ISSN: 0973-7510
E-ISSN: 2581-690X
Toxoplasma gondii is a protozoan parasite that is widely distributed in the human population and is responsible for corresponding global morbidity. Specifically, T. gondii causes toxoplasmosis, leading to miscarriage, stillbirth, and neural disorders. This parasite attacks different human organs and glands, such as the thyroid gland, and causes various corresponding health issues. Recently, studies have established a link between T. gondii and autoimmune thyroid diseases (AITD), which contributes to preterm delivery, miscarriage, low birth weight, and death. Therefore, the aim of this study was to detect the prevalence of toxoplasmosis and its association with AITD among pregnant women. A total of 180 blood samples were collected from pregnant women and examined using an enzyme-linked immunosorbent assay (ELISA). The patients were within the age range of 15–50 years old, and lived in Duhok City, Iraq; samples and clinical information was collected from August 2021 to February 2022. The corresponding blood samples were tested for anti-Toxoplasma IgG antibody, Toxoplasma IgG avidity, FT3, FT4, and TSH hormones, and TPO, Tg, and TSHR antibodies. Overall, our results showed that out of 180 pregnant women, 110 (61.1%) were seropositive for anti-Toxoplasma IgG antibody; specifically, 25 (22.7%) and 85 (77.3%) had recent and past infections, respectively. Approximately 54.4% (98) of the pregnant women had thyroid disorders; further, 22 (12.2%), 13 (7.2%), and 8 (4.4%) women had TPO, Tg, and TSHR antibodies, respectively. A total of 43 (23.8%) patients screened positive for AITD. Out of the 110 Toxoplasma IgG–positive women, 35 (31.8%) had AITD. The older women, rural residents, restaurant food consumers, and women with cat contact had relatively high infection rates. Toxoplasma seropositive women had more elevated autoantibodies than seronegative ones. In conclusion, this study demonstrated a high rate of toxoplasmosis and a corresponding association with thyroid hormones changes and AITD in pregnant women in Duhok, Iraq. Further, it is necessary to reduce overall infection rates through effective health and educational programs. Therefore, it is essential to measure Toxoplasma antibodies, screen for thyroid hormones and autoantibodies, and encourage gynecologist visits to reduce the risks to mothers and fetuses.
AITD, ELISA, IgG Avidity, Pregnancy, Thyroid Hormones, Toxoplasmosis
Toxoplasma gondii is an obligate coccidian protozoan parasite that can infect all warm blooded animals and humans, ultimately causing toxoplasmosis.1 The final hosts of T. gondii are wild and domestic cats, while all other animals and humans act as intermediate hosts.2 T. gondii has infected approximately one third of the world’s population; therefore, effective diagnosis, prevention, and management of toxoplasmosis is required.3 Specifically, T. gondii infection is acquired either by ingesting mature oocysts in food, water, and soil, which has been contaminated with cat feces, or consuming improperly cooked meat that contains tissue cysts.4 This parasite can transmit during organ transplantation, blood transfusion, or by reactivation of latent stages.5 Additionally, infections during pregnancy can cause congenital toxoplasmosis through vertical transmission from the infected mother to the fetus, ultimately resulting in miscarriage, stillbirth, hydrocephalus, microcephaly, and neurological disorders.6 Currently, several methods have been developed to diagnose toxoplasmosis such as the direct detection of T. gondii, immunological, and molecular techniques.7 The direct diagnosis of toxoplasmosis involves microscopic examination of tachyzoites or tissue cysts and strain isolation.8 In clinical approaches, the most widely used method is enzyme-linked immunosorbent assay (ELISA) for the detection of IgM and IgG antibodies.9 In recent decades, several molecular techniques have been developed, such as PCR, nested PCR, and real-time PCR; however, these techniques are in limited use because they require expensive equipment, complex procedures, and highly qualified technicians.10 T. gondii can attack many organs and glands, including the thyroid gland, which releases the thyroid hormones triiodothyronine (T3), tetraiodothyronine (T4), and calcitonin, which are essential for regulating heart, brain, and bone functions alongside metabolism.11
Little is known about T. gondii infections in thyroid gland. Nonetheless, some recent studies have illustrated that T. gondii is responsible for thyroid hormone alterations, which lead to resultant changes in thyroid morphology and function; initial infection with T. gondii, alters T3 andT4 secretion, resulting in TSH disturbance via a dramatic increases thyroid peroxidase (TPO) levels.12 In the late 1990s, Stahl and Kaneda reported that mice infected with T. gondii showed a decline in serum thyroxine. After determining that the thyrocytes were unimpaired, they concluded that the primary effect of T. gondii infection was a disturbance in the hypothalamic regulation of thyrotropin-releasing hormone; hence, the pituitary–thyroid (T4) feedback loop is secondarily affected.13
Thyroid gland abnormalities are classified as hypothyroidism and hyperthyroidism with different signs and symptoms. Hypothyroidism is a disorder in which the thyroid gland does not release enough thyroid hormones, whereas hyperthyroidism is a thyroid disorder in which the thyroid produces excessive thyroid hormones.14 Consequently, hypothyroidism leads to weight gain, cold sensitivity, hair loss, and slow heart rates, whereas hyperthyroidism causes sweating, anxiety, fatigue, and weight loss.15
