ISSN: 0973-7510
E-ISSN: 2581-690X
Virulent footrot represents as a significant contagious ailment affecting sheep, with Dichelobacter nodosus being the primary agent responsible for transmitting the disease. Vaccinations and various management practices are utilized to control the disease. In this study, we cultured D. nodosus in broth culture under reducing conditions and formulated a vaccine using Montanide oil as adjuvant. The efficacy of the vaccine was assessed in sheep to determine its ability to elicit an effective immune response and its therapeutic potential. D. nodosus serogroup B (JKS-07B strain) was grown in a modified TAS broth and a whole cell killed vaccine was prepared using 1.5 × 109 cells per dose, along with the oil adjuvant Montanide ISA 61 VG. The vaccine trial was conducted in sheep and two doses of the vaccine were given subcutaneously with a 30 days interval between the doses. The serum antibody titers in the vaccinated animals were measured at 0, 30, 60, 90, 120, and 150 days post-vaccination using the microplate agglutination test. Protective antibody titers (≥ 3000) were achieved by the 30th day following the primary vaccination in all animals, reaching their peak at the 60th day. The protective level of antibody titers was maintained up to 120 days following the primary vaccination. The vaccine also demonstrated a therapeutic effect in animals that were clinically affected. Animals with a lesion score of 2 took 18-21 days for recovery, while those with a lesion score of 3-4 took 40-47 days to recover from lameness.
Dichelobacter nodosus, Antibody Titer, Montanide ISA 61 VG, Vaccine, Therapeutic Effect
Ovine virulent footrot is an important infectious bacterial disease in sheep that leads to severe lameness. The disease is marked by an initial stage of painful interdigital dermatitis, which can advance to the separation of hoof horn from the underlying tissue. This progression results in severe lameness, accompanied by a distinctive foul smell at all stages of the disease.1,2 Footrot significantly reduces the productivity, breeding efficiency, and overall welfare of the affected animals.3-6 It is caused by the synergistic action of certain bacterial species, with Dichelobacter nodosus (D. nodosus) acting as the main transmitting agent.7 The disease is a result of the interaction between the bacterium and the hoof epithelium, facilitated by warm and humid environmental conditions.8,9 The clinical manifestation varies based on the severity of lesions, which is influenced by the strains of D. nodosus. Virulent isolates of D. nodosus are distinguished by their capacity to produce heat-stable proteases.10 Other organisms, particularly Fusobacterium necrophorum, may have a significant role in either aiding the development of footrot1 or intensifying its severity.11 There are multiple serogroups of D. nodosus (A-I and M) distinguished by their type IV fimbrial antigen and immunity to the bacterium is always serogroup specific.12-14 The expression of pili or fimbriae in bacteria is influenced by environmental factors such as temperature15 and the consistency of media.16
Vaccinations and various management practices are effective in controlling virulent footrot in sheep. Serogroup-specific vaccinations have proven successful in eradicating the disease from sheep flocks.17-19 Footrot in sheep and goats has been found to be endemic in Kashmir for last two decades, resulting in substantial economic losses for sheep farmers.20-22 Serogroups A, B, C, E, F and I of D. nodosus have been reported in India.21-23 Among these serogroups, B was found to be predominant (>90%) in Kashmir.22 Based on this information, a serogroup B specific vaccine was formulated in our laboratory (unpublished data) using D. nodosus grown on plate culture. The challenge of maintaining anaerobic conditions for the growth of this bacterium complicates the production of these vaccines, rendering the process both cumbersome and costly. Moreover, for large scale production of the vaccine, the bacterium needs to be grown in broth culture and requires anaerobic and fastidious growth conditions. In this study, we cultured D. nodosus in broth culture under reducing conditions and formulated a vaccine using Montanide oil as adjuvant. The efficacy of the vaccine was assessed in sheep to determine its ability to elicit an effective immune response and its therapeutic potential.
