ISSN: 0973-7510
E-ISSN: 2581-690X
Burkholderia pseudomallei, the causative agent of melioidosis, is endemic in Southeast Asia and tropical regions like Indonesia. This bacterium poses a challenge due to its uncommon and resistance to high-generation antibiotics. This study aims to detect B. pseudomallei in clinical samples from patients suspected of having melioidosis and to describe its clinical manifestations. A total of 118 clinical samples were collected and analyzed using Ashdown’s Selective Agar and routine bacteriological media, while the Vitek 2 Compact system was utilized for antibiotic identification and sensitivity testing. B. pseudomallei was detected in 9 (7.6%) samples, with the highest positivity rate in sputum at 6/59 (10.16%), followed by pus at 5.9% (1/17), pleural fluid at 10% (1/10), and bronchoalveolar lavage (BAL) at 4.5% (1/22). No B. pseudomallei growth was detected in blood and urine samples. Clinically, the positive cases exhibited symptoms akin to pulmonary tuberculosis in 3/9 (33%), combined hydropneumothorax and pulmonary TB in 2/9 (22%), and bilateral pleural effusions in 1/9 (11%). Other clinical presentations included a combination of bilateral pleural effusions and pulmonary TB, mediastinal pleural effusion with pulmonary TB and pneumonia, and burns on the soles of the feet. Antibiotic susceptibility testing revealed effective treatment options for ceftazidime, piperacillin-tazobactam, meropenem, and tigecycline. These findings highlight B. pseudomallei as a significant health concern in North Sumatra, emphasizing the need for improved diagnostic awareness and effective antimicrobial strategies to manage melioidosis cases.
Antibiotic Resistance, Burkholderia pseudomallei, Diagnostic Techniques, Melioidosis
Melioidosis is a severe infectious disease caused by the bacterium Burkholderia pseudomallei. This bacterium is resistant to many advanced-generation antibiotics, making it less familiar and challenging to detect in routine culture laboratories.1-3 Burkholderia pseudomallei is a saprophyte that thrives in soil, water, standing water, and rice fields.1 The disease poses a significant public health issue in Southeast Asia and northern Australia, where it is highly endemic to Eastern Thailand and Northern Australia, with causative organisms prevalent in soil and freshwater.4 Its diagnosis is increasing in other tropical areas, signaling an emerging and serious global threat.5
The clinical spectrum of melioidosis is broad and complex, including local skin lesions, sub-acute pneumonia, focal organ abscesses, musculoskeletal infections, and deadly fulminant pneumonia.6 It can account for up to 20% of all community-acquired sepsis cases in the tropics and 40% of sepsis-related deaths in northern Thailand. The overall mortality rate for the primary disease can reach as high as 50% in northeast Thailand and up to 20% in advanced medical settings in Northern Australia.7 The disease typically follows bacterial skin contamination and exposure to contaminated water or soil. Inhalation of contaminated dust can also lead to the pneumonia form of the disease. The potential for this bacterium to cause disease via inhalation has led to its classification as a potential biothreat agent by the Centers for Disease Control.8 Despite its clinical variability and diagnostic challenges, comprehensive data on the natural history, epidemiological risk factors, and geographic distribution of melioidosis remain limited.9 Melioidosis is primarily found in Southeast Asia, northern Australia, South Asia (including India), and China, with the majority of diagnosed cases in Thailand, Malaysia, Singapore, and northern Australia. Reports of cases have also emerged from Papua New Guinea and New Caledonia.10 In Indonesia, however, melioidosis cases are rarely reported; between 2013 and 2014, three cases were identified in the province of Makassar, South Sulawesi.11 In 2024, the Indonesian province of Riau, which is located on the island of Sumatera and borders Malaysia, reported a rather high number of melioidosis cases. This study discovered that the melioidosis mortality rate in Riau Province was 41%, which is equivalent to the 43% observed in a previous study carried out in Indonesia and higher than that of China (23%), Malaysia (33%), and other countries.12
The clinical diagnosis of melioidosis is challenging due to the absence of unique clinical signs specific to the disease.4 The gold standard for diagnosis remains conventional, yet B. pseudomallei is frequently misidentified as a culture contaminant or as other species such as B. cepacia, Bacillus spp., or Pseudomonas spp., particularly by laboratory personnel unacquainted with these organisms.13 There are currently no reliable, commercially available rapid diagnostic tests for melioidosis. Serological tests like the indirect hemagglutination test are commonly utilized but lack sensitivity and specificity. This study focuses on detecting B. pseudomallei in clinical samples from patients suspected of melioidosis at Universitas Sumatera Utara Hospital in Medan, Indonesia, and aims to document the clinical features associated with the infection.