Autoimmune thyroid disease(AITD) occurs following a dysregulation in immune tolerance in which the body’s immune system attacks the thyroid gland and hormones, causing damage and disruption to this hormone system. Specific auto antibodies are formed in AITD which targeting thyroid antigens; these antibodies are, consequently, observed in hypothyroidism and hyperthyroidism conditions.16 An example of AITD is Hashimoto’s thyroiditis, which is a common cause of hypothyroidism and is primarily caused by T cell–mediated autoimmune responses. Alternatively, Graves’ disease is an AITD that causes hyperthyroidism through humoral autoimmunity responses. Specifically during pregnancy, AITD can cause premature delivery, abnormal neural development, and miscarriage.17 The prevalence of AITD is increased by, and is associated with, various genetic and environmental factors, such as pathogens, substances, certain cytokines, sex, and other unknown factors that may contribute to its development.18 Little is known about AITD; nonetheless, some recent studies have demonstrated a specific link between T. gondii and AITD with elevated autoantibody levels. Additionally, the molecular similarities between thyroid auto antigens and T. gondii pathogen components, other autoimmune diseases, and family history are all considered to be risk factors for AITD.19
Prior studies have been conducted in Iraq to determine the prevalence of T. gondii infections. Corresponding studies in Duhok City, Iraq conducted by Atroshi and Mero20 and Ramadhan and Sarkees21 indicated Toxoplasma seropositivity rates as 27.7% and 44.4%, respectively. Additionally, Salih et al.22 performed another study in Duhokas sessing the seropositivity for Toxoplasma antibodies (36.3%). Additionally, another study23 demonstrated that the seroprevalence of T. gondii infection in Kirkuk Province, Iraq, was 36.17%. Moreover, Al-Khamesietal.24 revealed that the rates of chronic and acute toxoplasmosis among pregnant women in Baghdad City, Iraq were 68.75% and 31.25%, respectively. Therefore, the aim of this study was to detect the prevalence of toxoplasmosis and its association with AITD among pregnant women. The current study is the first to address the link between T. gondii, thyroid hormones, and AITD in pregnant women in Duhok City, Iraq. Through this study, we aim to advise pregnant women to be aware about the various health issues surrounding toxoplasmosis, thyroid disorders, and autoimmunity in order to reduce the risks and promote early management of these diseases during pregnancy.
This cross-sectional study included the assessment of 180 pregnant women. These patients attended obstetrics and gynecology hospitals in Duhok City, Iraq, from August 1, 2021 until February 28, 2022; the corresponding age range was 15–50 years old. The clinical information was collected from each woman using a special informative questionnaire, including name, age, residency, educational level, number of births, pregnancy period, food habits, and contact with cats. A total of 5mL of venous blood was obtained by vein puncture using a sterile disposable syringe, placed in a plane tube without anticoagulant, labeled, left for 20 min at room temperature to clot, and centrifuged at 3000 rpm for 10 min to obtain serum. All separated serum samples were poured into sterile 2 mL Eppendorf tubes; each tube was labeled, named, and stored at -20°C until use.25
Ethics of Study
Scientific and ethical approval for the study was granted by the Scientific Committee of the College of Medicine/Duhok University, and ethical approval was obtained from the Research Ethics Committee of the General Health Directorate, Duhok, Iraq. No. 13072021-7-3.
Inclusion Criteria
All pregnant women with different periods of pregnancy were included in this study.
Exclusion Criteria
Women who were excluded from this study included those with unknown pregnancy periods, those with other infectious diseases, and those with immunosuppressive or chronic diseases.
Study Design
In this cross-sectional study, all pregnant women were examined using ELISA and tested for anti-T. gondii IgG antibody (Bioactiva Diagnostics, Germany) and Toxoplasma IgG avidity (Novalisa, Germany). Determination of thyroid hormones levels of free triiodothyronine (FT3; pg/mL), free thyroxine (FT4; ng/dL) and thyroid-stimulating hormone (TSH; µIU/mL) were measured using an AccuBind ELISA kit (Monobind, USA). Detection of TPO (IU/mL), thyroglobulin antibodies (Tg; IU/mL) and thyroid stimulating hormone receptor (TSHR-U/L)was conducted using the Aeskulisa ELISA technique (Germany), according to manufacturer’s instructions. The optical density was measured at 450 nm with an ELISA plate reader (BioTek, USA).
Statistical Analysis
All data were statistically analyzed using the statistical program R Studio and a chi-square test. Descriptive statistics were used to describe the data using the means, standard deviation, range for numerical variables, and frequency (n)with percentage (%) for categorical variables. The data were represented using tables, pie charts, and histograms. A P-value <0.05 was considered statistically significant.
Detection of Anti-Toxoplasma IgG Antibodies by ELISA
The calibrators and serum samples were incubated in microplate wells coated with purified and inactivated T. gondii antigen. After incubating and washing the samples, the wells were treated with a conjugate composed of anti-human IgG antibodies labeled with horseradish peroxidase (HRP). After the second incubation and washing step, the wells were then incubated with 3,32,5,52 -tetramethylbenzidine (TMB). Finally, an acidic stop solution was added and the absorbance was read at 450nm using an ELISA microplate reader.
Detection of Toxoplasma IgG Avidity by ELISA
Microtiter plates were coated with specific antigens to bind to the corresponding antibodies in the sample; these samples were then incubated and washed to remove all unbound sample material. Next, a HRP-labeled conjugate was added to the wells to bind to the captured antibodies. Then, a second washing step was conducted to remove all unbound conjugates. The immune complex formed by the bound conjugate was visualized by adding TMB. Sulfuric acid was added to stop the reaction and the absorbance was measured at 450nm using an ELISA microplate reader.
