ISSN: 0973-7510
E-ISSN: 2581-690X
Nosocomial infections are proving to be a menace for the whole health care system, with methicillin resistant Staphylococcus aureus (MRSA) being a very notorious causative agent. Along with the role of mecA gene producing penicillin-binding protein (PBP2a), production of beta-lactamase enzyme by Staphylococcus aureus makes the organism resistant to all β-lactam agents. This study aims at phenotypic detection of methicillin resistance and β-Lactamase production in all S. aureus isolates by Cefoxitin disk diffusion test and Penicillin zone-edge test, respectively. In this prospective cross-sectional study, samples were obtained from patients admitted to different departments and were processed according to the standard laboratory protocols. As per the CLSI guidelines, phenotypic detection of resistance to methicillin was done by Cefoxitin Disk Diffusion test, whereas production of beta-lactamase enzyme was phenotypically detected by penicillin zone edge test. Among 179 isolates of S. aureus, 116 (64.8%) were MRSA, whereas the remaining 63 (35.2%) isolates were methicillin-sensitive Staphylococcus aureus (MSSA). Staphylococcus aureus infection among ICU and non-ICU patients were found to be 24(13%) and 155(86.6%) respectively. Among 24 ICU patients, 19 had MRSA infection, whereas among 155 non-ICU patients, 97 had MRSA infection. Out of 63 MSSA isolates, only 2 (3.17%) were found to be sensitive to Penicillin by Zone-edge test, 04 isolates showed presence of β-lactamase production, while remaining 57 isolates were resistant to Penicillin. At the same time, several class of antibiotics were found to be ineffective against these MRSA isolates. Cases of methicillin-resistant and b–lactamase producing Staphylococcus aureus infections are on the rise in our hospital settings.
Methicillin-resistant Staphylococcus aureus, Beta Lactamases, Multidrug Resistance
Methicillin resistant Staphylococcus aureus infections are the major contributors among different nosocomial infection worldwide, including India.1,2 Penicillin-resistance among Staphylococcus aureus (S. aureus) strains have been known since 1942, which further paved the way for semisynthetic penicillins, including methicillin,3 which is used in the treatment of infections caused due to penicillin resistant S. aureus strains. With the increasing number of infections due to S. aureus, the prevalence of Methicillin resistant Staphylococcus aureus (MRSA) infections has also increased in India.4 Since 1980, about 33% of the world’s population exhibiting increased incidence of MRSA infection in hospital settings belong to the Asia pacific region.5 It has been reported from different parts of Indian subcontinent at a rate of about 25% to 50%.6
MRSA has been linked to the causation of several hospital acquired infections which are rapidly progressive and fatal such as pneumonia, endocarditis, necrotizing fasciitis, osteomyelitis, toxic shock syndrome and severe sepsis, whereas, various risk factors that are commonly associated with MRSA infection are prolonged hospitalization, intensive care admission, invasive procedures, hemodialysis, recent antibiotic use, HIV infection and discharge with long-term indwelling urinary catheter or long term central venous access. Earlier, MRSA was known to cause infection among hospitalized patients, commonly known as hospital-associated MRSA (HA-MRSA) infections. But recently, these infections are engulfing a large part of population which have never come in contact with the hospitals, health care workers or hospitalized patients, known as community-associated MRSA (CA-MRSA) infections.7 CA-MRSA is generally known for causing skin and soft tissue infections like abscesses, furunculosis and cellulitis but it also causes severe morbidities like bacteremia, septic shock, necrotizing pneumonia and osteomyelitis.7,8
The Staphylococcal cassette chromosome mec (SCCmec) is a genomic island which when acquired, causes methicillin resistance. It expresses the mecA gene which helps in producing the penicillin-binding protein (PBP2a), making the organism resistant to all β-lactam antibiotics.9 Recently, several other related genes like mecB, mecC, and mecD have also been discovered.10 The mecA mediated resistance can be detected in laboratory by using Oxacillin (broth microdilution or agar dilution technique) or Cefoxitin (broth microdilution or disk diffusion technique).11 Cefoxitin (30µg) may be used as a surrogate marker for Oxacillin in detecting MRSA, if oxacillin MIC testing is not done and can prove to be a very effective method of detection of MRSA and MSSA (Methicillin sensitive Staphylococcus aureus) isolates in resource limited settings.
