ISSN: 0973-7510
E-ISSN: 2581-690X
To analyzes the association between Mannose binding lectin MBL gene and preterm labor, DNA was obtained from peripheral blood of 150 aborted women patients diagnosed as preterm labor and 150 healthy individual have no previous preterm history was extracted. Mannose binding lectin gene was genotyped by PCR-RFLP (Polymerase Chain Reaction – Restriction Fragment Length Polymorphism) method. The data were analyzed by odd ratio (OR) calculation with confidence intervals (CI 95%) and chi square x 2 results were statically analysis. Statistical significant were sent as p value £0.05. The allelic frequency between gene and preterm labor group was (94.5) in control compared with (70.7) in patients with significant differences. The homozygous were found to be more than heterozygous (32.3) whereas homozygous AA (43.4) and GG (33.3) with significant differences this work is considered the first study for understanding the correlation between Preterm birth and mannose protein level in Iraq.
Mannose binding lectin, polymorphism, preterm labor.
Genotyping of mannose binding lectin was involved three miss-sense variations in exon 1 (R52C, G54D and G57E). The mannose binding lectin (mbl) gene was located on chromosome 10q11.2-q21, each of which was related with decreased levels of circulating mannose binding lectin1, on the other hand two promoter variations known to impact the expression of the it gene. Plasma mannose binding lectin concentration was determined by an incorporation of genotypes was affected by mutations in the structural gene as well as by two dimorphic loci in the promoter region. Ultra-uterine infection and immune system defects causing susceptibility was a major cause of spontaneous preterm birth were suggest the MBL which need to reconsider the importance of this protein in the innate immune system. Mannose binding lectin (MBL) is an important element of the innate defense system. It was one of the recognition molecules of the complement system that binds to mannose N-acetyl glucosamine residues and focused on the cell target and activates the complement system through the lectin pathway . However, mannose binding lectin (MBL) in focus of attention because of its role as a recognition molecule in complement system. It is Ca+ -dependent collagenous lectin, synthesized in liver with main role to mediate innate immune defense against microorganisms2.
Mannose binding lectin Level in humans was determined genetically by a number of polymorphic sites in the promoter region of the mbl gene in the and structural gene. Phenotypic and genetic deficiency in producing of mannose binding lectin may susceptible to unusual, serious or recurrent infections in some groups of patients especially those with immune-compromised functions such as patient with malignancy or neutropenia.
MBL plays an important role in the maintenance of pregnancy and inflammation3. Low level concentration of mannose binding lectin has been proposed to be a risk factor for miscarriage4.
Premature birth which responsible for the majority of the congenital neurologic disabilities and deaths in the infant. The infection often originate from the intestinal tract or urinary. Some of single nucleotide polymorphisms are correlated with healthy complication, development of several disease and mannose binding lectin protein deficiency. The activation of complement by mannose binding lectin contribute to increasing the possibility of an insufficient vascularization at time of duration which lead to preterm birth5.
Samples collection
A total of 150 aborted women were taken their age ranged from 15 to 40 patients diagnosed as preterm labor by the physician (including those with bacterial vaginosis, urinary tract infections and aborted women) who were admitted to Babylon Maternity and pediatric hospital and Al-Hilla Teaching Hospital, and 150 health control subjects were recruited in the study at the period from February to October 2016.
Ethical approval
The necessary ethical approval from ethical committee of the hospitals and patients and their followers must obtain. The project was done and the samples were obtained after getting the agreement from the females (patients and controls).
Blood collection
Three milliliter of freshly venous blood from (patients and controls ) were collected and submitted to DNA extraction.
DNA Extraction from blood sample
The extraction of DNA was carried out according to the extraction kits of genomic DNA supplemented by manufactured company (Genaid).
Detection of MBL by specific primer
The primer and PCR conditions were used to amplify MBL 54 listed in Table 1. Each 25 µl of PCR reaction was contained 2.5 µl of each reverse and forward primers, 12.5 of master mix, 2.5µl of free nuclease water, and 5µl of DNA. The product was visualized by electrophoresis on 2% agarose for 50 min at 70 V. the amplicon size was determined by comparison with the allelic ladder (100bp).