Study setting
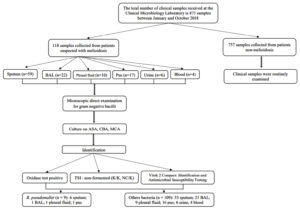
This study is a retrospective cohort analysis of clinical samples from infectious patients hospitalized at Universitas Sumatera Utara Hospital, Medan, Indonesia. Samples, including pus, sputum, pleural fluid, broncho-alveolar lavage (BAL), blood, and urine, were collected from individuals suspected of having melioidosis. Sampling was performed by doctors, nurses, or health professionals (Figure 1). Observations were conducted on patients admitted to Universitas Sumatera Utara Hospital from January to October 2018. Microbiological identification tests carried out included direct microscopy, culture, oxidase test, and antibiotic susceptibility testing.
Figure 1. Study protocol on detecting Burkholderia pseudomallei from suspects of melioidosis in USU Hospital
Microscopical examination
Gram staining (Color Gram 2- F, BioMerieux SA, Marcy l’Etoile, France) protocol was utilized for the direct microscopical examination of clinical samples to identify gram-negative rods, bacilli, and non-spore-forming bacteria. However, this examination is not specific for B. pseudomallei.14
Culture Maintenance, Identification, Biochemical and Antimicrobial Susceptibility Testing
Microbiological culture was maintained using selective and routine media. Ashdown’s Selective Agar (ASA) (Oxoid Ltd., UK), first described by LR Ashdown in 1979,15 was employed for the selective isolation of B. pseudomallei, which produces a characteristic colony morphology.16 Routine media used included Columbia Blood Agar (CBA) and MacConkey Agar (MCA) (both from Oxoid Ltd., UK). Cultures were incubated at 35 °C in an obligate aerobic atmosphere for 24-48 hours.17 Growth of bacterial colonies was then identified microscopically through Gram staining. An oxidase test was performed using oxidase strips (MB0266A from Oxoid, UK) on colonies suspected of being B. pseudomallei isolated on ASA, CBA, and MCA media exhibiting specific characteristics. A positive oxidase test was indicative of B. pseudomallei.18
The identification process began with the observation of bacterial colonies grown on ASA, CBA, and MCA media. On ASA media, the initial colonies of B. pseudomallei appeared as pinpoint formations with a clear to pale pink color, evolving into darker pink to purple, flat, slightly dry, and wrinkled with a metallic sheen after 2 days of incubation. On CBA, B. pseudomallei grew slowly; colonies are smooth, non-hemolytic, and dry, becoming clearly visible after 2 days. On MCA, B. pseudomallei formed non-lactose fermenting (colorless) colonies that also became dry and wrinkled after 2 days, whereas E. coli, a lactose fermenter, produced pink to red colonies that turned colorless with prolonged incubation.18 Further identification and antimicrobial susceptibility testing were carried out using the Vitek 2 Compact system, employing the GN ID Vitek card for identification and the AST GN93 Vitek card for susceptibility testing (VITEK® AST-GN93, bioMerieux, USA). Burkholderia pseudomallei is non-fermented (K/K) on TSI media, resistant to gentamicin (GN) and colistin (CL), and as sensitive to amoxicillin-clavulanic acid (AMC).19 In this study, amikacin and gentamicin are used to confirm the double disk diffusion, since colistin was not available in Indonesia.
Data analysis
Clinical data, including age, sex, type of clinical sample, and provisional diagnosis, were collected from culture request forms. Data analysis involved descriptive statistics, presenting clinical features, percentages, and prevalence of suspected melioidosis cases infected with B. pseudomallei, as well as patterns of antibiotic susceptibility.
