ISSN: 0973-7510
E-ISSN: 2581-690X
Dengue caused by the Dengue virus (DENV) is a vector-borne viral disease. It is a major public health issue in endemic regions such as India, where secondary infections pose a higher risk of severe illness due to antibody-dependent enhancement. Differentiating primary and secondary infections is crucial for disease management and surveillance. The aim of this study was to estimate the proportion of primary and secondary infections among Dengue cases using Capture IgM and IgG ELISAs and assess the performance of various ratio- and index-based classification methods. Archived serum samples from 784 hospitalized acute-phase Dengue cases in Thiruvallur district, Tamil Nadu, were analyzed, with diagnosis confirmed by non-structural protein (NS1) and/or IgM ELISAs. Capture IgG Enzyme-Linked Immunosorbent Assay (ELISA) classified 46.6% as primary and 53.4% as secondary infections. The IgM/IgG ratio of 2.6 showed the highest concordance (88.6%) with Capture IgG ELISA, while the Receiver Operating Curve (ROC) analysis identified an optimal Optical Density (OD) ratio of 3.08, yielding a sensitivity of 94.3% and specificity of 89.8%. The findings underscore the substantial burden of secondary infections, highlighting the need for continuous surveillance and preventive strategies to mitigate severe outcomes. Capture IgG ELISA remains a robust tool for distinguishing secondary Dengue cases and the IgM/IgG OD ratio of 2.6 serves as a reliable diagnostic alternative with minimal misclassification. These results support the integration of ratio-based methods in Dengue diagnostics to enhance classification accuracy and inform targeted public health interventions.
Primary Dengue, Secondary Dengue, ELISA, Capture IgM, Capture IgG, Diagnosis
Dengue is a vector-borne, neglected tropical illness caused by the Dengue virus (DENV), a member of the Flaviviridae family. DENV is a positive-sense single-stranded RNA virus with four distinct serotypes: DENV 1-4 and transmitted by female Aedes mosquitoes.1 Clinically, Dengue is categorized as Dengue with or without warning signs, and severe Dengue.2 Since the first documented outbreak in Jakarta in 1779,3 Dengue fever has been associated with high morbidity and mortality. It has emerged as a major public health concern with a notable increase in prevalence over the last several decades.2 The global pooled prevalence of Dengue during 2016 – 2024 was 32.9%.4 In India, Dengue remains endemic, with peak occurrences typically observed after the monsoon season and seroprevalence of 56.9%, with a case fatality ratio of 2%-6%.5
Primary infection with a single DENV serotype does not provide protection against future infections with heterologous serotypes, potentially due to antibody-dependent enhancement. This mechanism contributes to the high incidence of reinfections and increases the risk of developing Dengue Hemorrhagic Fever and Dengue Shock Syndrome, which can result in multi-organ complications.6
Beyond clinical management, distinguishing primary from secondary Dengue infections is vital for epidemiological assessments. The hemagglutination inhibition (HAI) test, traditionally used for this purpose has reliability issues7,8 due to inconsistent antibody titer rise (WHO criterion:1:1280), interference from non-specific inhibitors, specific need for chemical pre-treatment of samples, and the requirement of paired samples.7,9 In comparison, the Enzyme-Linked Immunosorbent Assay (ELISA) is easy to perform and serves as a better alternative to the HI test.10 The Dengue Capture IgG ELISA, introduced in the early 1990s, is widely used for detecting secondary infections.11 Alternate methods to classify primary and secondary infections based on Capture IgM ELISA Optical Density (OD)/IgG OD, Capture IgM index/Capture IgG index, and Capture IgG index/Capture IgM index have been proposed.11-17 Several ELISA-based methods, including avidity testing and IgM/IgG ratio assessment, have been compared to the HI test and demonstrated greater reliability.10,18 Although a few studies have reported interpretative cut-offs based on the analysis of IgG avidity, IgG titers and IgM/IgG ratios,19,20 none have compared the performance of ELISA with ratio or index-based methods. This study aimed to estimate the proportion of primary and secondary dengue infections using Capture IgM and IgG ELISAs and assess the performance of ratio- and index-based classification methods.