Detection of FT3, FT4, and TSH Hormones by ELISA
Microplates were coated with hormone antibodies, and the enzyme reagent solution was added to each well. The wells were incubated and washed with washing buffer to remove all unbound sample material. Then, a working substrate solution was added to the wells; after a second incubation, stop solution was added, and the absorbance was read at 450 nm using an ELISA reader.
Detection of TPO and Tg Antibodies by ELISA
Serum samples diluted 1:101 were incubated in the microplates coated with the specific antigen. The patient´s corresponding antibodies bound to the antigen and the unbound fraction was removed in the following wash step. Then, the samples were incubated with anti-human immunoglobulins conjugated to HRP (conjugate); this conjugated antibody reacted with the antigen–antibody complex of the samples in the wells. All unbound conjugate was washed off in the following step. Addition of TMB followed by stop solution was conducted before measuring absorbance at 450nm using an ELISA microplate reader.
Detection of TSHR Antibodies by ELISA
TSHR auto antibodies (TRAb) in the serum samples, calibrators, and controls were incubated with TSH receptors coated onto ELISA plate wells for two hours. Then, the samples were discarded, leaving the TRAb, which were bound to the immobilized receptor. A human monoclonal autoantibody against TSHR that was labeled with biotin (M22-biotin) was added in a second incubation step; this antibody specifically interacted with the immobilized TSH receptors that had not been blocked by the bound TRAb. The amount of M22-biotin bound to the plate was then determined in a third incubation step by the addition of streptavidin peroxidase, which bound specifically to biotin. Excess unbound streptavidin peroxidase was discarded and TMB was added. Finally, the stop solution was added, and the absorbance was read at 450 nm using an ELISA plate reader.
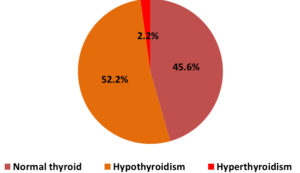
Figure 1 shows the frequency of normal thyroid and thyroid disorders among pregnant women. Out of 180 pregnant women, 82(45.6%)had normal thyroid, while 94(52.2%) and 4(2.2%) had hypothyroidism and hyperthyroidism, respectively.
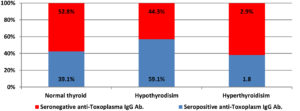
Figure 2. Distribution of normal thyroid and thyroid disorders among Toxoplasma IgG positive and negative women
Figure 2 shows the rates of normal thyroid and thyroid dysfunction among women with seropositive and seronegative anti-Toxoplasma IgG antibody. Out of 110 women with seropositive anti-Toxoplasma IgG antibody,43(39.1%)had normal thyroid, 65(59.1%) and 2(1.8%)had hypothyroidisim and hyperthyroidisim respectively. From 70 women seronegative anti-Toxoplasma IgG antibody, 37(52.8%) had normal thyroid, 31 (44.3%) and 2(2.9%)had hyothyroidism and hyperthyroidism respectively. Seropositive Toxoplasma IgG antibody associated with TSH level increase. An association was obtained between toxoplasmosis and thyroid status p-value 0.04.
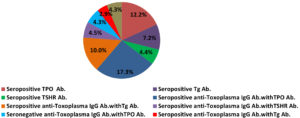
Figure 3 illustrates the percentages of TPO, Tg and TSHR antibodies among women. Out of 180 women, about 43 (23.8%)had autoantibodies, 22(12.2%), 13(7.2%) and 8(4.4%) had seropositive TPO, Tg and TSHR antibodies, respectively. From 110 women seropositive for Toxoplasma IgG antibody, 35(31.8%) had autoantibody, (17.3%), 11 (10.0%) and 5(4.5%) seropositive TPO, Tg and TSHR antibodies respectively. While, from 70 Toxoplasma negative women, 8(11.4%) had autoantibodies classified as 3(4.3%), 2(2.9%)and 3(4.3%) had TPO, Tg and TSHR antibodies. Toxoplasma seropositive women had more elevated TPO, Tg and TSHR antibodies than seronegative ones. T.gondii is linked with autoimmune thyroid disease with a statistically significance (p-value 0.001).
Overall,180 pregnant women were included in the current study; their mean age±standard deviation was 28±6.11 years (range:15–50 years). Of these women, 110(61.1%) were diagnosed as positive for anti-Toxoplasma IgG antibody, 25 (22.7%) had a recent infection with a low IgG avidity and 85 (77.3%) had past infection with a high IgG avidity. Approximately 23.9% (43 women) screened positive for AITD. Table 1 shows the distribution of anti-Toxoplasma IgG antibody among pregnant women compared to certain demographic characteristics. The highest toxoplasmosis rates were detected among old women, rural residents, those who were illiterate, those that had multiple births, those that ate food outdoors, and those that had contact with cats. The 2 women in the 45–50-year-old group (100%) were both seropositive for anti-Toxoplasma IgG antibody; in contrast, low rates of infection 5(29.4%) were observed in the 15–20-year-old group, while the infection rate of women living in rural areas was 78.0% (32 women). High infection rates were detected in women who were illiterate (17 women; 85.0%) compared to the lowest rate of infection, which was observed in those in the higher education group (23 women; 46.0%). Women with multiple children showed higher rates of infection than those with few children: 18(81.8%) and 42(47.7%), respectively. With regarding to eating habits, 16(80.0%) of women who ate al fresco were Toxoplasma IgGAb positive. Finally, the rate of seropositivity for anti-Toxoplasma IgG antibodies among women with cat contact was 80.6% (25 women).
