ISSN: 0973-7510
E-ISSN: 2581-690X
The ochratoxin A (OTA) is a mycotoxin which is present in food products as a contaminant, and it is one of the hazardous toxins causing health risks in animals and humans. One of the main health issues is the damage to kidneys. The most adopted technique used in detoxification of this mycotoxin is biodegradation. In this study, Acinetobacter calcoaceticus isolated from soil samples was used for the detoxification of ochratoxin, and also this study explains the antibiotic resistance potential of this organism. Acinetobacter calcoaceticus was tested to see if they could break down ochratoxin A(OTA). Acinetobacter calcoaceticus was shown to be able to break down OTA among the tested microorganisms. We tested the ability of A. calcoaceticus to degrade OTA in LB medium at 25 and 28°C, with OTA concentrations of 2ppm, 6ppm, and 10ppm. A. calcoaceticus was able to break down OTA from a starting concentration of 10 (g/ml) at these conditions. At 25 and 30°C, A. calcoaceticus removed an average of 0.1005 and 0.0636 (g/ml/h of OTA, respectively, from a medium containing an initial concentration of 10 (g/ml). A. calcoaceticus degraded ochratoxin A significantly during and after the log phase of cell development at both incubation temperatures. The hypothesis is that A. calcoaceticus degraded OTA into an ochratoxin with reduced toxicity. At the same time the potential of this microorganism strain was also measured using susceptibility testing and it showed the potential of development of its resistance. Strains of Acinetobacter calcoaceticus isolated from soil samples were tested for their susceptibility against different unrelated classes of antibiotics. A. calcoaceticus was resistant to multiple antibiotics. In vitro degradation assays were used exposing the toxin to the degrading enzyme or microorganism in a controlled laboratory environment. The degradation of the toxin was monitored using various techniques such as high-performance liquid chromatography (HPLC). The significance of this study is to highlight the capability of the Acinetobacter calcoaceticus in degrading ochratoxin A, so that health risks associated with it can be reduced; also, the antibiotic resistance potential measurement helps in development of optimum antimicrobial strategy.
Degradation, Ochratoxin A, Ochratoxin α, Acinetobacter calcoaceticus, Mycotoxins, Biodegradation
Mycotoxins are toxic substances produced by certain types of fungi that can contaminate food and feed crops.1 These toxins can have harmful effects on human and animal health, including carcinogenic, mutagenic, teratogenic, and immunosuppressive effects.2-4 Mycotoxins can also cause acute and chronic illnesses, including liver damage, respiratory distress, and neurological disorders.5-7
The most common types of mycotoxins include aflatoxins, ochratoxins, fumonisins, deoxynivalenol (DON), zearalenone (ZEN), and patulin. Contamination of food and feed with mycotoxins can result in economic losses due to crop damage and decreased quality, as well as trade restrictions and public health concerns.8 Therefore, detection, prevention, and control of mycotoxins are important for ensuring food safety and protecting human and animal health.
Numerous microorganisms, such as actinobacteria, bacteria, filamentous fungi, and yeast, have been found to break down and/or adsorb OTA. The most crucial biodegradation pathway for OTA is the transformation of OTA into less hazardous OT via hydrolysis of the amide bond. Cell wall components are the primary determinant of microorganisms OTA adsorption capacities. Several bacteria have been discovered to have high OTA degradation and/or adsorption capacity, and various OTA degradation enzymes9 have been identified or cloned from microbes and animal pancreas, offering the promising potential for use in the food and feed industries.10
“In addition to cereals, coffee beans, almonds, chocolate, pulses, beer, wine, spices, and dried vine fruits, ochratoxin A (OTA) is a mycotoxin that can contaminate other plant items. OTA contamination is not limited to food plants. Ochratoxins are cyclic pentapeptides with an attached L-phenylalanine moiety; they are produced from dihydroisocoumarin.11 In this context, ochratoxins are defined as a type of poison. An OTA was first identified in 1965 in a laboratory-grown strain of Aspergillus ochraceus.11 Since then, numerous reports of Aspergillus and Penicillium species that produce this mycotoxin have emerged. Studies have shown that OTA can damage the kidneys,12-14 suppress the immune system, result in congenital malformations, and even trigger cancer. It has been suggested that OTA may contribute to nephropathies in both humans and animals. This mycotoxin, for instance, is linked to kidney disorders in both humans and animals, such as the Nephropathy in a Danish pig model. Kidney atrophy, tubular degeneration, interstitial fibrosis, and hyalinization of the glomeruli are hallmarks of Balkan endemic nephropathy, for which OTA has been proposed as a possible etiological factor.14 This disease has been shown to affect humans. Strong evidence in experimental animals led to OTA’s 1993 classification as a possible human carcinogen (Group 2B) by the International Agency for Research on Cancer (IARC). In Tunisia, OTA has been associated with chronic karyomegalic interstitial nephropathy and chronic interstitial nephropathy, while in Egypt, it has been associated with urothelial tumors (end-stage renal disease).15
Many investigations have also targeted learning how OTA is taken up by bacterial cell walls. Two commercial yeast cell walls (YCW) were used to measure OTA adsorption capacity in stomach-like conditions (YCW1 contained 23.3% polysaccharides containing 17.4% glucans and 5.9% mannans, YCW2 contained 44.0% polysaccharides containing 23.0% -glucans, and YCW3 contained 23.3% polysaccharides containing 17.4% glucans and 23.0% mannans). It was found that the number of polysaccharides or -glucans/mannans present had no bearing on the adsorption capacity for OTA.16
Problem Statement
The fungal secondary metabolite ochratoxin A (OTA) has been found in a wide range of animal feeds and other products. This means OTA will always pose a risk to people and other animals. As a result, getting rid of OTA in agriculture and food will necessitate a variety of methods. Safety, flavor, nutritional quality, organoleptic attributes, availability, and cost-effectiveness are just few of the ways in which biological detoxification systems excel over their chemical and physical counterparts. This study provides a comprehensive overview of the most recent findings in the field of OTA biodetoxification utilizing enzymes, adsorption, and degradation.