Between January and October 2018, the Clinical Microbiology Laboratory received 875 clinical samples. Of these, 118 were from patients suspected of having melioidosis, with 9 of these 118 samples (7.6%) testing positive for B. pseudomallei, and the remaining 109 samples (92.4%) showing other bacteria. The other 757 samples were from patients not suspected of having melioidosis; in these, B. pseudomallei was not detected. However, 458 of these 757 samples (60.5%) tested positive for other bacteria, while 299 samples (39.5%) showed no bacterial growth or were culture-negative (Table 1).
Table (1):
Distribution of clinical samples and culture results
| Distribution of clinical samples (n = 875) | Total (%) | Types of microorganisms |
|---|---|---|
| Suspected of melioidosis | 118 (13.4%) | B. pseudomallei: 9 (7.6%) |
| Other bacteria: 109 (92.4%) | ||
| Not suspected of melioidosis | 757 (86.5%) | B. pseudomallei: 0 (0) |
| Other bacteria: 458 (60.5%) | ||
| Negative culture: 299 (39.5%) |
From the 118 samples under investigation for melioidosis, various types were collected and tested for microbial presence using routine (CBA and MCA) and selective (ASA) media. The sample distribution included 17 pus, 59 sputum, 10 pleural fluid, 22 bronchoalveolar lavage (BAL), 4 blood, and 6 urine samples. Out of these, 9 samples (7.6%) tested positive for B. pseudomallei. The highest positivity rates were seen in sputum samples, with 6 out of 59 (10.16%), followed by 1 out of 17 pus samples (5.9%), 1 out of 10 pleural fluid samples (10%), and 1 out of 22 BAL samples (4.5%). No growth of B. pseudomallei was detected in blood and urine samples (Table 2).
Table (2):
Types of microorganisms that grow in clinical samples of suspected melioidosis patients
| Microorganisms | Samples type n = 118 (%) | |||||
|---|---|---|---|---|---|---|
| Sputum n = 59 | BAL n = 22 | Pus n = 17 | Pleural fluid n = 10 | Urine n = 6 | Blood n = 4 | |
| Burkholderia pseudomallei | 6 (10.16%) | 1 (4.54%) | 1 (5.88%) | 1 (10%) | 0 | 0 |
| Others: | 32 (54.23%) | 19 (86.36%) | 10 (58.82%) | 6 (60%) | 3 (50%) | 2 (50%) |
| Pseudomonas aeruginosa, | ||||||
| Klebsiella pneumoniae | 5 (8.47%) | 2 (9.09%) | 0 | 0 | 0 | 1 (25%) |
| Escherichia coli | 4 (6.77%) | 0 | 1 (5.88%) | 1 (10%) | 1 (16.66%) | 0 |
| Acinetobacter baumannii | 10 (16.94%) | 0 | 0 | 0 | 0 | 1 (25%) |
| Burkholderia cepacia | 1 (1.69%) | 0 | 3 (17.64%) | 1 (10%) | 2 (33.33) | 0 |
| Stenotrophomonas maltophilia | 1 (1.69%) | 0 | 1 (5.88%) | 1 (10%) | 0 | 0 |
The growth of B. pseudomallei colonies was clearly visible on ASA media within 24 hours of incubation. However, on CBA and MCA media, the growth was not immediately apparent, posing challenges for laboratory technicians who might initially assume an absence of B. pseudomallei. Nevertheless, after 48 hours of incubation, colonies became visible, allowing for observation of their morphology (Figure 2). A comparison of the growth times of B. pseudomallei colonies at 24 and 48 hours is presented in Table 3.
