ISSN: 0973-7510
E-ISSN: 2581-690X
Coronavirus disease 2019 (COVID-19) infections can be related to vast spectrum of co-existent bacterial and fungal infections. A 49-year-old diabetic male was admitted with a history of fever, cough and breathlessness since 5 days. He developed persistent headache with right sided purulent nasal discharge. Relevant histo-pathological, biochemical, microbiological and imaging studies were performed which proved it to be a dual infection of Aspergillosis and Mucormycosis. We present one such case in a COVID-19 patient to highlight its unusual clinical features along with the diagnostic and therapeutic challenges.
Mucormycosis, Aspergillosis, COVID-19, Diabetes, Fungal Infections
The (COVID-19) infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be interconnected with vast spectrum of disease patterns which ranges from Rhino-Orbital Cerebral Mucormycosis (ROCM) to life-threatening pneumonia along with superadded co-existent bacterial and fungal infections. These co-infections can be due to a prior co-morbid condition. Most common comorbidity associated with co-existent infections is Diabetes Mellitus (DM) and India has higher frequency of Type II DM (8.9% of adults, that is around 77 million patients, which is a major risk factor for COVID-19 co-infections.1 India has faced the first wave of COVID-19 pandemic on 11th March 2020 and second wave of COVID-19 pandemic on 1st March 2021 and is currently undergoing third wave of COVID-19 pandemic. During this pandemic several cases of ROCM were noted. However, reports of combined dual infection of Mucormycosis and Aspergillosis are very rare and the exact incidence of such dual infection remains unknown. Hence current study highlights a case of combined Mucormycosis and Aspergillosis in a COVID-19 patient for its rare occurrence.
Case Report
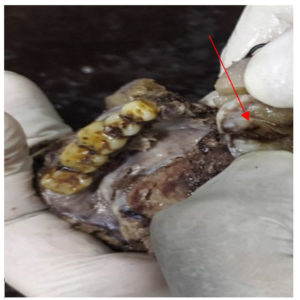
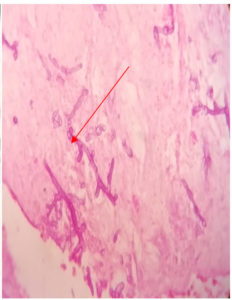
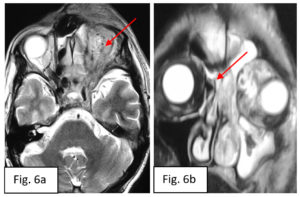
A 49-year-old male was admitted to our Hospital, with complaints of fever, cough and breathlessness since five days. He developed right sided headache which was gradually progressive, dull type of headache radiating to eyes, cheeks and forehead which occurred throughout the day. There was also history of right sided nasal discharge which was purulent, copious, non blood tinged and non foul smelling. Patient had history of type II DM since 12 years (on medication).No significant past history was noted. Patient appetite was adequate and patient complained of disturbed sleep. Local examination: Anterior rhinoscopy showed deviated nasal septum towards the right side with black crust noted on the middle turbinate. Bilateral nasal mucosa was congested. Left side nasal discharge was present. Right eye showed proptosis, congestion with peri-orbital edema and accumulation of tissue debris in the soft tissue surrounding medial half of upper and lower lids. The left eye was normal on examination with normal visual acuity. General examination: On examination: Temperature was 101°F, pulse: 72/minute, Blood pressure: 130/80 mmHg, respiratory rate: 20/minute and oxygen saturation of 88% on oxygen supplementation (10 liters/min). Physical examination: On clinical assessment all systems were within normal limits. Routine baseline investigations: Hb(hemoglobin): 11.5 gm/dl (normal 13-17 gm/dl), neutrophilia: 83% (normal 40-80%), increased ESR :70mm/hr(normal 0-20 mm/hr), elevated serum creatinine (1.68 mg/dl; normal 0.70-1.20 mg/dl). Biochemical investigations demonstrated: S. ferritin: 300 ng/ml (normal 20 to 250ng/ml), Serum free iron 180ug/dl (50 to 150 ug/dl). Radiological investigations: Nasopharyngeal swab was collected and RT-PCR was performed which turned out to be positive and diagnosis of COVID-19 was confirmed. Microbiological examination was done by preparing direct smear from pus and tissue using 10% KOH (potassium hydroxide) which demonstrated positive fungal elements. Culturing on SDA (Sabourauds Dextrose Agar) showed growth of Aspergillus