Table (1):
Primer and PCR conditions for MBL 54 genotyping.
| SNP-Coding Gene | |||||
|---|---|---|---|---|---|
| Gene name | Primer sequence | bp | condition | Reference | |
| MBL | F-5’CGGTATAGGCACACAATG GTGAG3′ R-5’GCAATGGTTCAAGCGATTC TTC3′ |
349 | 94°C 1min | 1x | [6] |
| 94°C 1min | 30x | ||||
| 60°C 1min | |||||
| 72°C 2min | |||||
| 72°C 10min | 1x | ||||
Detection of genotyping by RFLP- PCR
The amplicon size 394bp was cleaved by Ban enzyme provided by Bio lab Company as shown in Table (2) in suitable buffer at 37°C for 24 hr. which result in two fragment of 89bp and 260 for GG genotype, three fragments to AG 349, 260,89 bp respectively for heterozygous and single band 349bp for AA mutant type.
Table (2):
Restriction enzyme used in this study.
Restriction enzyme |
Primer |
Cutting sites |
Ref. |
|---|---|---|---|
BanI |
MBL-54 |
5’C CGC3’ 3’GGC G5′ |
[6] |
Statistical Analysis
This study used statistical analysis was done using SPSS version 20. Categorical variables were introduced as frequencies and percentages. the allele frequency of MBL-54 polymorphism were test and analysed by Fishers exact test [(Allele×2) + mixed/ (Total×2)]
The correlation among genotype and risk of preterm labour was evaluate by calculate of odd ratio with 95% CI. A p–value of £ 0.05 was considered as significant.
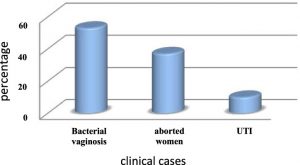
This study was included (150) Patient with different clinical characteristic as shown in figure 1 .
The result of current study was showed that only 15paitents which gave (10%) percentage belong to urinary tract infections7.
Also, the result was showed that only 55 patients who gave (37%) percentage among aborted women8. The high rate of aborted women is belonging to estrogen abnormality during the pregnancy period which converts the Glycogen to lactic acid.
On the other hand, the result was showed that from 150 patients only 80 patients with bacterial vaginosis which gave a results (53%) such as in result obtained by9.
By using PCR and gel electrophoresis the result of MBL -54 G/C was showed that the product was located in 349bp as shown in figure 2 , after that the PCR product was represented to the RFLP technique and the product was digested with BanI enzyme . All genotypes were clearly identified in both Patients and controls.
Fig. 2. Gel electrophoresis for detecting MBL gene amplicon product were separated by gel electrophoresis in an 1.5% agarose, at 70 volt for 45 min. lane: 1,2,3,4,5, 6 healthy control., lane 7,8,9,10,11,12 patients, 1500bp marker (Ladder). The size product is 349 bp
The result was showed that a single band with 349 bp when the enzyme does not cut and gave the homozygous mutant type (AA) but when its work gave the homozygous wild type (GG) , the enzyme cuts the two alleles and the PCR-product will appear as double band of 260bp and 89 bp whereas the heterozygous genotype (AG) will appear as the three bands with 349 bp, 260 bp and 89 bp as shown in figure 3 .
Fig. 3. Agarose gel electrophoresis of 1.5% at 70volt for 45min, show restriction digestion pattern of MBL -54 A/G polymorphisms of gene using BanI enzyme. L: 1500 DNA ladder. Lanes2, 3,6,8,11 homozygous wild type (GG). Lane 1,4,7,10,12: mutant genotype (AA). Lanes5, 9: heterozygous genotype (AG)
Also, table 3 was showed that the mutated homozygous (AA) genotype have frequency (43.3 %) in PLT (preterm labor) and (26.7 %) in control group, whereas the heterozygous (AG) was found to be (23.3%) in PLT and (20%) in control but the wild homozygous (GG) form was found to be (33.3%) in patients and (53.3%) in control.