Table (1):
Distribution of toxoplasmosis based on some demographic characteristics in pregnant women.
Variables |
n.(%) |
Seropositive Toxoplasma IgG Abs. n. (%) |
Seronegative Toxoplasma IgG Abs. n.(%) |
P-value |
|
|---|---|---|---|---|---|
Age |
15-20 |
17(9.4 ) |
5(29.4) |
12(70.6) |
*0.02 |
21-26 |
55( 30.6) |
32(58.2) |
23(41.8) |
||
27-32 |
65(36.1 ) |
40(61.5) |
25(38.5) |
||
33-38 |
34(18.9 ) |
25(73.5) |
9(26.5) |
||
39-44 |
7( 3.9) |
6(85.7) |
1(14.3) |
||
45-50 |
2(1.1 ) |
2( 100) |
0(0.0) |
||
Residency |
Rural |
41(22.8 ) |
32( 78.0) |
9(22.0) |
*0.01 |
Urban |
139 (77.2) |
78 (56.1) |
61(43.9) |
||
Educational level |
Illiterates |
20(11.1) |
17(85.0) |
3(15.0) |
*0.01 |
Primary level |
22(12.2) |
15(68.2) |
7(31.8) |
||
Secondary level |
88(48.9) |
55(62.5) |
33(37.5) |
||
Higher education |
50(27.8) |
23(46.0) |
27(54.0) |
||
Number of births |
(0-3) |
88(48.9) |
42(47.7) |
46(52.3) |
*0.0007 |
4-7)) |
70(38.9) |
50(71.4) |
20(28.6) |
||
7< |
22(12.2) |
18(81.8) |
4(18.2) |
||
Pregnancy period |
1st trimester |
43( 23.9) |
33( 76.7) |
10(23.3) |
0.05 |
2nd trimester |
49( 27.2) |
27(55.1 ) |
22(44.9) |
||
3rd trimester |
88( 48.9) |
50( 56.8) |
38(43.2) |
||
Type of eating foods |
Eating indoor |
139(77.2 ) |
79( 56.8) |
60(43.2) |
*0.04 |
Eating outdoor |
20( 11.1) |
16( 80.0) |
4(20.0) |
||
Eating indoor and outdoor |
21( 11.6) |
16(76.2 ) |
5(23.8) |
||
Cat contact |
Yes |
31( 17.2) |
25( 80.6) |
6(19.4) |
*0.01 |
No |
149(82.8 ) |
85( 57.0) |
64(43.0) |
*Statistically significance (p-value <0.05)
Table 2 shows the analysis of FT3, FT4 and TSH hormones among pregnant women. Women with seropositive anti –Toxoplasma IgG antibody had more abnormal FT3, FT4 and TSH levels than IgG negative ones 45.5%,44.5% and 49.1% p-value 0.03.
Table (2):
Frequency of FT3, FT4 and TSH hormones Toxoplasma IgG seropositive and seronegative women.
Hormones |
Cases |
Seropositive Toxoplasma IgG Abs. n. (%) |
Seronegative Toxoplasma IgG Abs. n.(%) |
P-value |
|---|---|---|---|---|
FT3 |
Normal |
60(54.5) |
42(60.0) |
0.51 |
Abnormal |
50(45.5) |
28(40.0) |
||
FT4 |
Normal |
61(55.5) |
42(60.0) |
0.54 |
Abnormal |
49(44.5) |
28(40.0) |
||
TSH |
Normal |
56(50.9) |
47(67.1) |
*0.03 |
Abnormal |
54(49.1) |
23(32.9) |
*Statistically significance (p-value <0.05)
Overall, 180 pregnant women were included in this study. 110(61.1%)women were seropositive for anti-Toxoplasma IgG antibody; specifically, 25(22.7%) and 85(77.3%) had recent and past infections, respectively. This study was in line with study by Eisa et al.26 performed in Sudan, the seroprevalance of Toxoplasma IgG antibody was 61.1%., Eskandarian et al.27 observed similar results to our findings, the serprevalence of Toxoplasma IgG antibody in pregnant women in Iran was 62.7%. Kalantari et al.28 revealed the positivity of Toxoplasma IgG antibody was 60.5%. The sample size, climatic similarity, nutritional habits, socioeconomic status, cat contact may play a role in this prevalence rate similarity.
The prevalence (percent ioeconomic status, and cat contact may have played a role in this prevalence rate similarity between the current study and prior studies. However, our findings disagreed with those in a study conducted by Al-Saeed et al.29 in which they determined a 10% prevalence of Toxoplasma IgG antibodies. Additionally, Muradetal.30 conducted a study in Duhok City; this study indicated that the seropositivity of Toxoplasma antibodies in pregnant women was 21.1%. Our results contrasted with those observed by Alvarado-Esquivel et al.31; in this study, they reported a relatively low Toxoplasma IgG positivity in Mexico(6.1%). The dissimilarities in the demographic characteristics, nutritional habits, socioeconomic status, and public awareness of toxoplasmosis may have led to the corresponding discrepancies observed in these studies compared to the current study. According to the phases of toxoplasmosis, the current study revealed similar findings to those observed by Saki et al.32; in particular, Saki et al.32 reported that the seroprevalence rates of recent and past infections were 22.7% and 77.3%, respectively. Further, Alver et al.33 showed an agreement with the current study by determining that the prevalence of recent toxoplasmosis infections was 27.6%, while the prevalence of past infections with toxoplasmosis was 60.1%.