There is also a second method which makes S. aureus resistant to all beta-lactams, which is by production of beta-lactamase enzyme by the organism, that hydrolytically destroy beta-lactams.12 The gene responsible for encoding β-lactamase is blaZ, which develops such resistance.13 Staphylococci, which are β-lactamase-positive, are resistant to penicillin, aminopenicillin, carboxpenicillin and ureidopenicillins. In the laboratory, penicillin disk diffusion zone-edge test and nitrocefin-based tests can be used for detecting β-lactamase production in S. aureus, phenotypically. However, penicillin zone-edge test has been found to be more sensitive and is recommended, if only one test is to be used for β-lactamase detection.11 As per the Clinical and Laboratory Standards Institute (CLSI), penicillin zone edge test14 has been recommended for screening S. aureus isolates for β-Lactamase production.11
This study aims at phenotypic detection of Methicillin resistance and β-Lactamase production in all S. aureus isolates by Cefoxitin disk diffusion test and Penicillin zone-edge test, respectively. S. aureus isolates were obtained from various clinical samples like pus, urine, blood, conjunctival swab, ET aspirate and synovial fluid.
A prospective cross-sectional study of one year duration from March 2020 to February 2021, was carried out in the Bacteriology laboratory, for the detection of Methicillin Resistance and β-Lactamase production in Staphylococcus aureus. Written and informed consent was taken from patients.
Inclusion and Exclusion Criteria
All patients having a surgical site infection, skin ulcers or burn, skin abscess, diabetic foot ulcers and infected traumatic wound were included in the study. All patients who did not give their consent were excluded from the study.
Sample Collection and Processing
Specimens were collected from various clinical departments. The specimens included in this study were swab/aspirate from soft tissue infection and abscess (pus), urine, blood, conjunctival swab, Endo-tracheal aspirate and synovial fluid. General culture media like Nutrient Agar and Blood Agar, whereas selective media like Mannitol salt agar (MSA) were used for inoculation purpose, which were subsequently incubated for 24 hours at 37°C, aerobically.
Detection of S. aureus was done following conventional methods of identification, like catalase test, tube coagulase test and mannitol salt agar. Furthermore, routine antimicrobial susceptibility test was carried out by Kirby Bauer disk diffusion method. Cefoxitin Disk Diffusion test was used for detection of methicillin resistance. Routine disk diffusion procedure was used to perform the Cefoxitin disk (30µg) test on S. aureus isolates, and isolates showing zone of inhibition of ≥ 22 mm were interpreted as MSSA or negative for mecA mediated resistance, whereas those showing zone of inhibition of ≤ 21 mm were interpreted as MRSA or positive for mecA mediated resistance. CLSI defines oxacillin resistance in S. aureus as an MIC ≥ 4µg and all strains meeting this definition are characterized as “MRSA” even though methicillin is no longer used either to test strains in the lab or as a therapeutic agent.11
Penicillin Zone-edge Test
For standard disk diffusion procedure, few bacterial colonies were used for making a normal saline suspension and the turbidity was matched up to 0.5 McFarland standard. It was further inoculated on Mueller–Hinton agar and 10-U penicillin G discs were applied, as per the CLSI guidelines. The plates were incubated at 35°C ± 2°C, in ambient air, for 16 – 18 hours. Zone diameters were then measured and interpretation of susceptibility was based on CLSI (zone diameter of ≥ 29 mm) recommendations. All zone edges were assessed for sharp or fuzzy appearance. A sharp zone edge (“cliff”) corresponds to the presence of b-lactamase, whereas a fuzzy zone edge (“beach”) indicates the absence of β -lactamase.11
Statistical Analysis
Data were entered into MS Excel spreadsheet and SPSS version 20 (IBM Corp) was used for data analysis purpose. Descriptive statistics were presented in the form of means, standard deviation was calculated for continuous variables and frequencies for categorical variables. Data were graphically presented using columns, graphs and pie charts. A chi-square evaluation was used for group comparison for categorical data. P value < 0.05 was considered as statistically significant.
Ethical Consideration
Mandatory ethical clearance was obtained for this study from the Institutional Ethical Committee (memo no.1602/IEC/IGIMS/2020)
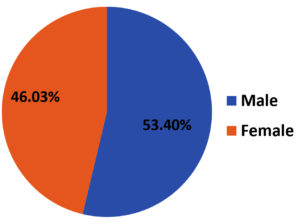
From March 2020 to February 2021, among 179 isolates of S. aureus, 116 (64.8%) were MRSA and 63 (35.2%) were MSSA. Among 116 MRSA isolates, 62 (53.4%) belonged to male patients [Figure 1]. The mean age of the patient was 35.08 with a standard deviation of 18.6.
