Yes | *** | 12.99 to 19.42 |
Note: ***Extremely significant (<0.001), ** Very significant (0.001 to 0.01),*Significant (0.01 to 0.05), ns- not significant (>0.05), CI- confidence interval, diff- difference.
Fig. 3. Box-whisker plots showing Blood parameters of all the Groups.
Note: A) ASO, B) CRP, C) Hemoglobin, D) RDW, E) Platelet, F) Neutrophil
Among the reactivation cases, one patient presented with known case of RHD (Rheumatic Heart Disease)- Mitral stenosis (MS) and she underwent Closed Mitral Valvotomy for about 22 years back and Balloon Mitral valvotomy for about 15 years back. While collecting the sample, she had the complaints of fever, cold, cough and Joint pain. More number of rheumatic fever cases were documented under 20 years of age and females (56.7%) were affected predominantly than the males (43.3%). (Fig. 2).
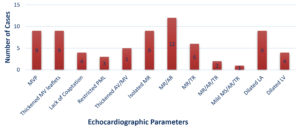
In ECG, prolonged PR interval was the commonest finding (n=13) followed by Sinus tachycardia (n=9).In Echocardiography, Color Flow Doppler findings and morphological changes of the valve with Mitral Regurgitation (MR), Aortic Regurgitation (AR), dilated left atrium (LA), left ventricle (LV) were seen (Fig. 4) Of 30 patients with Rheumatic Carditis, 19 were mild, seven were moderate and four had severe regurgitation. All the patients had normal ejection fraction (EF>55%).
Fig. 4. Echocardiographic Parameters in Group II.
Note: MVP-mitral valve prolapse, MV- mitral valve, AV-aortic valve, PML-posterior mitral leaflet, MR-mitral regurgitation, AR- aortic regurgitation, TR- tricuspid regurgitation, MS-mitral stenosis.
Genetic Marker
Combination of three genes, emm, spe B and sof were seen in three patients, presence of two genes like emm and spe B were seen in two patients, spe B and sof were seen in one patient. Single gene presentations of emm in two patients, spe B in five patients, sof in two patients were documented. The presence of emm gene along with the super antigens showed a significant changes in echocardiography. In that, presence of three genes showed severe regurgitation when compared with the presence of two genes and single gene. This genetic profile along with the implications of echocardiography is shown in Table 5.
Table (5):
Presence of virulent genes and the implications of Echocardiography.
No |
Genes |
No of Patients (n=7) |
Echocardiography |
|---|---|---|---|
1. |
emm |
1 |
Moderate MR |
2. |
Emm |
1 |
Moderate MR, Mild AR |
3. |
emm + spe B |
1 |
Moderate MR, Mild TR |
4. |
emm + spe B |
1 |
Moderate MR, Mild AR, Mild TR |
5. |
emm + spe B |
1 |
Moderate MR, Moderate AR |
6. |
emm+sof+ spe B |
2 |
Severe MR, Moderate AR |
Group III- Controls
This group of individuals were with history of sore throat had negative GAS culture, negative ASO titre and CRP. This group of patients were not under any other treatment. In ECG, Sinus tachycardia (n=27) alone was observed whereas Echocardiography was normal for all the patients.
In this study, Echocardiographic findings and Clinical profile of Pharyngitis, Rheumatic Fever patients along with the presence of virulent genes were analysed. A study done by Francis et al.,19 in aboriginal Australians have showed GAS positive culture comparison with the Jones criteria and Echocardiographic findings in Rheumatic Fever patients. A study done in Turkey documented that Hemoglobin and RDW was significantly lower in rheumatic carditis whereas Platelet count and Neutrophil was significantly higher when compared with control subjects.20 Similar finding was observed in our study when we compared RF/RHD and pharyngitis with the control subjects. High C-Reactive Protein (CRP), an indicator of bacterial infection is used to assess the disease severity.21 CRP level was found to be higher in Group I and Group II. Kim et al.,22 documented ASO levels were highest in the children. Martins et al.,23 documented ASO levels were significantly higher in RF patients when compared with Pharyngitis. Similarly, in our study Group I had raised ASO titre with the mean of 683.3±322.6, Group II had a mean value of 896.6±349.0. Thus ASO titre indicates that the patients were infected with GAS in the past or due to reinfection.