Cats contaminate the environment with Toxoplasma oocysts; additionally, there are several risk factors for toxoplasmosis, including social, cultural, and socio-demographic statuses.34 In the current study, the maximum seropositivity rate for Toxoplasma IgG antibody was observed among the 45–50 years age group (100%), compared to the minimum Toxoplasma IgG positivity observed among the 15–20 years age group (29.9%). The outcome of this investigation was in accordance with a study conducted by Agorzodo et al.35, which revealed a high Toxoplasma seropositivity in the >45 years age group (67.0%) and a low Toxoplasma positivity in the<18 years age group (25.4%). Our results were comparable to those of Mousavi-Hasanzada et al.,36 which found a higher seroprevalence of T. gondii infection in older age groups than younger age groups (53.9% and 28.7%, respectively). The current study demonstrated that the seropositivity of T. gondii antibody significantly increased with age (P<0. 05). This difference in prevalence with age may be because of a higher probability of contact with oocysts, prolonged exposure to risk factors and transmission routes, and a lack of prevention and control methods for toxoplasmosis. Contrastingly, Babaieetal.37 observed that Toxoplasma IgG seropositivity was higher in the 16–21 years age group (36.2%)than in the 32–47 years age group (35.5%). This discrepancy has been attributed to high income of the older group and the younger group eating junk food that was contaminated with infective stages of T. gondii.
Our study indicated that the seroprevalence of T. gondii IgG antibody in pregnant women who lived in rural areas (78.0%) was significantly higher than those in urban areas (56.1%) (P<0.05). Ramadhan and Sarkees21 conducted a study in Duhok, Iraq and found similar results to the current study; the seroprevalence rates of Toxoplasma IgG antibody in rural and urban residents were 46.7% and 43.8%, respectively. Further, Raissi et al.38 demonstrated that Toxoplasma seropositivity in rural areas was 26.3%, whereas in urban areas it was 16.0%. Therefore, it was postulated that rural areas contain numerous stray cats that live on farms that may contaminate the environment with oocysts that can directly infect humans or can infect livestock that will be later slaughtered for human consumption; additionally, rural women have more frequent contact with soil and farming, low educational levels, and lack awareness of prevention and control strategies for toxoplasmosis. However, Al-Aqeely et al.39 reported dissimilar results compared to our findings; in particular, they determined that the prevalence of toxoplasmosis was higher in pregnant women who lived in urban areas than those in rural areas:21.0% and 15.4%, respectively. Nonetheless, this finding can be attributed to the high income of urban areas and their different eating habits, including an increase in consumption of poultry and junk food from restaurants, which have been found to be a major source of T. gondii transmission.
Within this study, illiterate women were determined to possess a higher Toxoplasma seropositivity (85.0%) than higher educated women (46.0%). Evidence from Agrozodo et al.35 supported our findings; in this study, Toxoplasma positivity rates in illiterate and highly educated groups were determined to be 57.5% and 33.3%, respectively. Therefore, it was suggested that illiterate women were more likely to be older, live in rural areas, have contact with cats, and lack effective information about infection. In contrast, Mizanietal.40 reported a higher Toxoplasma seropositivity in the higher education group (38.3%) than in the illiterate group (32.8%). Overall, it was suggested that women with academic education should have more knowledge related to Toxoplasma biology and its corresponding prevention and control strategies. The lack of effective information about this disease, such as the route of transmission during pregnancy, and poor socioeconomic status can increase the risk of infection.
In our study, multi gravid women had a higher prevalence of T. gondii infection, which increased proportionally with an increasing number of children. The seroprevalence of Toxoplasma IgG antibodies among women with more than seven children was 81.8%, whereas women with few children had an infection rate of 47.7%. This result was in agreement with Mizani et al.40 who indicated a higher prevalence of infection in the multiple births group (50.4%) than in the few-birth group (39.3%). We hypothesized that most women with multiple births are illiterate and live in rural areas. Additionally, in the present study, the seropositivity rate for Toxoplasma IgG antibodies in the first trimester of pregnancy was higher than in the second and third trimesters (76.7%,55.1%, and 56.8%, respectively). These findings aligned with a study conducted in Egypt by Mandour et al.41; in this study, it was reported that the prevalence of infection in the first-trimester group was 68.2%, compared to third-trimester pregnancy group which was 60.2%. This difference in infection may be because most pregnant women in the first trimester of pregnancy live in rural areas and have low educational levels regarding contact with cats. However, a study conducted in Saudi Arabia by Majid et al.42 produced contrasting results to our findings; in this study, the seropositivity rate in the first and third trimesters of pregnancy were 7.1% and 31.2%, respectively.