Study population and samples
Archived serum samples from hospitalised Dengue cases in the acute phase (0-7 days) without any coinfections were included in the study. These cases were from acute febrile illness surveillance (2016-2019) at select secondary care health facilities in Thiruvallur district, Tamil Nadu, India. Dengue diagnosis was confirmed based on NS1 and/or IgM ELISA positivity.
Case definitions
Primary and secondary Dengue infections were classified using Dengue IgM ELISA and IgG Capture ELISA. A primary Dengue infection was defined by a positive IgM Capture ELISA and a negative IgG Capture ELISA, while a secondary infection was confirmed by positive results for both IgM and IgG Capture ELISAs.
Laboratory analysis
The Dengue IgM Capture ELISA (Panbio, Germany) was performed on all samples following the manufacturer’s guidelines. A cut-off greater than 11 Panbio units indicated either an active primary or secondary Dengue infection. The assay had a sensitivity of 94.7% (95% CI: 85.4-98%.9%) and specificity of 100% (95% CI: 95.7%-100%).
The Dengue IgG Capture ELISA (Panbio, Germany) was performed on IgM Capture positive sera following the manufacturer’s guidelines to detect secondary Dengue infections. The assay had a sensitivity of 80.9% (95% CI: 75.1%-86.7%) and specificity of 87.1% (95% CI: 80.6%-93.7%) and used a cut-off value greater than 22 Panbio units, which was equivalent to a hemagglutination inhibition (HI) titer of 1:2560.21
In addition to the classification of primary and secondary Dengue by the IgM and IgG ELISAs, the following ratio or index cutoffs were used for comparison.
- Capture IgM/IgG optical density (OD) ratio cutoffs (i) 1.212 (ii) 1.422 (iii) 1.7811 (iv) 1.815 and (v) 2.6,13 where a sample OD ratio greater than the particular ratio cut-off indicated primary Dengue whereas lesser value indicated secondary Dengue infections.
- IgM index/IgG index of 1.3214 [primary Dengue (>1.32); secondary Dengue (≤1.32)]
- IgG index/IgM index of (i) 1.1016 and (ii) 1.1417 [primary Dengue (<1.1); secondary Dengue (≥1.1)]
Data analysis
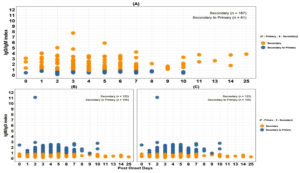
Descriptive statistics were applied to present categorical variables in terms of frequencies and percentages. Bar plots were used to visualize the distribution of Dengue cases by post-onset days. Dot plots were used to compare different classification ratios among Dengue IgG Capture positive cases, highlighting the discordance rates for each ratio threshold. Sensitivity and specificity were evaluated to determine the optimal cut-off value for IgM/IgG ratios, IgM/IgG index ratios, and IgG/IgM indices. Receiver Operating Characteristic (ROC) curves were generated and the area under the curve (AUC) was measured to assess the performance. The plots were created using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY) and R studio.
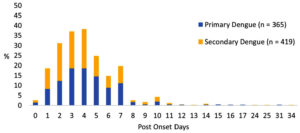
Among the 784 Dengue cases included in this analysis, 191 (24.4%) were NS1 positive, IgM negative, whereas the remaining 593 (75.6%) were NS1 negative, IgM positive. Based on Capture IgG testing, 365 (61.6%) and 228 (38.4%) of the 593 cases were categorized as primary Dengue and secondary Dengue infections. All 191 NS1-positive cases were secondary infections. In total, 365 (46.6%) of cases were identified as primary Dengue, while the remaining 419 cases (53.4%) were classified as secondary Dengue infections (Figure 1).