A study done by Negi et al.,24 documented RF/RHD is found to be more prevalent in females indicating that the pattern of valvular involvement has a strong association with the Gender. In our study, females were found to be predominantly affected in Rheumatic Fever. According to WHO25 and WHF 2021,26 the most common heart disease in young people <25 years of age is RHD. In our study, under 20 years of age were documented more in case of Rheumatic Fever. If left untreated, these individuals may go in to established RHD condition.
Some studies correlated the emm types, superantigen (SAg) gene profiles with antibiotic resistance genes,27 virulence factors with GAS,28 Vir and emm typing,29 emm typing30 alone or in relation with SAgs profile.17 Other investigators found SAg both in invasive and non-invasive infections.7,31 In genotyping; out of 107 isolates screened, 16.7% emm gene in Group I and 23.3% in Group II. This emm gene encoded M protein are known for producing heart reactive antibodies and responsible for crossreactivity.32
In the study conducted by Nandi et al.,33 spe A gene was detected in pharyngitis (6%) and RF⁄RHD (25%). Our study was in concordance with the study done by Mathur et al., in not detecting spe A gene as these are associated with rare invasive GAS infections (toxic shock syndrome, scarlet fever).33,34 In a study done for the detection of spe B gene in pharyngitis patients documented 45% and 78%by Balaji et al.,27 and Dhanda et al.28 Nandi et al.,33 documented spe B of 86.5% in pharyngitis and 100% in RF/RHD. Mathur et al.,34 detected 100% of spe B in throat samples of GAS. In our study, 56.67 % of spe B were documented in Pharyngitis and 73.33% in RF/RHD. Kumar et al.,18 detected sof gene in pharyngitis patients. But in our study, we detected 36.67% of sof gene in Pharyngitis, 40% in Rheumatic Fever.
In Group I, ECG showed sinus tachycardia and Echocardiogram showed normal study. There were no abnormalities detected in ECG and Echocardiography in Pharyngitis group as this Streptococcus pyogenes involved only in the Upper Respiratory Tract in the initial stage of infection. In Group II, prolonged PR interval followed by sinus tachycardia was observed and Mitral Regurgitation (MR) was the commonest doppler finding. Our study findings were similar with the study done by Bejiqi et al.,35 where he reported that MR is commonly encountered in RF patients due to hemodynamic changes and morphologic lesion in patients with rheumatic fever and tricuspid valve was rarely documented. Similarly, in our study tricuspid valve involvement was not identified.
In Group II, presence of three genes showed severe regurgitation whereas two genes in three subjects showed moderate regurgitation, presence of single gene (emm) also showed the moderate regurgitation. But in Group I, presence of three or two or single gene does not showed any abnormality in the heart.
Nucleotide sequence accession numbers
A total of 10 sequences are submitted to the NCBI database. The Genbank accession numbers were given as: OK632681, OK632677, OK632463, OK632376, OK632365, OK632282, OK632273, OK632107, OK632066 and OK632065.
To the best of our knowledge, rare documentations were done on studying the genetic relationship with the echocardiographic findings. When we compared the genetic relationship with the echocardiographic findings, presence of three genes showed the severe regurgitation in RF subjects. Whether these genes were responsible for cardiac abnormalities need to be correlated with the larger set of population. Limitation of this study is smaller sample size and the emm typing. Genetic profile and its correlation with the echocardiographic parameters in the presence of virulent genes among the established RHD condition will be the future plan.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Dr. R. Murugesan (former Director – Research) Chettinad Academy of Research and Education, for critical review of the manuscript and valuable suggestions. The corresponding author also thanks the Chettinad Academy of Research and Education (CARE), Chettinad Hospital and Research Institute (CHRI) for providing the research fellowship.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Chettinad Academy of Research and Education, India with reference number 619/IHEC/12-19.