and Mucormycosis. [Refer Figure 1]. Orbital exenteration and medial maxillectomy was the surgical procedure performed for the current case and same was sent for histopathological examination. Debrided sinus tissue specimen was sent for culture and sensitivity. Gross description of specimen of orbital exenteration received: Orbital exenteration specimen measuring 13 x 10.56 x 3.5 cm. External surface: unremarkable. Cut section: showed normal eyeball with areas of necrosis. [Refer Figure 2]. Gross description of Medial maxillectomy specimen: received medial maxillectomy specimen measuring 13 x 9 x 4.5cms. External surface: unremarkable. Cut section: showed area of necrosis. [Refer Figure 3] Microscopy: demonstrated characteristic fruiting bodies suggestive of Aspergillosis infection [Refer Figure 4] and also noted aseptate broad based hyphae suggestive of Mucormycosis infection [Refer Figure 5] suggestive of ROCM form of Mucormycosis. Non contrast Computed tomography(NCCT) [Refer Figure 6] of paranasal sinuses showed mild mucosal thickening in bilateral maxillary and right ethmoid sinuses, deviated nasal septum with bony septal spur towards the right side. MRI (Magnetic resonance imaging) of brain and orbit demonstrated maxillary sinus mucosal thickening and soft tissue swelling anterior to eyeball. [Refer Figure 6]. Patient was administered parenteral antiviral oseltamivir along with intravenous steroid (methylprednisolone) in accordance with hospital treatment protocol. For controlling Diabetes mellitus, Insulin was administered as per the sliding scale.
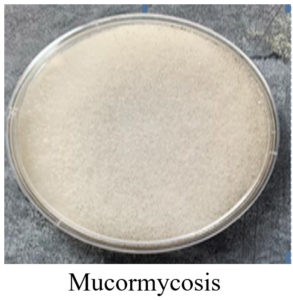
Figure 1a. Sabourauds dextrose agar culture (SDA): demonstrates clear cotton wool appearance suggestive of Mucormycosis
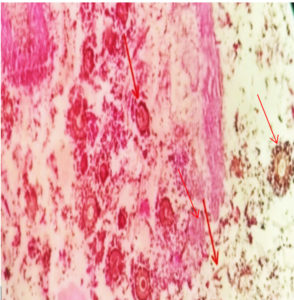
Figure 4. H & E (10 x) Demonstrates Both Aspergillosis and Mucormycosis. Fruiting bodies of Aspergillosis are observed along with broad, aspetate hyphae Resembling Mucormycosis
Figure 6. MRI(magnetic resonance imaging) brain and orbit demonstrated soft tissue swelling anterior to eyeball (Figure 6a) and maxillary sinus mucosal thickening (Figure 6b).
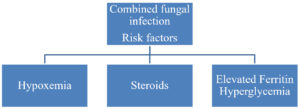
Mucormycosis is universally known as black fungus with prevalence rate of 0.14 cases per one thousand of Indian population.2 Mucormycosis is fatal deep fungal infection caused by the family Mucoraceae. There are six clinical forms of Mucormycosis, most common is the (ROCM) (44-48%) followed by the cutaneous type (10-19%), pulmonary (10-11%), disseminated (6-10%) and lastly the intestinal type (2-11%). Our case belongs to the ROCM form.3 Aspergillosis can manifest in two forms: Invasive Aspergillosis and pulmonary Aspergillosis.4 Aspergillosis and Mucormycosis co-infection frequently occurs in immune-compromised individuals. Invasive Aspergillosis of ROCM form can extend to adjacent areas and present as yellowish to black necrotic ulcers.5 Some of the frequent factors implicated for development of fungal infection in COVID-19 are listed as follows: hypoxemia, hyperglycemia either due to DM or due to steroids, increased iron levels secondary to elevated ferritin levels, reduced phagocytic activity of WBC’s (white blood cells) attributed to immunosuppressed state caused by virus or steroid therapy.6 [Refer Figure 7] COVID-19 infected patients commonly presents with hypoxemia which clinically present as breathlessness and cyanosis.7
(DM) is one of the entrenched comorbid conditions of COVID-19 and it is noted in 10% of admitted patients. Diabetes increases the severity of COVID-19 infection and uncontrolled hyperglycemia can lead to increased hospitalization in COVID-19 positive patients and simultaneously increasing the mortality rate of these patients.8 Various factors are attributed for increased mortality among COVID-19 patients with uncontrolled DM include: glycosylation of complement proteins, impaired binding of Oligosaccharides (C-type leptin), decreased Tumor necrosis factor alpha (TNF-a), Interleukin 10 (IL-10), and Interferon