Data analysis within the current study demonstrated that there was a significant relationship between Toxoplasma seropositivity and food habits (p <0.05). High Toxoplasma seropositivity was observed among the group that ate outdoor compared to the group that ate indoor (80.0% and 56.8%, respectively). Similar results were observed by Raissietal.38: in particular, the Toxoplasma positivity in the groups that ate outdoor or indoor were 35.7% and 20.7%, respectively. Further, the corresponding results in a study conducted by Kolbekova et al.43 supported these findings. Overall, we postulated that women who consume food outdoors may be infected with T. gondii as they lack information regarding toxoplasmosis transmission. Additionally, meat and vegetables are major sources of Iraqi meals; thus, the consumption of under cooked meat and unwashed vegetables leads to the transmission of parasites.
Our results demonstrated that Toxoplasma seropositivity was higher in women who had contact with cats (80.6%) than in those who did not have contact with cats (57.0%). This finding aligned with another study conducted by Al-Atroshi and Mero20 in Duhok, Iraq; Toxoplasma IgG seropositivity among women who had contact with cats (30.8%) contrasted with those who did not have contact with cats (25.7%). Additionally, a study conducted by Babaie et al.37 found similar findings; specifically, the seroprevalence of toxoplasmosis in women who had contact with cats and those who did not have contact with cats was 37.9% and 34.2%, respectively. Overall, most women who had contact with cats were from rural areas with poor socioeconomic status and hygiene. However, the results of the current study contradicted with a study conducted in Ethiopia by Fenta44; this alternative study indicated that a higher seropositivity of Toxoplasma was present among women who did not have contact with cats compared to those that did (82.7%and 80.0%, respectively).
In the current study, we determined that there were more pregnant women with thyroid disorders than healthy thyroid function; this aligned with Valizadah et al.,45 in which T. gondii was associated with changes in thyroid hormones and TSH disturbance. In the current study, the highest rates of thyroid diseases were reported in women with seropositive Toxoplasma antibodies, rather than seronegative ones. This finding was similar to those in studies conducted by Wu et al.15 and Alkhamesi24. In the current study, we reported an association between T. gondii with thyroid disorders and AITD; Alkhamesi et al.24 corroborated our findings that T. gondii is associated with thyroid disorders, showing a significant decrease in T3 and T4 levels and an increase in TSH levels among seropositive Toxoplasma women. During pregnancy, thyroid disorders pose risks to the mother and fetus, which may lead to miscarriage, preterm delivery, and fetal death. Raissi et al.38 reported in consistent results compared to our findings; they observed a seropositive Toxoplasma prevalence of 21.4% among individuals with thyroid disorders and did not find any correlation between T. gondii infection and thyroid dysfunction.
In the current study, out of 110 seropositive Toxoplasma women, 35(31.8%) had AITD, aligning with a study conducted by Kankova et al.46; specifically, Kankova et al.46 reported that 27.1%of 127 AITD women were positive for toxoplasmosis, T. gondii had an effect on thyroid production, and changes in thyroid hormone levels with highly elevated TPO antibodies were found among Toxoplasma IgG positive women compared to Toxoplasma seronegative women. Additionally, Valizada et al.45 tested1248 pregnant women and determined that acute and latent toxoplasmosis (LT)was observed in 3.4% and 29.6% of the women, respectively; the overall frequency of thyroid diseases was 18.8%,whereasapproximately27.9% of patients with LT had thyroid diseases. Further, 13.8% of pregnant women with LT only had AITD; a significant correlation and high elevation of TPO antibodies was also found among the seropositive Toxoplasma IgG antibody group compared to those in the Toxoplasma seronegative group. These findings can likely be attributed to the antigenic similarity of Toxoplasma and TPO leads to cross-reactivity in the immune system, potentially causing AITD. In contrast to Tozzoli et al.47, who determined that 65.5% of seropositive Toxoplasma women had AITD, only 31.8% of 110 Toxoplasma IgG women were positive for AITD in the current study. Nonetheless, Tozzoli et al.47 instead analyzed 120 AITD patients from different areas of Italy, rather than Duhok, Iraq. Our study indicated that pregnant women primarily possess forms of Hashimoto’s thyroiditis and few were identified as positive for Graves’ disease. However, differing results were obtained by Shapiraet al.19; these differences included the number of participating women, toxoplasmosis rate, number of patients with AITD, use of tests, and geographical areas. Overall, we demonstrated that T. gondii is associated with thyroid disorders as a result of autoimmunity, with corresponding abnormal levels of thyroid hormones observed in TPO-, Tg-, TSHR-, and T. gondii-positive women. AITD can cause miscarriage, preterm delivery, and low birth weight. Nonetheless, this study had some limitations, including the small number of patients with hypothyroidism and hyperthyroidism. Additionally, there were difficulties in the follow-up of the pregnant women and their fetuses, which would otherwise help further the understanding of the mechanisms by which Toxoplasma influences AITD.
Overall, pregnant women should be encouraged to practice good hygiene through preventive and control methods to reduce the risk of toxoplasmosis. The current study indicated the importance for screening for Toxoplasma antibodies and IgG avidity among pregnant women to distinguish between recent and past infection. Additionally, there is a requirement in the measurement of thyroid hormones and autoantibodies to provide early therapies for pregnant women. This study provides novel information to improve the understanding of AITD pathogenesis. Nonetheless, further studies are required to completely understand the link between toxoplasmosis and AITD, and to focus on the molecular mimicry observed between thyroid components and Toxoplasma antigens.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by Scientific Committee of the College of Medicine/Duhok University, and ethical approval was obtained from the Research Ethics Committee of the General Health Directorate, Duhok, Iraq. No. 13072021-7-3.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Hoz RML, Morris MI. Tissue and blood protozoa including toxoplasmosis, Chagas disease, leishmaniasis, babesia, acanthamoeba, balamuthia, and naegleria in solid organ transplant recipients guidelines from the american society of transplantation infectious diseases community of practice. Clini Transplant. 2019;33(9):e13546.