Culturing of D. nodosus in broth culture
The virulent strain JKS-07B of D. nodosus belonging to serogroup B (GenBank accession no NZ_SRJB00000000)24 and serotype B525 was used as a seed strain in the present study for broth culture vaccine formulation. In our laboratory, this strain was employed for vaccine formulation after being cultivated as a plate culture. The strain was revived on Trypticase Arginine Serine Hoof (TASH) agar plates and purity was checked. Subsequently, it was confirmed by serogroup specific PCR as described by Dhungyel et al.26 Trypticase Arginine Serine (TAS) broth was prepared according to Skerman27 with certain modifications.28 Briefly, medium components (all from HiMedia Laboratories Pvt. Ltd., India) namely tryptose 15 gm, proteose peptone 5 gm, HM peptone B 5 gm, yeast extract 5 gm, L-arginine 5 gm, DL-Serine 1.5 gm and Magnesium sulphate heptahydrate (MgSO4.7 H2O) 2 gm in 1 litre of distilled water along with sodium thioglycolate at the rate of 6 mM was added and pH was adjusted at 4.8. The medium was taken in 500 ml screw capped bottles leaving little space for proper autoclaving. Additionally, a separate solution of 20% sodium carbonate (Na2CO3) was prepared and autoclaved. It was then aseptically added to the autoclaved broth at a rate of one ml per 100 ml, resulting in a final concentration of 2 mg/ml and pH 6.8-6.9.28 The space left in the bottle prior to autoclaving was carefully filled, ensuring the absence of air bubbles, up to the brim with broth autoclaved separately. The bottle was subsequently placed in the incubator under airtight conditions overnight to check its sterility. Sterile broth was inoculated with pieces of agar containing well grown colonies of D. nodosus (strain JKS-07B) from the TASH agar plates and then incubated at 37 °C in a conventional laboratory incubator for 40 hrs. Then the broth was checked for presence of growth and visible turbidity was evident after 24 hrs of incubation; however, full bloom growth was observed after 40 hrs of incubation (OD600 = 0.5-1) when optimum pH (around 6.8 to 7.0) was attained. Beyond these pH range, the growth of the bacterium was either very slow or no growth was observed. Subcultures were made from this broth to fresh TAS broth prepared as above at the rate of 1 ml/100 ml and incubated as above.
Harvesting of D. nodosus and formulation of vaccine
D. nodosus in the broth culture were inactivated by 0.4% formalin and were subsequently harvested by centrifugation at 8000 x g. The pellets were collected in PBS in concentrated form and stored at -84 °C until use. At the time of vaccine formulation, the stored cells were thawed and mixed with Montanide ISA 61 VG oil adjuvant (Seppic, Paris, France) in a ratio of 40:60 following the manufacturer’s guidelines. The mixture was emulsified by a hand-held needle and syringe, and the emulsion was checked on ice cold water. The dosage was calibrated using the McFarland opacity standard to ensure that each ml of the vaccine contained around 1.5 × 109 colony forming unit (CFU) of D. nodosus. The formulation was tested for sterility under both aerobic and anaerobic conditions.
Immunization and testing for antibody titer
The experiment was approved by the Institutional Animal Ethical Committee vide reference No. AU/FVS/PS-23/2022/19465-96 dated 28-03-2022. The immunization trial was conducted at Mountain Research Centre on Sheep & Goat (MRCSG), FVSc & AH, Shuhama using 28 healthy sheep of around one year of age without any history of footrot. Prior to the proper immunization trial, 6 sheep were subcutaneously injected on the anterodorsal aspect of the neck with 1.5 ml of the vaccine formulation, followed by a 2nd injection 4 weeks later. One ml of the adjuvant was also administered to six animals as above. The sheep were carefully observed for any potential adverse reactions during the 15 days following the vaccinations.