Table (3):
Morphology of B. pseudomallei on CBA, MCA, and ASA media
Media |
>24 hours |
>48 hours |
Notes |
|---|---|---|---|
CBA |
Negative |
Positive |
Small colonies tend to be invisible, mixed colonies. No growth before 24 hours leads to false negative results |
MCA |
Negative |
Positive |
No growth before 24 hours leads to false negative results |
ASA |
Positive |
Positive |
Medium to large colonies after 24 hours of incubation in 37 °C. The first visible colonies of B. pseudomallei are pinpoint with a clear to pale pink color. Highly wrinkled circular purple colonies on ASA by 48 hours |
Figure 2. Characteristics of B. pseudomallei. (A) On Columbia Blood Agar (CBA), the colonies can be observed among other bacterial colonies after 48 hours of incubation. The colonies typically appear slightly convex inward, dry, sometimes wrinkled, and shiny. Due to the similarity in growth with other bacterial colonies, careful observation is required to accurately recognize and distinguish B. pseudomallei colonies. (B) On Mac-Conkey agar (MCA) colonies cannot ferment lactose, are almost colorless, may develop a metallic sheen with the appearance of rugose pink after 48 hours, colonies are scanty/non-dominant and sometimes resemble other gram-negative bacteria (difficult to distinguish). (C) On Ashdown selective agar (ASA) media, it has a faster colony growth time of 24-48 hours, clearer colony size, pink or pale color. It looks very clear and large at 48 hours of colonies with wrinkled colony morphology purple. (D) The microscopic appearance of the colony is rod-shaped, medium/fine in size, and gram-negative (red). (E) Triple sugar iron (TSI) show non-fermentation (K/K). (F) Positive oxidase test. (G) Screening for antibiotic sensitivity test using the disk diffusion method; amoxicillin/clavulanic acid (AMC), amikacin (AK), and gentamycin (CN)
Antibiotic sensitivity screening using the disk diffusion method revealed that amoxicillin/clavulanic acid (AMC) 30 produced an inhibition zone diameter of 33 mm, amikacin (AK) 10 showed an inhibition zone diameter of 9 mm, and gentamicin (CN) 10 resulted in an inhibition zone diameter of 0 mm (Figure 2). Appropriate antimicrobial treatment should be commenced immediately upon suspicion of melioidosis due to the high mortality rate associated with delayed or ineffective therapy. Following culture results, an antibiotic sensitivity test was performed for each patient to determine the definitive therapy against B. pseudomallei. All identified B. pseudomallei colonies demonstrated resistance to amikacin and gentamicin (n = 9/9, 100%). As empirical therapy at USU Hospital, ceftriaxone (n = 4), ciprofloxacin (n = 3), cefotaxime (n = 1), and levofloxacin (n = 1) were administered to most patients infected with B. pseudomallei (Table 4).
Table (4):
Antibiotic susceptibility patterns among B. pseudomallei isolates from clinical samples
| Antibiotics | % Antibiotic Sensitivity Testing (AST) (n = 9) | |
|---|---|---|
| Sensitive (%) | Resistant (%) | |
| Ampicillin/Sulbactam | 7 (82%) | 2 (18%) |
| Amoxicillin/Clavulanic Acid | 9 (100%) | 0 (0%) |
| Piperacillin/Tazobactam | 9 (100%) | 0 (0%) |
| Cefotaxime | 5 (55%) | 4 (45%) |
| Ceftriaxone | 4 (45%) | 5 (55%) |
| Ceftazidime | 9 (100%) | 0 (0%) |
| Cefepime | 9 (100%) | 0 (0%) |
| Aztreonam | 5 (55%) | 4 (45%) |
| Meropenem | 9 (100%) | 0 (0%) |
| Amikacin | 0 (0%) | 9 (100%) |
| Gentamicin | 0 (0%) | 9 (100%) |
| Ciprofloxacin | 3 (36%) | 6 (64%) |
| Tigecycline | 8 (91%) | 1 (9%) |
| Trimetoprim/Sulfametaxazole | 7 (82%) | 2 (18%) |
| Tetracycline | 6 (64%) | 3 (36%) |
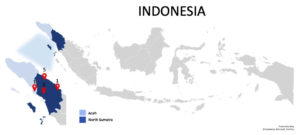
Out of 9 melioidosis-positive samples, 7/9 (78%) were elderly (aged 44-65 years), 1/9 (11%) were teenagers, and 1/9 (11%) were children. Geographically, 5 of the 9 positive cases were from Medan City in North Sumatra Province, two cases from Humbang Hasundutan District, a case from Labuhan Batu Utara District in North Sumatra, and a case from Aceh Singkil District in Aceh Province (Figure 3). Clinically, these patients presented with diverse symptoms including pulmonary TB in 3/9 (33%), hydropneumothorax combined with pulmonary TB in 2/9 (22%), bilateral pleural effusions in 1/9 (11%), and other complex conditions such as bilateral pleural effusions with pulmonary TB, mediastinal pleural effusion with pulmonary TB and pneumonia, and burns on the soles of the feet. Overall, these patients also have other co-morbidities (Table 5).