Strength of OTA Adsorption as Affected by strain and Status
The capacity of OTA to adsorb changed as a function of stress. Strains W28 and W46 of S. cerevisiae were reported to bind 34.51–70.17 percent of OTA (2 g/L) in grape must. However, 80%-85% of the OTA bound to S. cerevisiae was released back into the washing solution upon rewashing. The capacity of the S. cerevisiae-OTA complex to bind varied greatly between strains. Even though strain W13 of S. cerevisiae was more effective in removing OTA (2 g/L) from grape must than strains W28 and W46, the releasing-back percentage of 55% was still much lower. This demonstrated that offspring of S. cerevisiae TP5 and TT173 differ significantly in their adsorption ability for OTA when compared to their parents. Given the ionic and electrostatic interactions between OTA and mannoproteins, it is possible that the different adsorption capabilities of yeast cells can be attributed to their mannoprotein composition.17
Adsorption of OTA by the same bacterium varied in strength depending on whether or not the organism was alive or dead. Microbial cells’ attachment to OTA was only weakly reversible. According to reports, the OTA-cell complex formed by live cells was more stable than the OTA-cell complex formed by dead cells, with just 11% and 22% of the OTA-cell complex initially bound to release back into the PBS buffer, respectively. Schefferchys cerevisiae protoplasts the inability of BS to bind OTA suggests that the adsorption of OTA is tightly linked to components of the cell wall.
Recent studies in grapevine soils have led to the identification of a unique free-living strain of Acinetobacter sp. neg1, ITEM 17016, which can convert OTA into the non-toxic catabolic product OT17. The genome sequenced for ITEM 17016 was used to conduct a phylogenetic analysis, which revealed that Acinetobacter gyllenbergii is the closest relative to Acinetobacter sp. neg1. Peptidase genes are plentiful in Item 17016’s genome. To show how comparative transcription analysis can be used to locate candidate genes and pathways engaged in OTA degradation, we used it to illustrate a scenario. The development of biotechnological applications for the degrading activities of enzymes will benefit from this knowledge. The ability of the chosen peptidase to detoxify the drug was studied by cloning the enzyme in a heterologous system. The aims of the study are to identify both the detoxification potential of Acinetobacter calcoaceticus and the Antibiotic Resistance of Acinetobacter calcoaceticus.
Literature Review
Ochratoxin A (OTA) is a mycotoxin produced by certain species of fungi, such as Aspergillus and Penicillium, which can contaminate food and feed products, posing a risk to human and animal health. Various strategies have been proposed to detoxify OTA-contaminated matrices, including the use of microbial biodegradation.17,18 Acinetobacter calcoaceticus is a Gram-negative bacterium commonly found in soil, water, and plant environments. Recent studies have reported the ability of some Acinetobacter strains to detoxify OTA through degradation or adsorption mechanisms. Additionally, Acinetobacter strains have been studied for their potential as a source of novel antibiotics and resistance mechanisms.18
In a study, the authors aimed to evaluate the OTA detoxification potential and antibiotic resistance profiles of Acinetobacter calcoaceticus strains isolated from soil samples. The study involved the isolation and identification of Acinetobacter strains from soil samples, followed by screening for OTA detoxification potential using HPLC analysis. The antibiotic resistance profiles of the isolated strains were also determined using standard methods.19 The study found that some of the Acinetobacter strains isolated from soil samples exhibited OTA detoxification potential through enzymatic degradation, while others showed adsorption activity. The strains also showed resistance to various antibiotics commonly used in clinical and veterinary settings. The results of this study suggest that Acinetobacter strains have potential for use in OTA detoxification and as a source of novel antibiotics, but further studies are needed to fully explore their capabilities and safety.