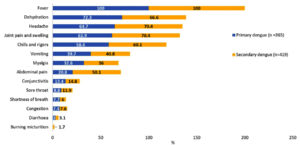
The median age of primary dengue cases was 24 years (IQR: 16-40), and secondary dengue cases were 25 years (IQR: 17-38). The median duration from onset of symptoms to sample collection was similar for both primary (4; IQR: 3-6 days) and secondary Dengue cases (4; IQR: 2-5 days). Fever, dehydration, headache, and joint pain were present in all cases, with abdominal pain, myalgia, and vomiting seen more often among the secondary Dengue cases (Figure 2).
Classification by different ratio-based methods:
IgM/IgG OD ratio
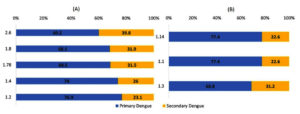
Among the 593 Capture IgM and IgG positive cases, the proportion of primary and secondary Dengue classified by different ratios were 1.2 [456 (76.9%), 137 (23.1%)]; 2.6 [357 (60.2%), 236 (39.8%)]; 1.78 [406 (68.5%), 187 (31.5%)]; 1.4 [439 (74.0%), 154 (26.0%)]; 1.2 [456 (76.9%), 137 ( 23.1%)], and 1.8 [404 (68.1%), 189 (31.9%)] respectively.
IgM/IgG index
Based on the ratio of 1.3, 403 cases (68.8%) were classified as primary Dengue, and 185 cases (31.2%) as secondary Dengue.
IgG/IgM index
Based on the ratio of 1.1, 459 (77.4%) and 134 (22.6%) cases were classified as primary and secondary Dengue, respectively. A similar proportion of primary 459 (77.4%) and secondary 134 (22.6%) cases were seen with an index ratio of 1.14 (Figure 3). Overall, 331 cases (55.8%) were consistently classified as primary and 129 (22.3%) as secondary based on both IgG Capture ELISA and IgM/IgG ratios (Figure 3).
Figure 3. Proportion of primary and secondary Dengue cases based on different classification ratios (N = 593). A. IgM/IgG ratio-based classification B. IgM/IgG index and IgG/IgM index-based classification
Relative performance of ratio-based methods versus IgG capture ELISA
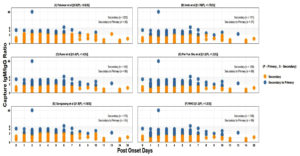
Among 228 secondary Dengue cases classified using the IgG Capture ELISA, the IgM/IgG ratio of 2.6 showed the lowest discordance 26 (11.4%) when compared to other ratio thresholds (Figure 4 & 5).
Figure 4. Comparison of different ratio-based classifications among secondary Dengue (IgG Capture ELISA Positive) cases (N = 228) A. Falconar et al. ratio ≥2.6 (primary) <2.6 (secondary) B. Innis et al. ≥1.78 (primary) <1.78 (secondary) C. Kuno et al. ≥1.4 (primary) <1.4 (secondary) D. Pei-Yun et al. ≥1.2 (primary) <1.2 (secondary) E. Sa-ngasang et al. ≥1.8 (primary) <1.8 (secondary) F. ≥1.2 (primary) <1.2 (secondary)
Figure 5. Comparison of index-based classifications among secondary Dengue (IgG Capture ELISA Positive) cases (N = 228). A. Harry prince et al. >1.31 (primary) <1.32 (secondary). B. Changal et al. <1.1 (primary) ≥1.1 (secondary) C. Cucunawangsih et al <1.14 (primary) ≥1.14 (secondary)
Optimal Cut-Off value for different classification ratios
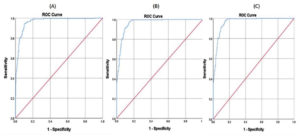
Based on ROC analysis, the estimated optimal cut-off value for IgM/IgG OD ratio was 3.08 (sensitivity 94.3%, specificity 89.8%, accuracy 91.5%); for IgG/IgM index was 0.41 (sensitivity 97.4%, specificity 85.5%, accuracy 90.0%), and for IgM/IgG index ratio was 2.42 (sensitivity 97.4%, specificity 85.5%, accuracy 90.0%) (Figure 6).