Following the safety trial, 10 sheep were administered one ml of the above vaccine formulation in the same manner as described previously. Other six animals designated as negative control, received one ml of PBS only. The booster was administered after 30 days of the primary vaccination. Blood serum samples were obtained from all the sheep via jugular venipuncture at intervals of day 30, 60, 90, 120, and 150 following primary vaccination. These samples were then examined for antibody titers using the microplate-agglutination test.29 Briefly, sera were diluted in PBS (pH 7.4) in a doubling dilution series to 100 µl volume in a micro-agglutination plate. An equal volume (100 µl) of formalin-killed D. nodosus antigen (5 × 108 CFU/ml) was added to the wells and mixed properly. Proper positive and negative controls were also kept. The aforementioned mixture was incubated at 37 °C overnight and agglutination was assessed. Agglutination titres were expressed as the reciprocal of the highest dilution of the serum which resulted in detectable agglutination.
Therapeutic trial of the vaccine
In addition to the above experiment, the vaccine formulation was also administered to 22 clinically affected sheep within three household flocks each exhibiting a lesion score of 2-430 in the villages of Shuhama and Saloora, district Ganderbal, J & K. Nine animals (three in each flock) with a lesion score of 2-3 were kept as control. The animals were regularly monitored on every alternate day for any signs of recovery. The serum samples were also collected from the animals as mentioned above. Besides, samples were also collected from the affected foot of the sheep with a sterile swab for detection and serogrouping of D. nodosus.
Immune response of sheep to the vaccine
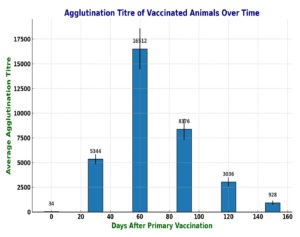
Based on its growth in TAS broth and considering our previous studies, JKS-07B strain of D. nodosus was selected for vaccine formulation.28 In the initial experiment, the vaccine formulation was deemed safe, even though the animals received a higher dose than the intended amount for the experiment. However, local reactions measuring approximately 3 and 5 cm in diameter, respectively, were observed at the site of injection. Details of the antibody titer of individual vaccinated animals on various days following primary vaccination, along with their means and standard deviations are presented in Table 1. All the vaccinated animals attained a protective agglutinin titre (≥ 3000) by the 30th day after primary inoculation. The serum mean antibody titer peaked at its maximum level (>16000) by the 60th day (30 days after the booster dose), followed by a gradual decline. The average protective titre was observed to remain up to 4 months after primary vaccination. The results are also depicted in Figure for easier understanding. The titre in the negative control group remained at 40 or less (≤ 40), throughout the study period. There was no significant difference in the agglutinin titre in the healthy and clinically affected animals that were vaccinated. Indurations ranging from 2 to 4 cm in diameter were observed at the injection sites. Bigger indurations softened at their centers within 1 to 1.5 months, and pus was drained out from them. The pus was found to be sterile upon culture on blood agar plates both aerobically and anaerobically. These lesions subsided by about 2-3 months.
Table (1):
Agglutination titre of animals vaccinated with killed whole cell broth culture vaccine
| Days after primary vaccination | Animal number | Mean | STD DEV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| 0 | 40 | 40 | 20 | 40 | 40 | 40 | 40 | 20 | 40 | 20 | 34 | 9.66 |
| 30 | 5120 | 4800 | 5600 | 5120 | 6400 | 4800 | 5600 | 4800 | 5600 | 5600 | 5344 | 516.67 |
| 60 | 16000 | 12800 | 19200 | 16000 | 19200 | 15360 | 16000 | 15360 | 19200 | 16000 | 16512 | 2085.87 |
| 90 | 8000 | 6000 | 9600 | 8960 | 9600 | 8000 | 8960 | 7680 | 8960 | 8000 | 8376 | 1083.89 |
| 120 | 3000 | 2000 | 3600 | 3000 | 3600 | 3000 | 3200 | 2560 | 3200 | 3200 | 3036 | 473.83 |
| 150 | 800 | 640 | 1200 | 800 | 1200 | 1000 | 1000 | 640 | 1200 | 800 | 928 | 220.95 |
Figure. Bar graph showing agglutination titre against D. nodosus in sheep on different days after vaccination
Therapeutic response of footrot affected sheep
Following the primary dose, therapeutic effect of the vaccine was recorded in the affected sheep. Observations revealed that sheep with a lesion score of 2 (13 sheep) took 15-21 days for recovery from lameness and wound healing. In contrast, those with lesion scores of 3 (6 sheep) and 4 (3 sheep) took 40-43 days and 48 days, respectively, to recover (Table 2). In the control group, there were no signs of improvement from lameness within the first 15 days; rather, the condition seemed aggravated. Therefore, the animals were treated with the candidate vaccine. However, they were not inspected regularly as before. They took similar time period to recover from the lameness as reported by the farmers. The samples collected for detection and serogrouping, only revealed the presence of serogroup B of D. nodosus.