Crossref - Afonso C, Paixao VB, Klaus A, etal. Toxoplasma-induced changes in host risk behavior are independent of parasite derived AaaH2 tyrosine hydroxylase. Scientific Reports. 2017;7(1):1-4.
Crossref - Peyron F, L’ollivier C, Mandelbrot L, et al. Maternal and congenital toxoplasmosis: diagnosis and treatment recommendations of a French multidisciplinary working group. Pathogens. 2019;8(1):24.
Crossref - DeBerardinis A, Paludi D, Pennisi L, Vergara A. Toxoplasma gondii a food borne pathogen in the swine production chain from a European perspective. Foodborne Pathog Dis. 2017;14(11):637-648.
Crossref - De la Luz Galvan-Ramirez M, Sanchez Orozco L, Gutierrez-Maldonado A, Perez L. Does Toxoplasma gondii infection impact liver transplantation outcomes A systematic review. J Med Microbiol. 2018;67(4):499-506.
Crossref - Shojaee S.,Teimouri A., Keshavarz H.,etal.,The relation of secondary sex ratio and miscarriage history with Toxoplasma gondii infection. BMC Infect Dis. 2018;18(1):1-6.
Crossref - Li R, Ma Y, Li J, et al. Application of Toxoplasma gondii GRA15 peptides in diagnosis and serotyping. Microbial Pathogenesis. 2020;143:104168.
Crossref - Xue Y, Kong Q, Ding H, et al. A novel loop -mediated isothermal amplification-lateral-flow-dipstick (LAMP-LFD) device for rapid detection of Toxoplasma gondii in the blood of stray cats and dogs. Parasite. 2021;28:41.
Crossref - Hegazy MK, Awad SI, Saleh NE, Hegazy MM. Loop mediated isothermal amplification(LAMP) of Toxoplasma DNA from dried blood spots. Exp Parasitol. 2020;211:107869.
Crossref - Rostami A, Karanis P, Fallahi S. Advances in serological, imaging techniques and molecular diagnosis of Toxoplasma gondii infection. Infection. 2018;46(3):303-315.
Crossref - Zhang W, Wang Y, Wei Z, et al. Endocrine Hypertension. In Secondary Hypertension. Springer. 2020:249-347.
Crossref - Wasserman EE, Nelson K, Rose NR, et al. Infection and thyroid autoimmunity: a seroepidemiologic study of TPOaAb. Autoimmunity. 2009;42(5):439-446.
Crossref - Stahl W, Kaneda Y. Impaired thyroid function in murine toxoplasmosis. Parasitology. 1998;117(Pt 3):217-222.
Crossref - Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316.
Crossref - Wu F, Xu Y, Xia M, Ying G, Shou Z. Hookworm anemia in a peritoneal dialysis patient in China. The Korean J Parasitol. 2016;54(3):315-317.
Crossref - McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252-265.
Crossref - De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018;6(7):575-586.
Crossref - Benvenga S, Santarpia L, Trimarchi F, Guarneri F. Human thyroid autoantigens and proteins of Yersinia and Borrelia share amino acid sequence homology that includes binding motifs to HLA-DR molecules and T-cell receptor. Thyroid.2 006;16(3):225-236.
Crossref - Shapira Y, Agmon-Levin N, etal. Prevalence of anti-Toxoplasma antibodies in patients with autoimmune diseases. J Autoimmun. 2012;39(1-2):112-116.
Crossref - Al-Atroshi AA, Mero WM. Seroprevalence of anti –Toxoplasma antibodies among women of child bearing age in Duhok Province. Science Journal of University of Zakho. 2013;1(1):44-49.
- Ramadhan DS, Sarkees AN. Seroprevalence and risk factors of toxoplasmosis among undergraduate female students of university of duhok. Journal of University of Duhok. 2019;22(2):243-250.
Crossref - Salih JM, Mero WM, Eassa SH. Seroprevalence and some demographic factors associated with Toxoplasma gondii infection among female population in Duhok province, Iraq. International Journal of Research in Medical Sciences. 2020;8(3):921-926.
Crossref - Salman Y, Mustafa W. Correlation between Toxoplasma gondii and thyroid function hormone levels in sera of patients attending private clinics and laboratories in Kirkuk City. Int J Curr Res Biosci Plant Biol. 2014;1(4):27-34.
- Al-Khamesi M. Effect of toxoplasmosis on lipid profile and thyroid hormones in aborted women. Al-Nahrain Journal of Science.2 016;19(4):122-126.
Crossref - Murad MA, Eassa SH, Ibrahim SA. Serosurvey of anti-Toxocara antibodies and risk factors in relation to vitamin d levels among pregnant women of Duhok City-Kurdistan Region. Duhok Medical Journal. 2021;15(2):52-62.
Crossref - Eisa I, Tayseer A, Al-Jaili M, et al. The relationship between people with toxoplasmosis and changes in thyroid hormone levels between 2017 and 2018, Khartoum, Sudan. Merit Research Journal of Medicine and Medical Sciences. 2019;7(9):308-315.