Early diagnosis of secondary dengue infection is essential, as it is a major risk factor for severe manifestations such as thrombocytopenia, haemorrhagic manifestations, hyperproteinaemia, and hepatic dysfunction.23 Therefore, distinguishing primary and secondary infections is critical for effective disease management, particularly in endemic countries such as India. This study documented a higher proportion (53.4%) of secondary dengue infections among hospitalized acute Dengue cases based on the detection of elevated IgG antibodies by Dengue IgG Capture ELISA. A multicentric study conducted across several states of India reported about two-thirds of study cases as primary Dengue, except in Tamil Nadu (Theni), Andhra Pradesh, and Karnataka, where two-thirds were secondary Dengue.24 A retrospective cross-sectional study from Uttar Pradesh, India, reported a higher proportion of primary Dengue cases of 90.0% (N = 198) during 2021-2022.23 In contrast, a hospital-based cross-sectional study in Thoothukudi district, Tamil Nadu, documented 53.57% (N = 15) of cases to be secondary Dengue.25 Similarly, a high proportion of secondary Dengue was seen in an outbreak investigation in Madurai district, Tamil Nadu, during 2017.26 Studies from Dengue-endemic countries such as Saudi Arabia,27 Colombia,28 Vietnam,29 and the Philippines30 have also highlighted a high proportion of secondary Dengue cases.
Various OD-based and index-based ratios were used to evaluate alternative criteria for differentiating primary and secondary infections. In this study, the relative performance of ratio-based methods on secondary Dengue cases revealed that the highest concordance was observed with the OD-based ratio of 2.613 for identifying secondary Dengue infections. The lower level of discordance with this ratio would result in fewer secondary cases being misclassified as primary Dengue infections. Compared to the OD-based ratios, the index-based evaluation had comparatively higher discordance with IgG capture ELISA. Overall, one-half of the cases were consistently classified as primary cases, whereas only a smaller proportion of cases were classified as secondary by all methods included in this study.
Using the IgM/IgG ratio-based classification is challenging due to the prolonged persistence of IgM,31 even up to eight months. IgG Capture ELISA detects elevated IgG from secondary infections, resulting in higher IgM/IgG ratios in primary infections. Over time, as IgM decreases and IgG increases in primary cases, the ratios may resemble those of recent secondary infections.14 This emphasizes the importance of knowing the time of sample collection while performing antibody-based assays.
The ROC analysis identified optimal cut-off values for each of the methods to detect secondary Dengue infections. However, since Dengue infection patterns vary by region, these cut-off values should be adjusted based on their geographical location. Using region-specific cut-offs improves detection, but changing them without considering prevalence in the area may reduce their reliability.20
This study underscores the importance of selecting the appropriate cut-off ratio for distinguishing between primary and secondary dengue infections using IgG/IgM capture ELISA. By systematically comparing several ratio-based methods to the reference capture IgG ELISA method, we demonstrate that diagnostic performance is highly sensitive to the choice of threshold values. Our findings highlight the need for standardized cut-off criteria to minimize misclassification and improve the validity of serological diagnosis. In addition, clinical and epidemiological contexts, including sample collection timing and population immunity, need to be taken into account when interpreting results to ensure accurate classification of Dengue infections.
Limitations
This study has certain limitations. First, we were unable to exclude/rule out the possibility of co-circulating flavivirus infections, which are known to induce cross-reactive antibodies with DENV. The presence of these antibodies could affect the measured IgM and IgG levels, potentially resulting in misclassification of primary and secondary Dengue infections. Specifically, elevated IgG levels from prior flavivirus exposure could lead to a low IgM/IgG ratio, resulting in misclassification of the infection as secondary Dengue. Second, our study relied on a single time-point sample collection and as a result, patients in the early stages of infection who may not yet have seroconverted may have been inadvertently excluded from the analysis.