Table (2):
Therapeutic effect of vaccine in footrot affected sheep (Lesion score 2-4)
Number of Animals |
Lesion Score |
Number of days to recover |
|---|---|---|
9 |
2 |
15 |
4 |
2 |
21 |
3 |
3 |
40 |
3 |
3 |
43 |
3 |
4 |
48 |
Dichelobacter nodosus is a Gram-negative, non-spore-forming rod and obligate anaerobic bacterium,31,32 sensitive to dryness and requires special medium for growth, which makes its culturing and isolation difficult. Hoof agar, TAS agar and Eugon agar are suggested for its isolation.33 Cultivating on solid medium requires a considerable amount of time and the strict maintenance of anaerobic conditions. Moreover, the reduced yield on solid media and the cumbersome harvesting procedure, in comparison to liquid media, contribute to increased costs in vaccine production. To overcome these challenges and facilitate the production of large biomass, liquid culture is a more advantageous option. Thomas34 employed a liquid medium containing a reducing agent, acid-hydrolyzed horn or wool, and auto-digested pancreas or trypsin, which facilitated substantial growth of cultures within 3 to 5 days under nitrogen. This medium was further modified by enriching it with yeast and liver extracts, bovine blood, amino acids and various protein digests.35-37 Skerman38 used TAS medium which also included lab-lemco, proteose peptone, yeast extract, MgSO4, thioglycolic acid and Na2CO3 for the growth of D. nodosus.
The fastidious and anaerobic nature of the organism poses a challenge for mass production of vaccines containing well-piliated organisms of representative serogroups of D. nodosus. In this study, we have addressed this problem by growing the organism in TAS broth in presence of Na2CO3 and reducing agent sodium thioglycolate. Although D. nodosus was grown in TASH agar at pH 7.8-8.0 in our laboratory, it was unable to grow at this pH in TAS broth. Therefore, we utilized the TAS medium as described by Skerman,38 but without the gas phase.
In the present study, D. nodosus serogroup B and serotype B5 (strain JKS-07B) was used in vaccine production as this serogroup/serotype was found to be predominant in Kashmir.22,25 Moreover, this current experiment utilized D. nodosus culture cultivated in broth. This choice was made due to concerns about potential fluctuations in the immune response caused by variability in the expression of bacterial fimbriae.15,16,39 However, the immune response was found to be at par with plate culture-based vaccine (unpublished data) in the present study although the peak titre was little less. This may be due to lesser expression of fimbriae by D. nodosus in broth culture or loss of fimbriae due to detachment and loss in the supernatant in the initial centrifugation process.
In this study, we employed Montanide ISA 61 VG as an adjuvant based on our earlier investigations, which demonstrated superior outcomes with this adjuvant compared to Montanide ISA 50 V, along with reduced incidences of local reactions. In India, Rani et al.40 reported development of inactivated whole cell vaccine against serogroup B and I of D. nodosus cultivated on TASH agar using alum and Montanide oil as adjuvants. The immune response was notably stronger when using an antigenic concentration of 2 × 109 bacteria/ml along with Montanide adjuvant, in comparison to an antigenic concentration of
1 × 109 bacteria/ml with alum adjuvant. However, they did not specify the type of Montanide oil used in the study. Employing Montanide ISA 61 VG as the adjuvant and administering a vaccination dose of 1.5 x 109 CFU per dose in our study, successfully triggered a strong protective immune response. This response exhibited a significant increase after a booster dose administered 30 days following the primary vaccination.