Table (3):
Genotype distribution of -54 G/A polymorphism in promotor region in MBL gene with preterm labor.
| Study variable | Study Group | χ2 | P-value | OR | 95 % CI | |
|---|---|---|---|---|---|---|
| Patients | Control group | |||||
| MBL | 13.26 | 0.001* | ||||
| AA | 65 (43.4) | 40 (26.7) | 1.393 | 0.744-2.607 | ||
| GA | 35 (23.3) | 30 (20.0) | 0.07 | 1.532-4.413 | ||
| GG | 50 (33.3) | 80 (53.3) | 2.6 | |||
| Total | 150 | 150 (100.0) | ||||
| (100.0) | ||||||
*p value ≤ 0.05 was significant
The result of the current study was showed that most patients were homozygous carry the mutant (A) alleles whereas the control individuals were have the (G) wild whereas origin GC type alleles dependent on the allelic frequency and the odd ratio results. Depending on this result there was significant correlated between study group and mannose binding lectin Polymorphism. The result was agreement with result obtained by10 who were observed a correlation between the maternal high mannose binding lectin genotype A and premature birth, and it was suggest that during pregnancy mannose binding lectin-associated inflammation caused by higher mannose binding lectin activity may lead to earlier delivery. Moreover, this result finding explain why some peoples are mannose binding lectin deficient in the general population.
On the other hand, the result of the present study was showed that A allele frequency was (16.11) preterm labor and (0.85) in control group whereas G allele frequency was (6.68) in the preterm labor group and (0.49) in the control group as show in table 4.
Table (4):
Allelic frequencies of -54 G/A polymorphism in MBL in preterm labor.
| Study variables | Group | χ2 | OR | P-value | |
|---|---|---|---|---|---|
| Patients | Control | ||||
| Alleles A |
1611 (70.7) | 844 (94.5) | 208.15 | <0.001 | |
| G | 49 (5.5) | 3.032 | |||
| Total | 688 (29.3) | 893 (100.0) | |||
| 2279 | |||||
| (100.0) | |||||
The allele frequencies and genotype of MBL -54 G/A polymorphisms were contrasted amongst patients with PT and control groups, risk of getting PT between various genotypes of – 54 G/A polymorphisms was evaluated.
Moreover, the result of the current study was exhibited that the frequency of polymorphism at codon 54 of the mannose binding lectin gene was essentially higher in the patients than in controls. This show the polymorphism of mannose binding lectin gene might be correlated with an increased risk for the preterm labour
In the codon 54, guanine to adenine substitution changes an aspartic acid to a glycine at the protein level. So, the codon 54 polymorphism was the most extensively studied polymorphisms of the mbl gene and it was observed that this polymorphism decrease mbl expression . The existence of variation alleles in codon 54 has been correlated with decrease the level of mbl in the vagina and in the circulation11,12.
The variation allele (G > A) at codon 54 of the mbl gene produces an unstable mannose binding lectin protein, which has been correlated with increment susceptibility to infections. These allele was not present in white women with a history of bacterial vaginosis, but more frequent in mostly in nonwhite women with the same condition. Maternal carriage of this allele was not related with spontaneous abortion in white women 13.
Earlier studies have been observed that higher levels of maternal mannose binding lectin which related with successful pregnancies and the levels of mannose binding lectin were increased in the first trimester of gestation, suggest the role of mannose binding lectin in placentation, implantation, and maintenance of pregnancy14.
MBL is included in the maintenance of pregnancy outcome. High levels of mannose binding lectin have been correlated with successful gestation while reduce levels are included in preterm labor (PTL) development15.
The persistence of various mannose binding lectin alleles at comparatively high frequency suggest that carriage may confer a relative advantage to the host under some environmental conditions. While a genetically related mannose binding lectin insufficiency increases susceptibility to bacterial infections16. Low levels of mannose binding lectin decrease the quantity of complement on the surface of intracellular microorganism, which utilize complement to invade and replicate in the host cells. codon 57 was associated with ethnicity found in Black population, whereas the 52 codon found in Cacasian population and related to yeast infection. Codon-54 mutation was studied in this research because it found in all population. MBL insufficiency might also predispose to the development of autoimmune disease. Therefore, a role of genetic variation involved in MBL, has been suggested. Ultra-uterine infection and immune system defects causing susceptibility a major cause of spontaneous preterm birth17.
The polymorphism in -54 G/A of the mbl gene was significantly correlated with the susceptibility to PTL. Nevertheless, the frequency of allele A carriers of this polymorphism was significantly higher in the PTL cases than that in controls. So, the codon 54 A variation of mannose binding lectin gene may have an increment risk for the PTL.