This study highlights the predominance of secondary dengue infections among the reported cases in the study region. This finding underscores the need for continuous surveillance and effective prevention efforts to identify and manage individuals with secondary Dengue, who are at higher risk of complications and adverse outcomes. The Capture IgG ELISA provides a reliable and accessible diagnostic option for detecting secondary dengue infections. When comparing the performance of various methods, an IgM/IgG OD ratio of 2.6 showed minimal misclassification, supporting its use as an effective tool for detecting secondary dengue.
ACKNOWLEDGMENTS
The authors acknowledge the technical support provided by Dr. G. Sathya Narayanan and Mr. T. Karunakaran, as well as the assistance of Mr. RA Sridharan and Mr. NN. Karthick in conducting the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest
AUTHORS’ CONTRIBUTION
PRA and GKCP conceived the study. PRA and GR performed the tests. VS and PRA conducted the data curation and formal analysis. PRA, SD, JP and GKCP conducted original drafting, review and editing. All authors critically revised and approved the final version of the manuscript.
FUNDING
None.
DATA AVAILABILITY
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee, ICMR-National Institute of Epidemiology under the protocol number NIE/IEC(A)07/2021/367.
- Messina JP, Brady OJ, Scott TW, et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 2014;22(3):138-146.
Crossref - World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control. New ed. TDR : World Health Organization; 2009.
Crossref - Wu W, Bai Z, Zhou H, et al. Molecular epidemiology of dengue viruses in southern China from 1978 to 2006. Virol J. 2011;8(1):322.
Crossref - Alageeli M, Madkhali AM, Abdullah A, et al. Global prevalence of dengue fever: a comprehensive systematic review (2016-2024). 2024;8(11):3286-3294.
Crossref - Ganeshkumar P, Murhekar MV, Poornima V, et al. Dengue infection in India: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(7):e0006618.
Crossref - Rivera L, Biswal S, Saez-Llorens X, et al. Three-year Efficacy and Safety of Takeda’s Dengue Vaccine Candidate (TAK-003). Clin Infect Dis. 2022;75(1):107-117.
Crossref - A-nuegoonpipat A, Prakong S, Sa-ngasang A, et al. Comparison between Haemagglutination Inhibition (HI) Test and IgM and IgG-capture ELISA in determination of primary and secondary dengue virus infections. Dengue Bull. 2006;30:141-145.
- Graham RR, Juffrie M, Tan R, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995-1996. Am J Trop Med Hyg. 1999;61(3):412-419.
Crossref - Kumari MP, Kumari BA, Begum MT. Serodiagnosis of Secondary Dengue Infection in a Tertiary Care Hospital, Mysuru. J Pure Appl Microbiol. 2024;18(3):1768-1775.
Crossref - Lukman N, Salim G, Kosasih H, et al. Comparison of the Hemagglutination Inhibition Test and IgG ELISA in Categorizing Primary and Secondary Dengue Infections Based on the Plaque Reduction Neutralization Test. Biomed Res Int. 2016;2016(1):523842.
Crossref - Innis BL, Nisalak A, Nimmannitya S, et al. An Enzyme-Linked Immunosorbent Assay to Characterize Dengue Infections Where Dengue and Japanese Encephalitis Co-Circulate. Am J Trop Med Hyg. 1989;40(4):418-427.
Crossref - WHO. World Health Organization. Regional Office for South-East Asia. Comprehensive Guideline for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. Revised and Expanded Edition. WHO Regional Office for South-East Asia.; 2011. https://iris.who.int/handle/10665/204894
- Falconar AKI, de Plata E, Romero-Vivas CME. Altered Enzyme-Linked Immunosorbent Assay Immunoglobulin M (IgM)/IgG Optical Density Ratios Can Correctly Classify All Primary or Secondary Dengue Virus Infections 1 Day after the Onset of Symptoms, when All of the Viruses Can Be Isolated. Clin Vaccine Immunol. 2006;13(9):1044-1051.
Crossref - Prince HE, Yeh C, Lape-Nixon M. Utility of IgM/IgG Ratio and IgG Avidity for Distinguishing Primary and Secondary Dengue Virus Infections Using Sera Collected More than 30 Days after Disease Onset. Clin Vaccine Immunol. 2011;18(11):1951-1956.