The protective immune response was found to persist for around 4 months in the vaccinated animals. Additionally, we noted tissue reactions at the vaccination sites, consistent with observations made by other researchers who utilized oil adjuvants, including Montanide oils.40,41 However, it was less in comparison to vaccine with Montanide ISA 50 V, where big tissue reactions as lumps of around 4-5 cm were recorded in most of the vaccinated animals (unpublished data).
Despite the potential adverse reactions, vaccination against footrot remains justified from both economic and animal welfare perspectives. The pain and hoof damage resulting from footrot far outweigh the potential adverse reactions associated with vaccination. The vaccine also demonstrated therapeutic potential. Other studies have similarly indicated that water-in-oil-based adjuvants are more effective for footrot vaccines.17,19,42 In our neighbouring country Nepal, a serogroup specific vaccine proved to be more effective in providing protection to animals, as the outbreak was associated with serogroup E strains only.17 Likewise, in Bhutan, an autogenous vaccine derived from D. nodosus (serogroup B) successfully eradicated the clinical manifestations of the disease.18 In addition to prophylaxis, the vaccine also demonstrated the therapeutic effects in the footrot affected sheep. Although, the number of days taken to recover varied in increasing order from low to high lesion score. Different studies on effect of vaccine against footrot also showed the therapeutic effects associated with vaccine in addition to prophylaxis.17,19,42,43 It has been found that high levels of anti-fimbrial (K) agglutinins responsible for both prophylactic and therapeutic efficacy developed in vaccinated sheep.44
Trials with the inactivated vaccine derived from the broth culture demonstrated optimal outcomes in generating a protective immune response that lasted for approximately four months, comparable to the vaccine produced through plate culture. Besides its prophylactic effect, therapeutic benefits of the vaccine were also observed. As such, it can also be utilized during an outbreak of footrot in the favorable season and for treating chronic cases. This will aid in the effective control and prevention of footrot when combined with other management practices in Kashmir. However, local tissue reaction is an issue. Therefore, D. nodosus fimbriae based subunit vaccine may be tried.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Yasham Speciality Ingredients Pvt. Ltd., Mumbai, India, for providing the Montanide ISA 61 VG used in the present study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This research was funded by the Department of Biotechnology (DBT), Ministry of Science and Technology, Govt. of India, through the project approved under sanction number BT/PR45210/NER/95/1935/2022, dated March 8, 2022.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, SKUAST Kashmir, India, with registration number 1809/GO/ReBiS/Rel/15/CPCSEA.
- Egerton JR, Yong WK, Riffkin GG. Foot Rot and Foot Abscess of Ruminants. CRC Press Inc., Boca Raton, Florida. 1989.
- Kennan RM, Han X, Porter CJ, Rood JI. The pathogenesis of ovine footrot. Vet Microbiol. 2011;153(1-2):59-66.
Crossref - Egerton JR, Seaman JT, Walker RI.X. Eradication of virulent footrot from New South Wales. Proceedings of the 13th National Symposium and 5th Conference on Lameness in Small Ruminants, Maribor, Slovenia. 2004.
Crossref - Nieuwhof GJ, Bishop SC. Costs of the major endemic diseases of sheep in Great Britain and the potential benefits of reduction in disease impacts. Anim Sci J. 2005;81(1):23-29.
Crossref - Wassink GJ, King EM, Grogono-Thomas R, Brown JC, Moore LJ, Green LE. A within farm clinical trial to compare two treatments (parenteral antibacterials and hoof trimming) for sheep lame with footrot. Prev Vet Med. 2010; 96(1-2):93-103.
Crossref - Storms J, Wirth A, Vasiliadis D, et al. Prevalence of Dichelobacter nodosus and ovine footrot in German sheep flocks. Animals. 2021;11(4):1102.
Crossref - Thomas JH. The pathogenesis of footrot in sheep with reference to protease of Fusiformis nodosus. Aust J Agric Res. 1964;15(6):1001-1016.
Crossref - Beveridge WIB. Foot-Rot in Sheep: A Transmissible Disease due to Infection with Fusiformis nodosus(n. sp.). studies on Its Cause, epidemiology, and Control. 1941. H.E. Daw for Council for Scientific and Industrial Research; Melbourne.