Most experts agree that the most effective way to combat mycotoxins’ deleterious effects on animal and human health is to stop the fungi in the field from growing and producing mycotoxins in the first place. Because mycotoxin-producing moulds typically only colonize injured plant sections, it is important to take precautions against mechanical processes and insects that may cause damage to crops. Furthermore, contamination can be avoided by reducing the number of weeds and agricultural leftovers that serve as inoculum sources. Reducing plant stress and employing GAPs like crop rotation and harvesting at the right times of year are important steps in growing healthy plants. In fact, Rousseau and Blateyron both stressed that with proper vineyard management, OTA levels in wine might be cut by as much as 80%.20
Fungicides are commonly applied to field crops before to harvest to limit the prevalence of infection. The chemical makeup of the fungicide, the treatment rate, the sort of crop, the fungal species, and the storage circumstances all play a role in the fungicide’s ability to prevent mould growth and the production of mycotoxins. The amount of OTA produced by A. sulphureus, P. verrucosum, and A. ochraceus was found to be suppressed by the organophosphate fungicide dichlorvos. The fungicide iprodione was discovered to have the potential to lower OTA production in A. westerdijkiae; this fungicide has a long history of secure and effective usage in agricultural commodities against a wide variety of fungal species, including those that produce OTA. The fact that this fungicide has been employed previously in agricultural products paved the way for our current discovery. A number of lab-based studies have looked into how fungicide treatments affect OTA concentrations in wines. Previous research has found that the black aspergilli that permeate grapefruit can be efficiently controlled by using a combination of the sulfamide-type fungicide Euparen with either the captan or mycodifol fungicide. Use of certain pesticides, such as azoxystrobin (a derivative of strobilurin) or Dinocap (a derivative of dinitrophenyl), in combination with sulphur has been shown to effectively lower the OTA content of wines in recent years. Combinations of insecticides like Carbendazim and Chorus, for example, have been demonstrated to be ineffective against aspergillus sour rot. However, trials conducted in France, Spain, Greece, and Italy showed that a different form of pesticide called Switch greatly reduced the number of black aspergilli present on grapes. In addition to the pyrimidine fungicide cyprodinil, Switch also includes the pyrrolnitrin fungicide fludioxonil. Given that pyrrolnitrin has been shown to be effective against black aspergilli, the fact that fludioxonil can be used against aspergilli should come as no surprise. Another study found that the fungicides Switch, Scala (which contains the pyrimidine fungicide pyrimethanil), and Mikal reduced both the amount of OTA in wines and the frequency of fungal colonisation in wines (containing fosetyl-Al and the dicarboximide folpel). Other fungicides that have been shown to be effective against fungal growth or OTA levels in grapevine include mepanipyrim, pyrimethanil, fluazinam, and iprodione. Mepanipyrim has fungicidal properties as well. Based on the results of a study that compared the effectiveness of 26 different fungicides against A, a combination of cyprodinil and fludioxonil, azoxystrobin, and penconazole greatly reduced both the infection rate and the amount of OTA produced. carbonarius infection and OTA generation on grapes and in synthetic medium. However, studies have shown a correlation between specific fungicide applications and an increase in OTA production in grapes. Fenhexamid, mancozeb, and copper hydroxide + copper have all been proven to boost infection and OTA production in grapes, whereas carbendazim has been shown to reduce fungal biota while boosting OTA synthesis. Antifungal fusapyrone, generated by Fusarium semitectum, and the antibiotic natamycin have shown promising effects in the recent management of OTA-producing aspergilli and OTA concentrations in vineyards.21
In most cases, plant breeding will result in an increase in a host plant’s resistance to the invasiveness of fungi. These kinds of efforts have shown some potential in combating problems such as the infection caused by Fusarium in wheat and corn. Researchers found that kernels of wheat, rye, and barley from different genotypes exhibited varying degrees of resistance to attack by fungi and accumulation of OTA. As a consequence of this, strains that have a stronger resistance to the invasion of fungi while they are being stored could be selected. In spite of this, to the best of our knowledge, grains have not been developed to be resistant to the accumulation of OTA.22 In addition, there is a lack of information concerning the accumulation of OTA that is caused by black aspergilli and the degree to which various grape varieties are vulnerable to infection. However, following an artificial infection with a combination of five OTA-producing strains, three of the twelve types that were investigated showed low levels of OTA contamination. These varieties included “Bianco di Alessano,” “Pampanuto,” and “Uva di Troia.” Cabernet Sauvignon was the most susceptible of the varieties. In a similar vein, there is a dearth of knowledge concerning the extent to which different coffee cultivars can withstand the accumulation of OTA. However, a lot of effort has been spent into finding and breeding cultivars that are resistant to the coffee berry borer. These cultivars should have lower levels of OTA because of the work that has been put into finding and developing them. Because of the natural resistance mechanisms in their genetic makeup, the Coffea abeokutae, Coffea excelsa, and Coffea kapakata species of coffee are exceptionally resistant to the coffee berry borer.23
Production and Isolation of Ochratoxin
To obtain, isolate, and produce ochratoxin A suitable microbial strain such as Aspergillus specie, was selected for ochratoxin production. The strain was grown on growth medium under optimal conditions for ochratoxin production, which included a temperature of 25-30°C and a relative humidity of 90-95%. The culture was monitored for ochratoxin production over time using techniques such as high-performance liquid chromatography (HPLC). Once sufficient quantities of ochratoxin were produced, the toxin was extracted from the culture using solid-phase extraction. The extracted ochratoxin was purified using technique of preparative HPLC to obtain a highly purified form of the toxin. The purified ochratoxin was quantified and characterized. The purified ochratoxin was used for further research studies, such as in vitro assay and susceptibility testing.