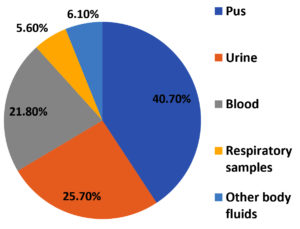
Staphylococcus aureus was predominantly isolated from pus (73) 40.7% samples from skin and soft tissue infections, followed by urine (46) 25.7%, blood samples (39) 21.8% and respiratory samples (10) 5.6% including sputum, bronchoalveolar lavage, endotracheal aspirate, pleural fluid and throat swab [Figure 2]. Rest of the samples were corneal swab, ascitic fluid, conjunctival and corneal swab and CSF.
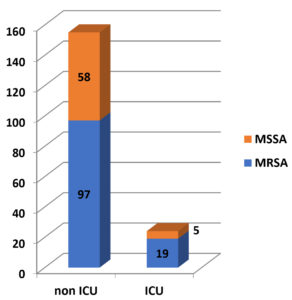
Staphylococcus aureus infection was present in 24 (13%) ICU patients, whereas it was present in 155 (86.6%) non-ICU patients. Among 24 ICU patients, 19 (79.16%) had MRSA infection, whereas among 155 non-ICU patients, 97 (62.58%) had MRSA infection. [Figure 3].
Pearson Chi-square test was carried out to find any association of more incidence of MRSA infection among ICU patients. Value of Pearson’s Chi-square was 2.507, degree of freedom was 1 and p value was 0.113. With a p value of p > 0.05, it is evident that there is no association between the incidence of MRSA infection among ICU patients.
As expected, all 116 MRSA isolates in our study were found to be Penicillin resistant (< 29 mm). Out of 63 MSSA isolates, only 2 (3.17%) were found to be sensitive to Penicillin by Zone-edge test having zone >29 mm with fuzzy edge at the zone indicating absence of β-lactamase production. Four of the MSSA isolates showed zone >29 mm with sharp edge at the zone indicating presence of β-lactamase production. Remaining 57 isolates had zone diameter < 29 mm [Table 1].
Table (1):
Zone edge test for detection of b-lactamase production in MSSA (n=63).
Pattern at edge |
Number (%) |
|---|---|
Fuzzy edge with > 29 mm Zone diameter |
2 (3.17 %) |
Sharp edge with > 29 mm Zone diameter |
4 (6.34 %) |
< 29 mm Zone diameter |
57 (90.47 %) |
In our study, Antibiotic susceptibility testing data for other antibiotics revealed that MRSA isolates were resistant to many other antibiotics like Ciprofloxacin (71.8%), Erythromycin (70.6%), Clindamycin (59.3%), Gentamicin (36.8%) and Cotrimoxazole (34.1%). There was no resistance documented against vancomycin and linezolid [Table 2].
Table (2):
Resistance of MRSA isolates to other Antibiotics by Kirby Bauer Disc Diffusion method (n=116).
Antibiotics tested |
Resistance pattern of MRSA (%) |
|---|---|
Penicillin |
100 |
Erythromycin |
70.6 |
Clindamycin |
59.3 |
Ciprofloxacin |
71.8 |
Gentamicin |
36.8 |
Cotrimoxazole |
34.1 |
Vancomycin |
0 |
Linezolid |
0 |
In this study, analysis of 179 S. aureus isolates from different clinical samples revealed that the overall rate of MRSA infection was as high as 64.8%, which is definitely alarming. Similar high prevalence rates have been registered by Gupta N. et al.,15 and Tiwari et al.16 A similar study from Andhra Pradesh showed a CA-MRSA prevalence of 64.7%.17 Other reports on CA-MRSA in India were by Shenoy et al.,18 from Mangalore, D’Souza et al.19 from Mumbai and Bouchiat et al. from Bangalore.20 A nasal carriage rate of 72.7% for MRSA was reported by Goud et al among healthy individuals in Bangalore, India.21
There are also studies from India showing lesser prevalence of MRSA, ranging from 26.14% to 43%.22,23 An overall 41% prevalence of MRSA was reported by a study undertaken by the Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India, from January 2008 to December 2009, among 15 tertiary care centers.4 Reason behind such variation could be many like different geographical area, varied sample size, methods used for testing and infection control status etc.