gamma(IFN-g).9-11 Hyperglycemia doubles the risk of co-infection in COVID-19 subjects. Role of Steroid therapy in COVID-19 patients is still debatable. Deleterious effects of steroid therapy are supposed to be due to lack in management of steroid-induced hyperglycemia as steroids negates the immune-modulatory effect of steroids.12 So, Physicians should be aware of the adverse implications from steroids and strict glycemic control should be followed for reducing hyperglycemia.9-11 Diagnosis of dual infection presents as a challenging scenario. Pre-requisite for diagnosing fungal infection includes: high clinical suspicion of the disease, clinically patient presents with sinus pain, proptosis, peri-orbital swelling and all these symptoms are considered to be “red flag” symptoms. So, along with high index of clinical suspicion, judicious utilization of imaging techniques (CT, MRI) coupled with diagnostic modalities such as histopathology examination (HPE), microbiological and biochemical assessment which can help in early and accurate diagnosis.13 HPE of Mucormycosis: demonstrates aspetate, irregular hyphae with branching angle of 90°. Characteristic finding is “tissue necrosis” which differentiates it from Aspergillus. The other differentiating points in favour of Aspergillus includes thinner hyphae, regular septations and 45° degree angle seen in Aspergillosis but HPE has certain limitation in differentiating Aspergillus and Mucormycosis.14 Fungal hyphae are noted only in 50% cases of HPE as hyphae are frangible in nature and can be damaged during manipulation of tissue so excessive tissue homogenization should be avoided. Mucorales grow rapidly on fungal culture media (SDA) incubated at temperature of 25°C to 30°C. Microaerophilic environment can augment the yield of fungus on culture media.15,16 Invasive Aspergillosis (IA) in Covid-19 remains a diagnostic challenge because of multiple factors. HPE using special stains such as: Periodic Acid Schiff (PAS) and Grocott-Gromori’s methenamine-silver stains (GMS) can be also equally effective in demonstrating Aspergillus specimen, but it is difficult to conclusively rule out other filamentous fungi such as Fusarium specimen etc. Hence a more sensitive diagnostic method may be required for a conclusive opinion and thereby requiring the following interventions a) Direct microscopy using optical brightener methods (Calcoflour or Blankophor) to increase the diagnostic sensitivity and specificity b)Molecular assays targeting ribosomal DNA (rDNA) can also be utilized for definitive diagnosis. Further similar studies are required to standardize the laboratory procedure.17 Molecular based diagnostic methods: PCR (Polymerase chain reaction), RFLP (Restriction fragment length polymorphism) can also be utilized for detection of Mucorales species targeting mainly 18srRNA gene. The considerable drawback for molecular methods remains less number of subjects studied for the efficacy of molecular methods.18 Lastly, Review of scientific data from various studies demonstrated that Mucormycosis is more prevalent in males and DM is the most common comorbidity.19-24 Our case was a male diabetic suffering with severe COVID-19 disease, admitted in ICU care and was on parenteral steroids. The comparative analysis of our observation is shown in Table.19-24 Garg et al.19 showed similar observation with an elderly male patient having combordities including Hypertension and Diabetes. Johnson et al.23 similar observations were also noted as compared to the current study as it presented with 76 year old male being affected with Diabetes and coexistent infection of Aspergillus and Mucormycosis which was demonstrated by BAL examination (Bronchoalveolar lavage). Bellanger et al.24 also identical findings with 55 year old male with co-existent infection of Aspergillus and Mucormycosis which was demonstrated on BAL and tracheal aspirate.
Table (1):
Comparison of various case reports of COVID-19 associated comborbidities and co-existing fungal infections.