Strain identification using molecular and conventional techniques
The A. calcoaceticus was allowed to grow on agar medium after isolation. Light and scanning electron microscopy was used for studying colony characteristics and morphological structure identification.
Application of 1% tetramethyl-p-phenylenediamine discs to colonies on LB plates determined the Oxidase activity by assessing violet colour formation within 10 seconds. The addition of 3% H2O2 to single colonies on an LB plate determined the Catalase activity by evolution of O2.
Molecular characterization of the specie was determined by 16S rDNA sequencing and amplified by PCR using the primers, 27f: 5′-AGAGTTTGATCMTGGCTCAG-3′ and 1492r: 5′-TACGGYTACTTGTTACGACTT-3′.24
dnastar software was used for DNA sequences manipulation. The software is developed by DNASTAR, Inc., a company based in Madison, Wisconsin, USA. The version used is Lasergene 17, which was released in 2021.
Ochratoxin A degradation assay
The bacteria were placed in an incubator with maximum growth medium for 2 to 4 days to enable the medium to reach its usual pH. Then allow picking the colonies from Petri dishes and moving it to flasks containing 200 ml LB media.
Two, six, and ten parts per million (ppm) of OTA were tested against a bacteria-free control group. The biomass was kept constant at 20°C with a glycerol content of 15%. Part of the bacterial biomass was thawed, then activated by a single passage in liquid LB medium, and then cultured for 24 hours at 37°C, before each experiment.25
Reference for ochratoxin A. Ochratoxin The stock solution of the standard was kept in 200 ppm absolute ethanol at 28°C.
Chemical Analysis
Chemical analysis was done using HPLC (High-Performance Liquid Chromatography). We used acetonitrile, water, and glacial acetic acid to dilute the supernatants by a factor of 10. The samples were filtered through RC 0.2 m micro spin filter tubes. Injecting 50 L into the HPLC apparatus with the use of a full loop injection mechanism was performed. It was feasible to infuse the liquid culture directly because there were no competing peaks eluting throughout the retention times of OT (ochratoxin) and OTA (ochratoxin A). Gallo et al.26 guidelines for quantifying toxin were mostly followed, with a few minor changes. Comparisons of peak areas to calibration curves yielded retention times of 7.75 and 10.36 minutes for OT and OTA, respectively. At 0.1 ng/mL, both OTA and OT could be detected (based on a signal-noise ratio 3:1). With the help of HPLC-HRMS (High-Performance Liquid Chromatography High-Resolution Mass Spectrometry), we were able to verify OT’s structure and find new putative degradation metabolites. When HPLC and HRMS are combined, the HPLC separates the components in a complex mixture, and the HRMS detects and identifies the separated components based on their mass-to-charge ratios with high resolution and accuracy. Further, an HPLC-HRMS analysis was performed on 20 L of supernatants using the specified procedure.
Determination of sensitivity of bacteria to the presence of ochratoxin A by conventional method
The disc diffusion method was used to test the strains of bacteria for their sensitivity to ochratoxin A. The bacteria were introduced to the LB agar medium as a suspension at a concentration that yielded a final cell density of about 106 per cubic centimeter of the medium. The medium was properly mixed and then placed onto 15 cm3 sterile Petri dishes. On the surface of the medium, it positioned sterile paper discs (10 mm in diameter) soaked with solutions made from standard ochratoxin A solution at the quantity of 20 l. The disc contained 0.1; 0.5; 1, 5 and 10 g of ochratoxin A. After 48 hours of incubation at 37°C, zones limiting the development of the researched microorganisms were checked around discs with particular OTA concentrations in the thermos shaker. Transferring the strains to an LB medium was also used to regulate their viability.27
The bacterium Acinetobacter calcoaceticus was shown to be capable of OTA degradation. There was an investigation into the bacterial decomposition of OTA in broth. LB medium (pH 7.50) was used to cultivate Acinetobacter calcoaceticus cells. A. calcoaceticus was cultured for 24 hours in 0.2 ml, yielding approximately 106 cells/ml, and then inoculated into flasks containing LB medium containing OTA concentrations of 2ppm, 6ppm, and 10ppm. The incubation process was carried out in a thermos shaker at a temperature of 30°C. Reciprocal shaking at a rate of 90 strokes per minute was used to aerate the flasks.
Determination of sensitivity of bacteria to the presence of ochratoxin A by Advanced method
Supernatants were analysed for ochratoxin A concentration using a Waters 515 system equipped with a 515 HPLC pump, 717 plus autosampler, 2489 UV/Vis detector (DAD), and 474 fluorescence (FLD) detector. Maintaining a temperature of 30°C on the Agilent 5 TC-C18, 250 4.6 mm, 5 m reversed-phase column was performed. The entire volume injected was 20 litres, at a rate of 1 ml per minute. The mobile phase was made up of acetonitrile, water, and acetic acid (57: 41: 2). (built on an isocratic foundation) The FLD detector used excitation and emission wavelengths of 330 nm and 460 nm. In order to quantify ochratoxin A, a linear external calibration between 25 and 2000 g l1 was utilised.