Most of the isolates (40.7%) were obtained from Skin and soft tissue infection (SSTI), proving it to be a major cause of S. aureus disease in the community, as well as among hospitalized patients. Similar findings were reported by Tiwari et al. from Varanasi (42%) and Mallick and Basak from Maharashtra (61.4%).24,25 The predominance in pus sample could be due to over-exposure of wound to environmental microorganisms. At the same time, presence of S. aureus as skin commensals makes the wound more prone for infection.
When gender distribution was compared, MRSA was found to be more prevalent among male patients (53.4%). Similar observations were reported by Rao et al.26 There were no significant differences in the clinical presentations between both genders, as regards to the prevalence of MRSA.
Antimicrobial resistance pattern for other antibiotics in this study shows that MRSA strains present maximum resistance against Ciprofloxacin (71.8%), Erythromycin (70.6%), Clindamycin (59.3%), Gentamicin (36.8%) and Cotrimoxazole (34.1%). Similar findings were reported by Raj Kumar et al.27
Almost all MRSA isolates were discovered to be sensitive to Linezolid and Vancomycin in our study, which is at par with the study done by Abbas et al.28
In this study, no association was found between increased incidence of MRSA infections and patients admitted in ICU (p value 0.113). This suggests that MRSA infection is not confined to severely ill patients nowadays and is getting more prevalent among communities.
Among 63 isolates of MSSA, only two isolates (3.17 %) were found to be Penicillin susceptible (absence of b – lactamase) by Penicillin zone edge test. Both the isolates were from pus sample and belonged to male patient. Simultaneously, four isolates (6.34 %) were found to be b-lactamase producers. SS Richter et al29 found around 4.46% b–lactamase producers among 448 MSSA, which is slightly lower than that observed in this study. Also, very large proportion of MSSA isolates (90.47 %) were found to be resistant to Penicillin, which is really worrisome.
High prevalence of b-lactamase production and methicillin-resistance among Staphylococcus aureus is a cause of concern. Along with the prevalence of HA-MRSA infection, there is an increase in the incidence of CA-MRSA infection as well. A patient with MRSA infection is exhibiting increased resistance to many classes of antibiotics, except Vancomycin and Linezolid, a fact which is more challenging for the health care providers and also for the clinical researchers. We recommend effective surveillance of MRSA at hospital and community level to know the exact burden and to follow all standard measures to reduce the spread of MRSA, including strict infection control and hand hygiene practices.
Limitations
Major limitations of this study are small sample size, lack of molecular detection of blaZ gene and smaller study duration. Moreover, the study involves only clinical isolates from a single tertiary care center.
ACKNOWLEDGMENTS
The authors would like to thank all faculty members, residents and technical staff members of Microbiology Department, Indira Gandhi Institute of Medical Sciences, Bihar, India for their contribution and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NS, KS contributed in conceptualization, study design, literature review, data collection, data analysis, interpretation, writing original draft and editing. NK contributed in study design, data analysis, writing review and editing. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India with reference number 1602/IEC/IGIMS/2020.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Dozois A, Thomsen I, Jimenez-Truque N, et al. Prevalence and molecular characteristics of Methicillin-resistant Staphylococcus aureus among skin and soft-tissue infections in an emergency department in Guyana. Emerg Med J. 2015;32(10):800-803.
Crossref - Tong SYC, Kearns AM. Community-associated MRSA from the Indian subcontinent. Lancet Infect Dis. 2013;13(9):734-735.
Crossref - Wu M, Tong X, Liu S, Wang D, Wang L, Fan H. Prevalence of methicillinresistant Staphylococcus aureus in healthy Chinese population: a system review and meta-analysis. PLoS One. 2019;14:e0223599.
Crossref - Joshi S, Ray P, et al. Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence and susceptibility pattern. Ind. J Med. Res. 2013;137(2):363-369.
- Lim WW, Wu P, Bond HS, et al. Determinants of methicillin-resistant Staphylococcus aureus (MRSA) prevalence in the Asia-Pacific region: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2019;16:17-27.
Crossref - Chatterjee A, Rai S, Guddattu V, Mukhopadhyay CSK. Is methicillin-resistant Staphylococcus Aureus infection associated with higher mortality and morbidity in hospitalized patients? A cohort study of 551 patients from South Western India. Risk Manag Healthc Policy. 2018;11:243-250.
Crossref - Otter JA, French GL. Molecular epidemiology of community-associated methicillin-resistant Syaphylococcus aureus in Europe. Lancet Infect Dis. 2010;10(4):227-239.
Crossref - Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated methicillin resistant S. aureus. Lancet. 2010;375(9725):1557-1568.