Crossref - Egerton JR, Roberts DS, Parsonson IM. The aetiology and pathogenesis of ovine footrot. I. A histological study of the bacterial invasion. J Comp Pathol. 1969;79(2):207-216.
Crossref - Stauble A, Steiner A, Normand L, Kuhnert P, Frey J. Molecular genetic analysis of Dichelobacter nodosus proteases AprV2/B2, AprV5/B5 and BprV/B in clinical material from European sheep flocks. Vet Microbiol. 2014;168(1):177-184.
Crossref - Witcomb LA, Green LE, Kaler J, et al. A longitudinal study of the role of Dichelobacter nodosus and Fusobacterium necrophorum load in initiation and severity of footrot in sheep. Prev Vet Med. 2014;115(1-2):48-55.
Crossref - Claxton PD, Ribeiro LA, Egerton JR. Classification of Bacteroides nodosus by agglutination tests. Aust Vet J. 1983;60(11):331-334.
Crossref - Claxton PD. Antigenic classification of Dichelobacter nodosus. In: Footrot and Foot Abscess of Ruminants. (Egerton JR, Young WK, Riffkin GC, eds.). CRC Press Inc, Boca Raton. 1989:155-166.
- Ghimire SC, Egerton JR, Dhungyel OP, Joshi HD. Identification and characterization of serogroup M among Nepalese isolates of Dichelobacter nodosus, the transmitting agent of footrot in small ruminants. Vet Microbiol. 1998;62(3):217-233.
Crossref - Salzer R, Kern T, Joos F, Averhoff B. Environmental factors affecting the expression of type IV pilus genes as well as piliation of Thermus thermophilus. FEMS Microbiol Lett. 2014;357(1):56-62.
Crossref - Hendrickx APA, Bonten MJM, van Luit-Asbroek M, Schapendonk CME, Kragten AHM, Willems RJL. Expression of two distinct types of pili by a hospital acquired Enterococcus faecium isolate. Microbiol. 2008;154(Pt 10):3212-3223.
Crossref - Egerton JR, Dhungyel OP, Abbott KA, et al. Eradication of virulent footrot from sheep and goats in an endemic area of Nepal and an evaluation of specific vaccination. Vet Rec. 2002;151(10):290-295.
Crossref - Gurung RB, Dhungyel OP, Tshering P, Egerton JR. The use of an autogenous Dichelobacter nodosus vaccine to eliminate clinical signs of virulent footrot in a sheep flock in Bhutan. Vet J. 2006;172(2):356-363.
Crossref - Dhungyel OP, Schiller N, Eppleston J, et al. Outbreak-specific monovalent/bivalent vaccination to control and eradicate virulent ovine footrot. Vaccine. 2013;31(13):1701-1706.
Crossref - Wani SA, Samanta I, Bhat MA, Buchh AS. Molecular detection and characterization of Dichelobacter nodosus in ovine footrot in India. Mol Cell Probes. 2004;18(5):289-291.
Crossref - Farooq S, Wani SA, Hussain I, Bhat MA. Prevalence of ovine footrot in Kashmir, India and molecular characterization of Dichelobacter nodosus. Indian J Anim Sci. 2010;80(9):826-830.
- Wani SA, Farooq S, Kashoo ZA, et al. Determination of prevalence, serological diversity, and virulence of Dichelobacter nodosus in ovine footrot with identification of predominant serotype as a potential vaccine candidate in J&K, India. Trop Anim Health Prod. 2019;51(5):1089-1095.
Crossref - Sreenivasulu D, Vijayalakshmi S, Raniprameela D, Karthik A, Wani SA, Hussain I. Prevalence of ovine footrot in the tropical climate of southern India and isolation and characterization of Dichelobacter nodosus. Rev Sci Tech. 2013;32(3):869-877.
Crossref - Qureshi S, Wani SA, Farooq S, et al. Genome sequence of Dichelobacter nodosus JKS-07B isolate from J&K, India associated with virulent footrot of sheep. Sci Prog.2021;104(4):1-14.