HPLC was used to quantify the amount of ochratoxin A in the samples, and UPLC-MS/MS was used to detect any ochratoxin A metabolites. Ochratoxin A and ochratoxin were detected using a Thermo Scientific UltiMate 3000 UPLC system coupled with a Thermo Scientific Q Exactive mass detector with HESI controlled by EMPOWER 3 software. Immediately after collection, the sample was introduced into the 40°C analytical column (100 x 2.1 mm x 1.6 m; Waters Cortecstm UPLC C18).
Two litres injected at a rate of thirty-three milliliters per minute. Solvent A consisted of methanol, while solvent B contained water, 0.01% formic acid, and 1 mmol/l ammonium acetate. A depiction of the elution gradient is shown below. A 90% B is awarded for the first two minutes, an 80% B for the third, a 79% B for the fourth, a 74% B for the fifth, a 40% B for the tenth, a 5% B for the thirteenth, and a final 90% B for the final eleven minutes and twenty-one seconds.
To recap, these were the HESI values: Single ion monitor in positive mode with a m/z width of 4 Da; collision energy of 35 eV; ion transfer tube temperature of 320°C; spray capillary voltage of 3.2 kV; collision energy of 35 eV; HESI at 300°C; sweeping with N2 at 35 atm; mass resolution of 70 000 FWHM; mass/mass resolution of 17 500 FWHM; m/z range of 200-800 Da; etc.
Screening of the strains capable of reducing the amount of ochratoxin A in model media
In the experiment, 10 cm3 of liquid LB medium with 0.05 cm3 of ochratoxin A standard solution was utilized. At first, there were 200 ml of ochratoxin A in the medium. At a density of 107 CFU/cm3, biomass suspended in a physiological salt solution (5% vol) was used to inoculate the media. Amounts of ochratoxin A were measured in the supernatant after bacterial cultures were grown in static conditions at 37°C for 120 hours. Percentages were used to show how much the toxin concentration in the medium had dropped from the start.
Assessment of changes in the amount of ochratoxin A during cultivation
Ochratoxin 200 ccs of LB medium was spiked using a standard solution. At time = 0, the OTA concentration was carefully measured to be 2, 6, and 10 ppm. The microorganisms in the exponential growth phase were suspended in normal saline and used as inoculum (107 cells ml1). The optimal temperature for the culture’s growth was 37°C. Using a Thermos shaker, it is measured the concentration of ochratoxin A in the post-culture liquid and the biomass at 5, 15, 24, and 40 hours. The standard plate technique on LB medium was used to determine the number of bacterial cells at the same intervals.28
Ochratoxin A analysis
Technique for quantitatively analyzing immunoenzymatically responses After the biomass was centrifuged, the concentration of ochratoxin A in both the biomass and the post-culture media was measured using ELISA. R-Ridascreen Ochratoxin Biopharm’s A from Darmstadt, Germany, was used for the analysis. The extraction method used was carried out as recommended by the manufacturer. With this test, a concentration of 80 ppt is the minimum level of detection.
Susceptibility testing against antibiotics
An agar dilution approach was employed to determine minimum inhibitory concentrations (MICs).19 On the day of the experiment, plates were covered with tubes containing various concentrations of antibiotics and 20 ml of melted Diagnostic Sensitivity Test agar (Hi Media, Bombay, India). Antibiotic concentrations ranged from 2-1024/zgm1-1. The agar plates were then injected with 22 spots, each containing 104–105 bacteria from exponentially developing cultures, and dried at 37 for 1 hour. As controls, DST agar plates without antibiotics were infected with test organisms. After 24 hours of incubation at 30°C, the plates were read. The maximum concentration that permitted microbial growth was known as the subinhibitory concentration (SIC), while the lowest concentration that prevented it was known as the MIC. The phrase “resistant strain” refers to a species that is not inhibited by 25/zgml-I of antibiotics.
Statistical Analysis
In this study, it gives the mean of three independent measurements. The results of a one-way analysis of variance (ANOVA) were analyzed.
Characterization of ochratoxin A biodegradation by Acinetobacter calcoaceticus
This strain of A. calcoaceticus was shown to have a high biodegradation ability, as it was able to totally degrade ochratoxin A in the culture medium. After several iterations of cultivation in OTA-LB, ochratoxin A was nearly eradicated (up to 100%) over a range of inoculum sizes, temperatures, and ochratoxin A concentrations. Increasing the inoculum size from 01 to 1% enhanced the ochratoxin A conversion rate. In tests with greater inocula, the lag time was reduced. It’s possible that the time it took for bacteria to develop, to begin biodegrading ochratoxin A, and to completely change ochratoxin A to ochratoxin was quite short. When exposed to OTA-LB, cells quickly adapted and began producing enzymes to break down the toxin. Inoculum size has an effect on the rate of ochratoxin A degradation from 24 to 48 hours. When the inoculum size was increased from 01 to 1%, a 38% and 22% boost was seen after 24 and 36 hours, respectively. It took 24 hours for 50% of the ochratoxin A to be degraded when a concentrated inoculum was utilized.