- Eskandarian A. Seroepidemiology of toxoplasmosis in admitted pregnant women in maternity ward of Kowsar teaching and cure center in Qazvin-2006. Iran J Med Microbiol. 2009;3(2):73-79.
- Kalantari N, Ghaffari S, Bayani M, et al. Serological study of toxoplasmosis in pregnant women in the city of Babol, northern Iran, 2012-2013. J Ilam Univ of Med Sciences. 2014;22:102-108.
- AL-Saeed AT, Eassa SH, Murad MA. Detection of toxoplasmosis among women with abortion using molecular and serological tests in Duhok city. Duhok Medical Journal. 2016;10(2):56-68.
- Murad MA, Eassa SH, AL-Saeed AT. Serodiagnosis of TORCH infections among aborted women in Duhok city/Kurdistan Region/Iraq. Journal of Duhok University.2019;22(2):185-94.
Crossref - Alvarado-Esquivel C, Ramos-Nevarez A, Guido-Arreola CA, etal., Association between Toxoplasma gondii infection and thyroid dysfunction: a case-control seroprevalence study. BMC Infect Dis. 2019;19(1):1-5.
Crossref - Saki J, Zamanpour M, Najafian M, Mohammadpour N, Foroutan M. Detection of acute and chronic Toxoplasma gondii infection among women with history of abortion in the southwest of Iran. J Parasitol Res. 2021;6:1-6.
Crossref - Alver O, Payaslyoglu A, SaglykI. Serological prevalence of Toxoplasma gondii in faculty of medicine of Bursa uludag university during 2016-2018. Turkiye Parazitolojii Dergisi. 2021;45(4):257-261.
Crossref - Mohammed JM. Seroprevalence and epidemiological correlates of Toxoplasma gondii infections among people with regards to Interleukin-10 Profile in Duhok city, Kurdistan Region/Iraq. PhD. Thesis, College of Medicine, University of Duhok. 2019.
Crossref - Agordzo SK, Badu K, Addo MG, Owusu CK, Mutala AH, Tweneboah A, Abbas DA, Ayisi-Boateng NK. Seroprevalence, risk factors and impact of Toxoplasma gondii infection on haematological parameters in the Ashanti region of Ghana: a cross-sectional study. AAS Open Research. 2019;2. 2020;2:166,1-6.
Crossref - Mousavi-Hasanzadeh M, Sarmadian H, Ghasemikhah R, et al. Evaluation of Toxoplasma gondii infection in western Iran:seroepidemiology and risk factors analysis. Tropical Medicine and Health.2 020;48(1):1-7.
Crossref - Babaie J, Amiri S, Mostafavi E et al. Seroprevalence and risk factors for Toxoplasma gondii infection among pregnant women in Northeast Iran. Clinical and Vaccine Immunology. 2013;20(11):1771-1773.
Crossref - Raissi V, Alizadeh G, Bayat F, et al. Association between T3, T4, and TSH hormones proportion and Toxoplasma gondii anti-IgGseroprevalence in patients suffering from clinical and drug-controlled thyroid dysfunctions in southeastern Iran. Turkish Bulletin of Hygiene & Experimental Biology. 2021;78(3):299-306.
Crossref - Aqeely H, El-Gayar E, Khan P, et al. Seroepidemiology of Toxoplasma gondii amongst pregnant women in Jazan Province, Saudi Arabia. Journal of Tropical Medicine. 2014:1-6
Crossref - Mizani A, Alipour A, Sharif M, et al. Toxoplasmosis seroprevalence in Iranian women and risk factors of the disease: a systematic review and meta-analysis. Tropical Medicine and Health. 2017;45:1-13.
Crossref - Mandour A, Mounib M, Eldeek H, Ahmad A, Abdel-Kader A. Prevalence of congenital toxoplasmosis in pregnant women with complicated pregnancy outcomes in Assiut governorate, Egypt. J Adv Parasitol. 2017;4(1):1-8.
Crossref - Majid A, Khan S, Jan AH, Taib M, Adnan M, Ali I, Khan SN. Chronic toxoplasmosis and possible risk factors associated with pregnant women in Khyber Pakhtunkhwa. Biotechnol Biotechnol Equip. 2016;30(4):733-6.
Crossref - Kolbekova P, Kourbatova E, Novotna M, Kodym P, Flegr J. New and old risk-factors for Toxoplasma gondii infection: prospective cross-sectional study among military personnel in the Czech Republic. Clin Microbiol Infect. 2007;13(10):1012-7.
Crossref - Fenta DA. Seroprevalence of Toxoplasma gondii among pregnant women attending antenatal clinics at Hawassa University comprehensive specialized and Yirgalem General Hospitals, in Southern Ethiopia. Fenta BMC Infect Dis. 2019;19:1056.
Crossref - Valizadeh G, Khamseh M, Kashaniyan M, Rafiei-Sefiddashti R, Hadighi R. Role of Toxoplasma gondii in Thyroiditis of Pregnant Women. Russian Journal of Infection and Immunity.2 022;12(5):947-952.
Crossref - Kankova S, Prochazkova L, Flegr J., Calda P, Springer D, Potlukova E. Effects of latent toxoplasmosis on autoimmune thyroid diseases in pregnancy. PloS one. 2014;9(10):e110878.
Crossref - Tozzoli R, Barzilai O, Ram M, et al. Infections and autoimmune thyroid diseases:Parallel detection of antibodies against pathogens with proteomic technology. Autoimmun Rev. 2008;8(2):112-115.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.