Table (5):
Demographic characteristics of the melioidosis patients
| Characteristic | Total (%) (n = 9) |
|---|---|
| Gender | |
| Male | 6 (66.6%) |
| Female | 3 (33.3%) |
| Age group | |
| Elderly (44-65 years old) | 7 (77.7%) |
| Teenager (13-18 years old) | 1 (11.1%) |
| Children (0-12 years old) | 1 (11.1%) |
| Provisional diagnosis | |
| • Pure pulmonary TB | 3 (33.3%) |
| • Hydropneumothorax + pulmonary TB | 2 (22.2%) |
| • Bilateral pleural effusions | 1 (11.1%) |
| • Bilateral pleural effusions + Pulmonary TB | 1 (11.1%) |
| • Mediate pleural effusion + Pulmonary TB + pneumonia | 1 (11.1%) |
| • Burns on the soles of the feet | 1 (11.1%) |
| Empirical Therapy | |
| • Ceftriaxone | 4 (44.4%) |
| • Ciprofloxacin | 3 (33.3%) |
| • Levofloxacin | 1 (11.1%) |
| • Cefotaxime | 1 (11.1%) |
One hundred and eighteen clinical samples were collected and processed for culture during the study period. These samples were obtained from patients with relevant infections who were receiving care in various hospital settings, including inpatient rooms and intensive care units such as the ICU, PICU, and NICU. Sputum samples were predominant, reflecting that pneumonia is the most common manifestation of melioidosis.20 Burkholderia pseudomallei was detected in a limited number of sputum samples. Other prevalent pathogenic bacteria in sputum included P. aeruginosa, observed in 32/59 (54.23%) of cases. P. aeruginosa showed similar predominance in BAL, pus, and sputum samples., with occurrences of 19/22 (86.36%), 10/17 (58.82%), and 6/10 (60%), respectively. Additional pathogenic bacteria grown on routine media (CBA and MCA) from these clinical samples included K. pneumoniae, E. coli, A. baumannii, B. cepacia, and S. maltophilia. On ASA media, P. aeruginosa was also observed, although its colony morphology distinctly differs from that of B. pseudomallei,21 demanding further identification to confirm its species.
This study utilized three different media types (selective: ASA; routine/non-selective: CBA and MCA) to cultivate and assess the selectivity for B. pseudomallei (Figure 2). The results indicated that all three media were suitable for the growth of B. pseudomallei. However, ASA demonstrated a faster colony growth within 24-48 hours. In contrast, on CBA and MCA media, the colonies appeared very small and were nearly invisible, potentially leading to false-negative results. On CBA (Figure 2A), the colonies were not clearly visible as they grew amidst other bacterial colonies, making them obscured due to their small and smooth nature, thereby necessitating a longer incubation period of over 48 hours for visible growth. The colonies eventually developed a metallic luster and could appear dry or wrinkled after extended incubation.22 Similarly, on MCA media (Figure 2B), the colonies that could not ferment lactose were almost colorless and could develop a metallic luster and pink rugose appearance after 48 hours.23
ASA media displayed colonies with a clearer, larger size, showcasing a pink or pale color, and by 48 hours, the colonies exhibited a distinct purple, wrinkled morphology (Figure 2C). Observations from this study noted that ASA media was slightly oily and challenging to scratch due to its glycerol content. It is recommended to incubate sterile ASA media at 37 °C for at least 24 hours before use. This pre-incubation significantly improves the separation and purity of colony streaks in the 3rd and 4th quadrants. The microscopic appearance of the B. pseudomallei colony showed rod-shaped, medium/fine in size, and gram-negative (red) (Figure 2D). The selective growth of Burkholderia colonies on ASA media stems from their inherent resistance to polymyxin and gentamicin,23 with ASA media containing 4 mg/L of gentamicin, which suppresses the growth of non-Burkholderia bacteria. The TSI reaction indicates that the slant/butt is alkaline and non-fermented (K/K) (Figure 2E). Oxidase testing confirmed that all colonies of B. pseudomallei were oxidase-positive (Figure 2F). Antibiotic susceptibility testing demonstrated marked sensitivity to amoxicillin/clavulanic acid, limited susceptibility to amikacin, and complete resistance to gentamicin, as indicated by the absence of an inhibition zone (Figure 2G). These findings highlight the potential efficacy of amoxicillin/clavulanic acid against the tested isolates.