- Frakking FN, Brouwer N, Zweers D, Merkus MP, Kuijpers TW, Offringa M, Dolman KM. High prevalence of mannose-binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol., 2006; 145:5–12 .
- Vardar F, Pehlivan S, Onay H, Atlihan F, Guliz N, Ozkinay C, Ozkinay F: Association between mannose binding lectin polymorphisms and predisposition to bacterial meningitis. Turk J Pediatr., 2007; 49:270–273.
- Koturoglu G, Onay H, Midilli R, et al. Evidence of an association between mannose binding lectin codon 54 polymorphism and adenoidectomy and/or tonsillectomy in children. Int J Pediatr Otorhinolaryngol, 2007; 71: 1157-1161.
- Hansen S, Holmskov U. Structural aspects of collectins and receptors for collectins, Immunobiology, 1998, 165-189.
- Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation, Lancet, 1989,. 1236-1239.
- Ling Z, Liu X, Chen X, Zhu H, Nelson KE, Xia Y, Li L, and Xiang C.: Diversity of cervicovaginal microbiota associated with female lower genital tract infections. Microb. Ecol., 2011; 61(3): 704–714.
- Sebnem Çalkavur, Gülin Erdemir, Hüseyin Onay, Özge Altun-Köroðlu, Mehmet Yalaz, Osman Zekioðlu, Güzide Aksu, Ferda Özkýnay, Fuat, Akercan, Nilgün Kültürsay . Mannose-binding lectin may affect pregnancy outcome. The Turkish Journal of Pediatrics, 2015; 57: 26-33
- Gravett, M.G. Hummel ,D. and Eschenbach ,, D.A. Preterm labor associated with sub clinical Amniotic fluid. Infection and with bacterial vaginosis. ObsteteGynecol 1986. 67:229-237.
- Marrazo , J.M. Antonio, M.Agnew,K.and Hiller, S.L. Distrubition of gential Lactobacillus strains shared by female sex partners. J. Infect Dis., 2009; 199(5):680-683.
- Peterson ,C. Hedges, S. Stenqvist, KSuppressed antibody and interleukin -6 responses to acute pyelonephritis in pregnancy, Kidney., 1994; 145:571-577.
- Garred P, Larsen F, Madsen HO, Koch C: Mannose-binding lectin deficiency – revisited. Mol Immunol., 2003; 40:73–84.
- Babula O, Danielsson I, Sjoberg I, Ledger WJ, Witkin SS. Altered distribution of mannose-binding lectin alleles at exon I codon 54 in women with vulvar vestibulitis syndrome, Am J Obstet Gynecol, 2004, pg. 762-766.
- Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, Svejgard A. Interplay between promoter and structural gene variants controls basal serum level of mannan-binding protein, J Immunol, 1995, pg. 3013-3020.
- Koroglu O.A.a · Onay H.b · Erdemir G.a · Yalaz M.A. Cakmak B.A. Akisu M.A. Ozkinay F.B. Kultursay N.A. Mannose-Binding Lectin Gene Polymorphism and Early Neonatal Outcome in Preterm Infants. Division of Neonatology. 2010.
- Dagle JM, Lepp NT, Cooper ME, Schaa KL, Kelsey KJ, Orr KL, Caprau D, Zimmerman CR, Steffen KM, Johnson KJ, Marazita ML, Murray JC: Determination of genetic predisposition to patent ductus arteriosus in preterm infants. Pediatrics, 2009; 123:1116–1123.
- Amirchaghmaghi E, Taghavi SA, Shapouri F, Saeidi S Rezaei A, Aflatoonian R. The role of toll like receptors in pregnancy. Int J Fertil Steril., 2013; 7: 147-154.
- Onay b Gulin Erdemir a, Ozge Altun Koroglu a Huseyin Mehmet Yalaz a Bilin Cakmak a Mete Akisu a Ferda Ozkinay b Nilgun Kultursay a. Mannose-Binding Lectin Gene Polymorphism and Early Neonatal Outcome in Preterm Infants. Neonatology, 2010; 98:305–312 .
- Genc MR, Onderdonkb a A. Endogenous bacterial flora in pregnant women and the influence of maternal genetic variation. Department of Obstetrics, Gynecology and Reproductive Biology b Department of Pathology, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA.2010. DOI: 10.1111/j.1471-0528.2010.02772.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.