Crossref - Bhat MA, Wani SA, Hussain I, Magray SN, Muzafar M. Identification of two new serotypes within serogroup B of Dichelobacter nodosus. Anaerobe. 2012;18(1):91-95.
Crossref - Dhungyel OP, Whittington RJ, Egerton JR. Serogroup specific single and multiplex PCR with pre-enrichment culture and immunomagnetic bead capture for identifying strains of D. nodosus in sheep with footrot prior to vaccination. Mol Cell Probes. 2002;16(4):285-296.
Crossref - Skerman TM. Vaccination against foot-rot in sheep. NZ Vet J. 1971;19(5):112.
Crossref - Quraishi A, Ul Tarfain N, Hussain I, et al. Optimization of growth conditions for Dichelobacter nodosus in a modified reducing broth without gas phase. Int J Adv Biochem Res. 2024;8(7):734-738.
Crossref - Raadsma HW, O’Meara TJ, Egerton JR, Lehrbach PR, Schwartzkoff CL. Protective antibody titres and antigenic competition in multivalent Dichelobacter nodosus fimbrial vaccines using characterized rDNA antigens. Vet Immunol Immunopathol.1994;40(3):253-274.
Crossref - Egerton JR, Roberts DS. Vaccination against ovine foot-rot. J Comp Pathol. 1971;81(2):179-185.
Crossref - Garrity GM, Bell JA, Lilburn T. Thiotrichales ord. nov. In: Brenner, D.J., et al. Bergey’s Manual® of Systematic Bacteriology. Springer, Boston,MA; 2005.
Crossref - Raadsma HW, Egerton JR. A review of footrot in sheep: aetiology, risk factors and control methods. Livest Sci. 2013;156(1-3):106-114.
Crossref - Stewart DJ, Claxton PD. Ovine footrot clinical diagnosis and bacteriology. In: Corner LA, Bagust TJ (eds). Australian Standard Diagnostic Techniques for Animal Diseases. CSIRO Information Services, Melbourne. 1982:1-27.
- Thomas JH. A liquid medium for the growth of Fusiformis nodosus. Aust Vet J. 1963;39(11):434-437.
Crossref - Parsonson IM, Egerton JR, Roberts DS. Ovine interdigital dermatitis. J Comp Pathol. 1967;77(3):309-313.
Crossref - Marsh H, Claus, KD. The diagnosis of footrot in sheep. Cornell Vet. 1970;60:309-317.
- Egerton JR. Significance of Fusiformis nodosus serotypes in resistance of vaccinated sheep to experimental footrot. Aust Vet J. 1974;50(2):59-62.
Crossref - Skerman TM. Determination of some in vitro growth requirements of Bacteroides nodosus. Microbiol. 1975;87(1):107-119.
Crossref - Mattick JS, Anderson BJ, Mott MR, Egerton JR. Isolation and characterization of Bacteroides nodosus fimbriae: structural subunit and basal protein antigens. J Bacteriol.1984;160(2):740-747.
Crossref - Rani KS, Prameela DR, Sreenivasulu D, Vijayalakshmi S, Karthik A. Development of inactivated whole cell vaccine against ovine footrot. Inter J Vet Sci. 2016;5(2):103-105.
- Ross AD, Titterington DM. Injection site lesions of footrot vaccines in sheep. NZ Vet J. 1984;32(1-2):6-8.
Crossref - Dhungyel OP, Lehmann DR, Whittington RJ. Pilot trials in Australia on eradication of footrot by flock specific vaccination. Vet Microbiol. 2008;132(3-4):364-371.
Crossref - Egerton JR. Treatment of ovine foot-rot by vaccination with the specific aetiological agent Bacteroides nodosus. Comp Immunol Microbiol Infect Dis. 1979;2(1):61-67.
Crossref - Stewart DJ. The role of various antigenic fractions of Bacteroides nodosus in eliciting protection against foot-rot in vaccinated sheep. Res Vet Sci. 1978;24(1):14-19.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.