A. calcoaceticus dramatically decreased the OTA concentration over the course of six days. The results of the research demonstrated that OTA was transformed to OT by A. calcoaceticus. There was an average decline of 54% (RSD, 12%) in OTA after three days at 24°C, and a decline of 91% (RSD, 1%) after six days when compared to the control (100% OTA). In studies conducted at temperatures ranging from 22 to 28°C, our data showed that the bacterial degrading activity was slightly more effective at 24°C. Once incubated for just one day, the degrading activity was already functional. The results showed that enzymes generated by A. calcoaceticus were capable of converting OTA to OT. Microbial breakdown of OTA results in the production of OT and L-phenylalanine by hydrolysis of the amide bond, as predicted by the OTA’s chemical structure. HPLC-HRMS was used to identify the metabolite produced by OT.
Microbial screening
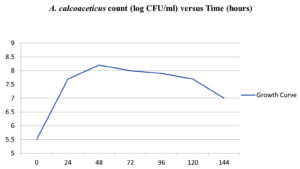
The results of the plate experiment demonstrated that none of the microorganisms examined affected the original hue of OTA or rendered its fluorescence nonexistent. In their research, Galtier and Alvinerie claimed that OTA fluorescence had disappeared. Since no microorganisms in the study were able to completely eradicate OTA fluorescence, it implies that none of the species examined were able to break the isocoumarin ring of OTA. Under ultraviolet (UV) light, A. calcoaceticus fluorescence assays in test tubes showed two colours: blue and green. Green fluorescent dots had an Rf (radio frequency) of 0.69, while blue fluorescent dots registered at 0.33. Value (R) for the ochratoxin A reference standard was 0.69. As expected, A. calcoaceticus treatment resulted in blue spots on TLC plates with the same fluorescent colour and R(values as ochratoxin a. An extracellular esterase is present on the surface of A. calcoaceticus and in the cell-free medium in which it grows. Kaufman’s research suggests that certain soil bacteria are capable of degrading pheny1carbamate herbicides (general formula, C6Hs -NH-CO-R). A single Acinetobacter spp. isolate may be capable of hydrolyzing two widely used pheny1carbamate herbicides, 2-chloroethyl-N-(3-chlorophenyl)-carbamate (CEPC) and isopropyl-N-(3-chlorophenyl)-carbamate (IPC) (CIPC). Soil microorganisms are thought to hydrolyze the amide bond or the ester linkage to degrade these two herbicides. Like Acinetobacter does with CEPC and CIPC, A. calcoaceticus probably broke down OTA by hydrolyzing the amide link. The generation of ochratoxin a during OTA degradation provided compelling evidence that A. calcoaceticus degraded OTA to ochratoxin a by cleaving the amide bond in test tube assays. However, more investigation is needed to confirm this theory. If you put A. calcoaceticus in LB medium at 28° C, what happens to it, and how does OTA degrade? The growth curve of A. calcoaceticus in LB medium devoid of OTA at 28°C is shown in Figure 1. On Figure 2, we see A. calcoaceticus growing in LB medium with a 10ppm OTA concentration at 28°C and the resulting breakdown of OTA. Approximately Log 5.6 CFU/ml of A. calcoaceticus was used for the first inoculation. A. calcoaceticus cells proliferated most rapidly in a growing medium with an initial OT A concentration of 10 ppm after 48 hours (Figure 2). At 10ppm OTA Alml, the growth curve of A. calcoaceticus (Figure 2) is more steeply declining after the initial peak than the growth curve in medium without OTA (Figure 1). At 10 ppm OTAalml, cells began to recover when OTA was degraded in the medium (Figure 2). At a dosage of 10 ppm, OT A had a noticeable negative effect on A. calcoaceticus cells near the end of the log phase. Other researchers have found that OT A has the same antibacterial effect.
Figure 2. Changes of ochratoxin A concentration and viable A. calcoaceticus in LB medium with ochratoxin A. concentration of 10ppm at 28°C
After 24 hours, the original 6ppm of OTA in the medium had dropped to a level that was statistically significant (p 0.05). At 28°C, in a medium containing six ppm OTAlml, no OT A was found (Figure 2). 0.1005 Ilg/ml/h was the average rate of OTA decline. Analysis of A. calcoaceticus and OTA breakdown in LB medium at 28°C. Figure 3 displays the A. calcoaceticus growth curve in LB medium without the addition of OTA at 28°C. Figure 4 depicts what happens to A. calcoaceticus, and the OTA as it degrades in LB medium with an initial OTA concentration of 6ppm at 28°C. A. calcoaceticus was initially inoculated at a concentration of around Log 6.0 CFU/ml. After a 24-hour lag period, A. calcoaceticus cell counts in growth medium with an initial OT A concentration of 6ppm reached a maximum of Log 9.0 CFU/ml at 48 hours (Figure 4). Whereas A. caleoaceticus grew exponentially in LB medium with or without OT A at both 25 and 30°C, a 24-hour lag phase was seen at 25°C while none was seen at 28°C. Within inoculation, cell counts in 6ppm OT All media dropped somewhat but recovered to maximum levels after 48 hours. With an increase in cell number, ochratoxin A levels dropped significantly (p 0.05) during 24 and 48 hours. At 48 hours, OTA declined significantly (p 0.05) in medium with 6ppm OT All, and the subsequent decline was linear (r > 0.95). After 144 hours, only six ppm of OT A was left in the medium. After 48 hours of incubation, when the number of A. calcoaceticus colonies was at its greatest, the average OTA concentration dropped by 0.0636 Ilg/ml/h (Figure 4). More ochratoxin A was degraded (p 0.05) when the medium was 30° C and included six ppm OTAlml than when it was 28°C. Since the lag phase was so long at 28°C, this makes sense. After 120 hours at 28°C, no ochratoxin A was found. However, trace levels of OTA remained. Our initial tests showed that LB medium (no ethanol added) containing OT A was not suitable for the growth of A. calcoaceticus. That A. calcoaceticus couldn’t get its carbon needs met by OT A alone was clear evidence of that. Therefore, all experiments utilized LB medium supplemented with ethanol as a carbon source.