Authors |
Age range |
Comorbidity |
Mucormycosis diagnosis method |
Other fungus |
|---|---|---|---|---|
Garg et al[19] |
55, male |
Diabetes, Hypertension |
Sputum |
– |
Kanwar et al[20] |
55, male |
End stage Renal Disease(ESRD) |
Sputum, Pleural fluid |
– |
Hanley et al[21] |
22, male |
– |
Autopsy |
– |
Placik et al[22] |
49, male |
– |
Thoracotomy |
– |
Johnson et al[23] |
76, male |
Diabetes, Hypertension |
BAL (Bronchioalveolar lavage) |
Aspergillus |
Bellanger et al[24] |
55, male |
Lymphoma |
Tracheal Aspirate, BAL |
Aspergillus fumigatus |
Present case |
49, male |
Diabetes |
Histopathological examination, SDA culture |
Aspergillus |
Limitations
The limitation of present study is that limited reporting of such cases and not many dual fungal infection cases are published in English literature. However, with the increasing number of such cases it is essential to provide satisfactory explanation for the increase in fungal co-infections in COVID-19 infected patients for providing better health care and decrease associated mortalities and morbidities.
Our case report highlights the fact that a high index of clinical suspicion, proper assessment of risk factors, diagnostic methods, advantages and disadvantages of various diagnostic methods and clinical settings in COVID-19 subjects needs to be followed for immediate management in order to significantly reduce the morbidity and mortality occurring from these fungal co-infections.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
N and SD Conceptualized the study, collected data, performed analysis and interpretation of data. N, RVSL and PSS performed supervision. N, SD, RVSL and PSS drafted the manuscript. N, RVSL and PSS edited and critically revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Sri Devaraj Urs Medical, College, India (SDUMC/KLR/IEC/219/2021-22).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi. 2020;6(2):91-95.
Crossref - Chander J, Singla N, Kaur M, et al. Saksenaea erythrospora, an emerging mucoralean fungus causing severe necrotizing skin and soft tissue infections-A study from a tertiary care hospital in north India. Infect Dis. 2017;49(3):170-177.
Crossref - Rai DK, Kumar S. Dual Invasive fungal infection by Aspergillus and Mucor in COVID-19 patient: A rare case report with literature review. Arch Med Sci. 2021;9:278-282.
- Aqsa A, Droubi S, Glaser A. Aspergillus and Rhizopus fungal coinfection in a patient with multiple myeloma. Cureus. 2020;12(5):805-809.
Crossref - Kumari M, Malhotra P, Bhardwaj M. Coinfection of mucormycosis and aspergillus in a diabetic patient: A rare entity. IP Int J Med Microbiol Trop Dis. 2019;5(4):241-243.
Crossref - Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102-112.
Crossref - Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: A harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243-2246.
Crossref - Skiada A, Pavleas I, Apiranthitou DM Epidemiology and diagnosis of mucormycosis: An update. J Fungi. 2020;6(4):265-268.
Crossref - Ilyas R, Wallis R, Soilleux EJ, et al. High glucose disrupts oligosaccharide recognition function via competitive inhibition: A potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011;216(1-2):126-131.
Crossref - Mazade MA, Edwards MS. Impairment of type III group B Streptococcus-stimulated superoxide production and opsonophagocytosis by neutrophils in diabetes. Mol Genet Metab. 2001;73(3):259-267.
Crossref - Price CL, Hassi HO, English NR, et al. Methylglyoxal modulates immune responses: Relevance to diabetes. J Cell Mol Med. 2010;14(6B):1806-1815.
Crossref - Garcia VC, Sanjuan G, Moreno GE, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin Microbiol Infect. 2021:27(1):83-88.
Crossref - Skiada A, Floerl CL, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol J. 2018;56(Suppl 1):93-101.
Crossref - Florl CL. Diagnosing invasive fungal diseases-limitations of microbiological diagnostic methods. Expert Opin Med Diagn. 2009;3(4):461-470.
Crossref - Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54(Suppl 1):55-60.
Crossref - Lackner M, Caramalho R, Florl CL. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol. 2014;9(5):683-695.
Crossref - Jain A, Taneja S. Post-COVID fungal infections of maxillofacial region:a systematic review. Oral Maxillofac Surg. 2021;7:1-7.
- Alvarez E, Sutton DA, Cano J. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J Clin Microbiol. 2009;47(6):1650-1656.
Crossref - Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (COVID-19) associated mucormycosis (CAM): Case report and systematic review of literature. Mycopathologia. 2021;186(2):289-298.
Crossref - Kanwar A, Jordan A, Olewiler S,et al. A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J Fungi. 2021;7:174.
Crossref - Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe. 2020;1:245-53.
- Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep, 2020;15:2378-81.
- Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64-7.
- Bellanger AP, Navellou JC, Lepiller Q, Brion A, Brunel AS, Millon L, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now, 2021;51:633-5
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.