Crossref - Sa-Ngasand A, Anantapreecha S, A-Nuegoonpipat A, et al. Specific IgM and IgG responses in primary and secondary dengue virus infections determined by enzyme-linked immunosorbent assay. Epidemiol Infect. 2006;134(4):820-825.
Crossref - Changal KH, Raina AH, Raina A, et al. Differentiating secondary from primary dengue using IgG to IgM ratio in early dengue: an observational hospital based clinico-serological study from North India. BMC Infect Dis. 2016;16(1):715.
Crossref - Cucunawangsih, Lugito NPH, Kurniawan A. Immunoglobulin G (IgG) to IgM ratio in secondary adult dengue infection using samples from early days of symptoms onset. BMC Infect Dis. 2015;15(1):276.
Crossref - Palabodeewat S, Masrinoul P, Yoksan S, Auewarakul P, Komaikul J. A modified IgG avidity assay for reliability improvement of an in-house capture ELISA to discriminate primary from secondary dengue virus infections. J Virol Methods. 2021;289(August 2020):114043.
Crossref - de Souza VAUF, Tateno AF, Oliveira RR, et al. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J Clin Virol. 2007;39(3):230-233.
Crossref - Agarwal A, Jain RK, Chaurasia D, Biswas D. Determining the optimum cut-off IgM/ IgG ratio for predicting secondary dengue infections: An observational hospital based study from Central India. Indian J Med Microbiol. 2022;40(4):492-495.
Crossref - Panbio Dengue IgG Capture ELISA | Abbott Point of Care. Accessed February 28, 2025. https://www.globalpointofcare.abbott/ww/en/product-details/panbio-dengue-igg-capture-elisa.html
- Kuno G, Gomez I, Gubler DJ. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods. 1991;33(1-2):101-113.
Crossref - Dinkar A, Singh J, Kumar N, Kumar K, Singh SK, Singh AK. Impact of secondary infections on dengue presentation: A cross-sectional study in a tertiary care hospital in Uttar Pradesh, India. J Infect Public Health. 2023;16(12):1925-1932.
Crossref - Rao C, Kaur H, Gupta N, et al. Geographical distribution of primary; secondary dengue cases in India – 2017: A cross-sectional multicentric study. Indian J Med Res. 2019;149(4):548-553.
Crossref - Gopal K, Kalaivani V, Anandan H. Prevalence of Dengue Fever and Comparative Analysis of IgM and IgG Antibodies in Dengue Fever in Thoothukudi- Southern Coastal City, Tamil Nadu. Ann Int Med Dent Res. 2016;2(6):4-7.
Crossref - Sankar SG, Sundari TM, Anand AAP. Emergence of Dengue 4 as Dominant Serotype During 2017 Outbreak in South India and Associated Cytokine Expression Profile. Front Cell Infect Microbiol. 2021;11:1-24.
Crossref - Ashshi AM. The prevalence of dengue virus serotypes in asymptomatic blood donors reveals the emergence of serotype 4 in Saudi Arabia. Virol J. 2017;14(1):107.
Crossref - Velandia-Romero ML, Coronel-Ruiz C, Castro-Bonilla L, et al. Prevalence of dengue antibodies in healthy children and adults in different Colombian endemic areas. Int J Infect Dis. 2020;91:9-16.
Crossref - Thai KTD, Nishiura H, Hoang PL, et al. Age-specificity of clinical dengue during primary and secondary infections. PLoS Negl Trop Dis. 2011;5(6):e1180.
Crossref - Biggs JR, Sy AK, Brady OJ, et al. A serological framework to investigate acute primary and post-primary dengue cases reporting across the Philippines. BMC Med. 2020;18(1):364.
Crossref - Chen WJ, Hwang KP, Fang AH. Detection of IgM antibodies from cerebrospinal fluid and sera of dengue fever patients. Southeast Asian J Trop Med Public Health. 1991;22(4):659-663.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.