Figure 3. Changes of ochratoxin A concentration and viable A. calcoaceticus in LB medium with ochratoxin A. concentration of 6ppm at 28°C
Figure 4. Changes of ochratoxin A concentration and viable A. calcoaceticus in LB medium with ochratoxin A. concentration of 2ppm at 28°C
Antibiotics Resistance Potential of Acinetobacter calcoaceticus
Except for carbenicillin and cefotaxime, to which it tested resistant to 55% and 21%, respectively, of all beta-lactam group antibiotics, Acinetobacter calcoaceticus exhibited resistance. With the exception of neomycin, it was also resistant to the majority of aminoglycoside antibiotics. The most toxic antibiotics, rifampicin and nalidixic acid, prevented the growth of A. calcoaceticus even at extremely low concentrations. Since isolates of A. calcoaceticus were found to be resistant to 12 of the 18 tested antibiotics, it was determined that this genus was the most resistant. The strains of A. calcoaceticus showed a significant level of multiple resistance, which was also noted.
When it comes to mycotoxins, ochratoxins are among the most valuable economically. Agriproducts are vulnerable to contamination by the ochratoxin-producing fungus at any stage of the supply chain, including before harvest, after harvest, and during processing. The presence of ochratoxin in contaminated food and feed is extremely dangerous to both animal and human health. To protect people and animals from OTA’s harmful effects, agricultural goods that are destined for human or animal consumption must not include more of the toxin than is legally allowed. Extensive research into the important points of OTA presence in the production chain of numerous affected commodities, including cereals, coffee, and wine, has been conducted, and detoxification techniques have also been explored. The best method for dealing with OTA contamination is to take preventative measures before and after harvest. The creation of OTA cannot be stopped fully, but its accumulation can be reduced. Effective ways for cleansing tainted goods have also received more attention. When agricultural products tainted with mycotoxins are repurposed as animal feeds, decontamination or detoxification treatments are helpful. Mycotoxins can be found in a wide variety of commodities, and while some treatments have been shown to reduce levels of individual mycotoxins, no universal technique has been discovered. The utilization of microorganisms or their enzymes for decontamination purposes is among the most promising options with regard to OTA. More in-depth study is required to find more effective organisms that can be employed safely for OTA decontamination.
Recommendation
Acinetobacter calcoaceticus was found to be capable of degrading OTA in LB medium at 25 and 30°C with an initial OTA content of 10ppm, as suggested by the above study. TLC tests confirmed that ochratoxin a was the result of OT A degradation by A. calcoaceticus, albeit this needs to be verified experimentally. It has demonstrated that ochratoxin A is significantly less harmful than previously thought. Because of this, some have proposed that A. calcoaceticus’s ability to degrade OTA constitutes a microbiological detoxication of OTA. For this experiment, researchers used an in vitro testing system and monitored environmental parameters. Despite the encouraging findings, these findings do not prove that food or feed can degrade OTA in an in-situ system. The results of this study encourage additional research into the microbial decomposition of OT A. Research on the potential for OTA degradation in foods and feeds is necessary, as is characterizing the chemical components involved in this process. The effects of OTA at greater concentrations on A. calcoaceticus require further investigation.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods. 2020;9(2):137.
Crossref - Eskola M, Kos G, Elliott CT, Hajslova J, Mayar S, Krska R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit Rev Food Sci Nutr. 2020;60(16):2773-2789.
Crossref - Cuciureanu M, Tuchilus C, Vartolomei A, Tamba BI, Filip L. An Immunoenzymatic Method for the Determination of Ochratoxin A in Biological Liquids (Colostrum and Cow’s Milk). Toxins. 2021;13(10):673.
Crossref - Hashemi SMB, Roohi R, Abedi E, Sayadi M. Kinetics and mathematics modeling of ochratoxin a detoxification in maize dough by Lacticaseibacillus casei subs. casei subjected to continuous and pulsed ultrasound. J Food Process Preserv. 2021;45(4):e15336.