AVAILABILITY OF DATA
All datasets generated or analysed during this study are included in the manuscript.
- Pilon PA, Savard N, Aho J, et al. Invasive group A streptococcal infection outbreaks of typeemm118 in a long-term care facility, and of type emm74 in the homeless population, Montreal, Quebec. Canada Communicable Disease Report Releve des Maladies Transmissibles au Canada. 2019;45(1):24-31.
Crossref - Koutouzi F, Tsakris A, Chatzichristou P, et al. Streptococcus pyogenes emm types and clusters during a 7-year period (2007 to 2013) in pharyngeal and nonpharyngeal pediatric isolates. J Clin Microbiol. 2015;53(7):2015-2021.
Crossref - Bahnan W, Hashwa F, Araj G, Tokajian S. emm typing, antibiotic resistance and PFGE analysis of Streptococcus pyogenes in Lebanon. J Med Microbiol. 2011;60(1):98-101.
Crossref - Latronico F, Nasser W, Puhakainen K, et al. Genomic characteristics behind the spread of bacteremic group A Streptococcus type emm 89 in Finland, 2004-2014. J Infect Dis. 2016;214(12):1987-1995.
Crossref - Athey TB, Teatero S, Sieswerda LE, et al. High incidence of invasive group A Streptococcus disease caused by strains of uncommon emm types in Thunder Bay, Ontario, Canada. J Clin Microbiol. 2016;54(1):83-92.
Crossref - Kaushal A, Kumar D, Khare S, Kumar A. speB gene as a specific genetic marker for early detection of rheumatic heart disease in human. Cell Mol Biol. 2012;58(1):50-54.
Crossref - Lintges M, van der Linden M, Hilgers RD, et al. Superantigen genes are more important than the emm type for the invasiveness of group A Streptococcus infection. J Infect Dis. 2010;202(1):20-28.
Crossref - Musser JM, Hauser AR, Kim MH, Schlievert PM, Nelson K, Selander RK. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci. 1991;88(7):2668-2672.
Crossref - Hytonen J, Haataja S, Gerlach D, Podbielski A, Finne J. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol Microbiol. 2001;39(2):512-519.
Crossref - Courtney HS, Pownall HJ. The structure and function of serum opacity factor: a unique streptococcal virulence determinant that targets high-density lipoproteins. J Biomed Biotechnol. 2010;2010:956071.
Crossref - Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131(20):1806-1818.
Crossref - Kebede D, Admas A, Mekonnen D. Prevalence and antibiotics susceptibility profiles of Streptococcus pyogenes among pediatric patients with acute pharyngitis at FelegeHiwot Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Microbiol. 2021;21(1):135.
Crossref - Clinical Laboratory of Standard Institute (CLSI). Performance standard for antimicrobial susceptibility testing for streptococci spp beta-hemolytic bacteria. 30th edition, CLSI guideline. CLSI Standard M100- Wayne. 2020;40(1):88-91. https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf. Accessed November 15th 2021.
- Altschul SF, Madden TL, Schcaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389-3402.
Crossref - Khalaf NZ, Abdul-Jalil AA, Najeeb LM. Isolation and Amplification of Emm Gene From Streptococcus Pyogenes Isolated from Iraqi Children. International Journal of Pharmaceutical Research. 2020;14(1).
Crossref - Schmitz FJ, Beyer A, Charpentier E, et al. Toxin-gene profile heterogeneity among endemic invasive European group A streptococcal isolates. J Infect Dis. 2003;188(10):1578-1586.
Crossref - Altun M, Yapici BM. Detection of Group A Beta Hemolytic Streptococci Species, emm, and Exotoxin Genes Isolated from Patients with Tonsillopharyngitis. Curr Microbiol. 2020;77(9):2064-2070.
Crossref - Kumar A, Bhatnagar A, Gupta S, Khare S. sof gene as a specific genetic marker for detection of Streptococcus pyogenes causing pharyngitis and rheumatic heart disease. Cell Mol Biol. 2011;57(1):26-30.