Crossref - Sit PS, Teh CSJ, Idris N et al. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infection and the molecular characteristics of MRSA bacteraemia over a two-year period in a Tertiary Teaching Hospital in Malaysia. BMC Infect Dis. 2017;17(1):274.
Crossref - Lakhundi S, Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4):e00020-18.
Crossref - CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2021.
- Fuda CC, Fisher JF, Mobashery S. Beta-lactam resistance in Staphylococcus aureus: the adaptive resistance of a plastic genome. Cell Mol Life Sci. 2005;62(22):2617-2633.
Crossref - Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265-1273.
Crossref - Gill VJ, Manning CB, Ingalls CM. Correlation of penicillin mini-mum inhibitory concentrations and penicillin zone edge appearance withstaphylococcal beta-lactamase production. J Clin Microbiol. 1981;14(4):437-440.
Crossref - Gupta N,Yadav M, Sharma H, et al. Prevalence and Sensitivity Pattern of Methicillin Resistant Staphylococcus aureus in a Tertiary Care Hospital in Jaipur. SAS J Med, 2021;7(8):370-373.
- Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006;6(1):1-6.
Crossref - Alvarez-Uria G, Reddy R. Prevalence and Antibiotic Susceptibility of Community-Associated Methicillin-Resistant Staphylococcus aureus in a Rural Area of India: Is MRSA Replacing Methicillin-Susceptible Staphylococcus aureus in the Community? ISRN Dermatol. 2012;212:248951.
Crossref - Bhat GK, Kishore A, Hassan MK, Shenoy MS. Significance of MRSA strains in community associated skin and soft tissue infections. Indian J Med Microbiol. 2010;28(2):152-154.
Crossref - De Lencastre H, Oliveira D, Tomasz A. Antibiotic resistant Staphylococcus aureus: A paradigm of adaptive power. Curr Opin Microbiol. 2007;10(5):428-435.
Crossref - Bouchiat C, El-Zeenni N, Chakrakodi B, Nagaraj S, Arakere G, Etienne J. Epidemiology of Staphylococcus aureus in Bangalore, India: Emergence of the ST217 clone and high rate of resistance to erythromycin and ciprofloxacin in the community. New Microbes New Infect. 2015;7:15-20.
Crossref - Goud R, Gupta S, Neogi U, Agarwal D, Naidu K, Chalannavar R. Commuinty prevalence of methicillin and vancomycin resistant Staphylococcus aureus in and around Bangalore, Southern India. Rev Soc Bras Med Trop. 2011;44(3):309-312.
Crossref - Lohan K, Sangwan J, Mane P, Lathwal S. Prevalence pattern of MRSA from a rural medical college of North India: A cause of concern. J Fam Med Prim Care. 2021;10(2):752-757.
Crossref - Dalela G, Gupta S, Jain DK, Mehta P. Antibiotic resistance pattern in uropathogens at a tertiary care hospital at Jhalawar with special reference to ESBL, AmpC beta lactamase and MRSA production. J Clin Diagn Res. 2012;6(4):645-651.
- Mallick SK, Basak S. MRSA-Too many hurdles to overcome: A study from Central India. Trop Doct. 2010;40(2):108-110.
Crossref - Tiwari HK, Sapkota D, Sen MR. High prevalence of multidrug resistant MRSA in a tertiary care hospital of Northern India. Infect Drug Resist. 2008;1:57-61.
Crossref - Rao BN, Srinivas B. A prospective study of Methicillin resistant Staphylococcus aureus (MRSA) in a teaching hospital of rural setup. J Evol Med Dent Sci. 2012;1:37-40.
- Rajkumar S, Sistla S, Manoharan M, et al. Prevalence and genetic mechanisms of antimicrobial resistance in Staphylococcus species: A multicentre report of the indian council of medical research antimicrobial resistance surveillance network. Indian J Med Microbiol. 2017;35(1):53-60.
Crossref - Abbas A, Nirwan PS, Srivastava P. Prevalence and antibiogram of hospital acquired-methicillin resistant Staphylococcus aureus and community acquired-methicillin resistant Staphylococcus aureus at a tertiary care hospital National Institute of Medical Sci. Community Acquir Infect. 2015;2(1):13-15.
Crossref - Richter SS, Doern GV, Heilmann KP, et al. Detection and prevalence of penicillin-susceptible Staphylococcus aureus in the United States in 2013. J Clin Microbiol. 2016;54(3):812-814.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.