Crossref - Abrunhosa L, Paterson RRM, Venancio A. Biodegradation of ochratoxin a for food and feed decontamination. Toxins. 2010;2(5):1078-1099.
Crossref - Reddy L, Bhoola K. Ochratoxins-food contaminants: Impact on human health. Toxins. 2010;2(4):771-779.
Crossref - Gu K, Ryu D, Lee HJ. Ochratoxin A and its reaction products affected by sugars during heat processing. Food Chem. 2021;348:129038.
Crossref - Adebo OA, Kayitesi E, Njobeh PB. Reduction of Mycotoxins during Fermentation of Whole Grain Sorghum to Whole Grain Ting (a Southern African Food). Toxins. 2019;11(3):180.
Crossref - Liuzzi VC, Fanelli F, Tristezza M, et al. Transcriptional Analysis of Acinetobacter sp . neg1 Capable of Degrading Ochratoxin A. Front Microbiol. 2017;7:1-9.
Crossref - Wang L, Hua X, Shi J, et al. Ochratoxin A: Occurrence and recent advances in detoxification. Toxicon. 2022;210:11-18.
Crossref - El-Khoury AE, Atoui A. Ochratoxin a: General overview and actual molecular status. Toxins. 2010;2(4):461-493.
Crossref - Ferenczi S, Cserhati M, Krifaton C, et al. A new ochratoxin a biodegradation strategy using cupriavidus basilensis Or16 strain. PLoS One. 2014;9(10):109817.
Crossref - Lee HJ, Kim HD, Ryu D. Protective Effect of alpha-Tocopherol Against Ochratoxin A in Kidney Cell Line HK-2. J Food Prot. 2023;86(5):100082.
Crossref - Kosicki R, Buharowska-Donten J, Twaruzek M. Ochratoxin A levels in serum of Polish dialysis patients with chronic renal failure. Toxicon. 2021;200:183-188.
Crossref - Yang Q, Dhanasekaran S, Ngea GLN, Tian S, Li B, Zhang H. Unveiling ochratoxin a controlling and biodetoxification molecular mechanisms: Opportunities to secure foodstuffs from OTA contamination. Food Chem Toxicol. 2022;169:113437.
Crossref - Pfliegler WP, Pocsi I, Gyori Z, Pusztahelyi T. The Aspergilli and Their Mycotoxins: Metabolic Interactions With Plants and the Soil Biota . Front Microbiol. 2020;10.
Crossref - Sheikh-Zeinoddin M, Khalesi M. Biological detoxification of ochratoxin A in plants and plant products. Toxin Rev. 2019;38(3):187-199.
Crossref - Campos-Avelar I, Colas de la Noue A, Durand N, et al. Minimizing Ochratoxin A Contamination through the Use of Actinobacteria and Their Active Molecules. Toxins. 2020; 12(5):296.
Crossref - Kowalska-Krochmal B, Dudek-Wicher R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens. 2021;10(2):165.
Crossref - Adegoke AA, Singh G, Stenstrom TA. Biosensors for monitoring pharmaceutical nanocontaminants and drug resistant bacteria in surface water, subsurface water and wastewater effluent for reuse. Nanoparticles in Pharmacotherapy. 2019:525-559.
Crossref - Slizewska K, Cukrowska B, Smulikowska S, Cielecka-Kuszyk J. The Effect of Probiotic Supplementation on Performance and the Histopathological Changes in Liver and Kidneys in Broiler Chickens Fed Diets with Aflatoxin B₁. Toxins. 2019;11(2):112.
Crossref - Cinar A, Onbasi E. Mycotoxins: The Hidden Danger in Foods. In: Sabuncuoglu S, ed. Rijeka: Intech Open. 2019.
Crossref - Al-Nussairawi M, Kriszt B, Krifaton C, Cserhati M. Transcriptome analysis of an ochratoxin-A biodegrading bacteria. COLUMELLA – Journal of Agricultural and Environmental Sciences. 2020;7(2):33-42.
Crossref - dos Santos HRM, Argolo CS, Argolo-Filho RC, Loguercio LL. A 16S rDNA PCR-based theoretical to actual delta approach on culturable mock communities revealed severe losses of diversity information. BMC Microbiol. 2019;19(1):74.
Crossref - Kharayat BS, Singh Y. Chapter 13 – Mycotoxins in Foods: Mycotoxicoses, Detection, and Management. In: Holban AM, Grumezescu AMBTMC and FD, eds. Handbook of Food Bioengineering. Academic Press. 2018:395-421.
Crossref - Gallo A, Ghilardelli F, Atzori AS, et al. Co-Occurrence of Regulated and Emerging Mycotoxins in Corn Silage: Relationships with Fermentation Quality and Bacterial Communities. Toxins. 2021;13(3).
Crossref - Zhao G, Wang YF, Chen J, Yao Y. Predominant Mycotoxins, Pathogenesis, Control Measures, and Detection Methods in Fermented Pastes. Toxins. 2020;12(2):78.
Crossref - Atungulu G, Mohammadi-Shad Z. Reference on Mycotoxins Occurrence, Prevalence, and Risk Assessment in Food Systems. 2019:294-343.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.