Crossref - Francis JR, Gargan C, Remenyi B, et al. A cluster of acute rheumatic fever cases among Aboriginal Australians in a remote community with high baseline incidence. Aust N Z J Public Health. 2019;43(3):288-93.
Crossref - Kar YD, Gullu UU. The Role of Whole-blood Parameters in Predicting the Severity of Acute Rheumatic Carditis in Children. Medical Bulletin of Haseki/Haseki Tip Bulteni. 2021;59(2):178-183.
Crossref - Christensen AMG, Thomsen MK, Ovesen T, Klug TE. Are procalcitonin or other infection markers useful in the detection of group A streptococcal acute tonsillitis?. Scand J Infect Dis. 2014;46(5):376-383.
Crossref - Kim S, Lee NY. Asymptomatic infection by Streptococcus pyogenes in schoolchildren and diagnostic usefulness of antideoxyribonuclease B. J Korean Med Sci. 2005;20(6):938-940.
Crossref - Martins TB, Hoffman JL, Augustine NH, et al. Comprehensive analysis of antibody responses to streptococcal and tissue antigens in patients with acute rheumatic fever. Int Immunol. 2008;20(3):445-452.
Crossref - Negi PC, Kandoria A, Asotra S, et al. Gender differences in the epidemiology of Rheumatic Fever/Rheumatic heart disease (RF/RHD) patient population of hill state of northern India; 9 years prospective hospital based, HP-RHD registry. Indian Heart Journal. 2020;72(6):552-556.
Crossref - www.who.int/health-topics/rheumatic-heart-disease.Accessed November 15, 2021.
- Rheumatic heart disease infographic. World-heart-federation.org/resource/rheumatic-heart-disease-infographic/. Accessed November 15, 2021.
- Balaji K, Thenmozhi R, Prajna L, Dhananjeyan G, Pandian SK. Comparative analysis of emm types, superantigen gene profiles and antibiotic resistance genes among Streptococcus pyogenes isolates from ocular infections, pharyngitis and asymptomatic children in south India. Infect Genet Evol. 2013;19:105-112.
Crossref - Dhanda V, Vohra H, Kumar R. Group A Streptococcus virulence factors genes in north India & their association with emm type in pharyngitis. Indian J Med Res. 2011;133(1):110-115. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3100139/. Accessed November 15, 2021
- Sagar V, Bakshi DK, Nandi S, Ganguly NK, Kumar R, Chakraborti A. Molecular heterogeneity among north Indian isolates of Group A Streptococcus. Lett Appl Microbiol. 2004;39(1):84-88.
Crossref - Dey N, McMillan DJ, Yarwood PJ, et al. High diversity of group A Streptococcal emm types in an Indian community: the need to tailor multivalent vaccines. Clin Infect Dis. 2005;40(1):46-51.
Crossref - Khan RM, Anwar S, Pirzada ZA. Streptococcus pyogenes strains associated with invasive and non-invasive infections present possible links with emm types and superantigens. Iran J Basic Med Sci. 2020;23(1):133-139.
Crossref - Guilherme L, Fae KC, Oshiro SE, Tanaka AC, A Pomerantzeff PM, Kalil J. T cell response in rheumatic fever: crossreactivity between streptococcal M protein peptides and heart tissue proteins. Curr Protein Pept Sci. 2007;8(1):39-44.
Crossref - Nandi S, Chakraborti A, Bakshi DK, Rani A, Kumar R, Ganguly NK. Association of pyrogenic exotoxin genes with pharyngitis and rheumatic fever/rheumatic heart disease among Indian isolates of Streptococcus pyogenes. Lett Appl Microbiol. 2002;35(3):237-241.
Crossref - Mathur P, Bhardwaj N, Mathur K, et al. Clinical and molecular epidemiology of beta-hemolytic streptococcal infections in India. J Infect Dev Ctries. 2014;8(03):297-303.
Crossref - Bejiqi RA, Retkoceri R, Zeka N, Bejiqi H, Retkoceri A. Heart lesion after the first attack of the rheumatic Fever 22 years experience in single centre. Medical Archives. 2015;69(1):49-53.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.