The diagnosis of melioidosis presents significant challenges in microbiology laboratories, often leading to underdiagnosis due to a lack of clinical awareness, which can contribute to the high mortality rates associated with the disease due to inappropriate or delayed initial treatment.24 In hospitals, empirical therapy is generally based on clinical judgment and diagnosis,25 administered to prevent severe infections while awaiting the results of microbiological cultures. In this study, patients received empirical therapies, including ceftriaxone, cefotaxime, ciprofloxacin, and levofloxacin. However, these therapies are often not effective as B. pseudomallei is intrinsically resistant to many common antimicrobial agents, including penicillins (except amoxicillin/clavulanic acid), third-generation cephalosporins (except ceftazidime), aminoglycosides, quinolones, and macrolides.26 Antibiotic sensitivity testing revealed high resistance levels to the four antibiotics, with all isolates showing resistance to amikacin and gentamicin. Comparatively, dominant gram-negative bacteria found in clinical samples not suspected of melioidosis, such as E. coli, K. pneumoniae, P. aeruginosa, and A. baumannii, remain over 80% sensitive to amikacin. This disparity highlights the difficulty in selecting effective antibiotic therapy for melioidosis, given the limited options available.
The percentage results for antimicrobial sensitivity utilized cutoff values established by the European Unit Reference Laboratory for Antimicrobial Resistance (EURL-AR) in 2013 to guide antimicrobial therapy in hospitals. These cutoff values are crucial for comparing antimicrobial sensitivity, aiding in the management of resistance, and assisting microbiologists in interpreting test results. The criteria are defined as follows: sensitivities below 60% are not recommended for therapy due to high resistance rates; sensitivities between 60-80% may be considered in consultation with the hospital’s antimicrobial resistance control program; sensitivities above 80% are generally recommended for therapeutic use. Antibiotics such as piperacillin-tazobactam, ceftazidime, cefepime, tigecycline, trimethoprim-sulfamethoxazole, amoxicillin-clavulanic acid, and meropenem, which demonstrated 100% sensitivity, are recommended as definitive therapy options for melioidosis patients.
Most B. pseudomallei isolates were identified in patients displaying clinical features of pulmonary tuberculosis and pleural effusion, aligning with typical manifestations of melioidosis. Melioidosis often presents as chronic pneumonia, which can closely mimic tuberculosis, usually regarded together in clinical assessments.22,26 Among these patients, 5/9 (55.5%) were diagnosed with tuberculosis alongside other comorbidities. These patients were treated with antituberculosis drugs such as rifampicin, ethambutol, and isoniazid, which may contribute to increased antibiotic resistance. Concurrent infections of M. tuberculosis and B. pseudomallei were identified, although co-infection is rare. A similar case of concurrent tuberculosis and melioidosis was also documented in Singapore in 2014, marking the first reported instance of these conditions occurring simultaneously in the site of infection.27 This study highlighted cases where both tuberculosis and melioidosis occurred simultaneously in the same patient. Although antibiotic therapy can alleviate the acute symptoms of melioidosis, the infection is known for its potential to relapse, with symptoms sometimes reappearing months or even years after apparent clinical resolution.28
Melioidosis is infrequently reported in Indonesia, with many cases noted only by their clinical symptoms without confirmatory laboratory evidence. This study documents the occurrence of B. pseudomallei infection in North Sumatra Province and its surrounding areas, including a notable case of coinfection with pulmonary tuberculosis. Approximately half of the melioidosis cases present with pneumonia, which may progress to fatal septicemia, mimicking unilateral pneumonia or other chronic diseases. Chronic forms of melioidosis often manifest as persistent pneumonia or non-healing skin sores.11,13,15,16,20 In this study, we identified a pediatric case where a child with a burn wound was infected by B. pseudomallei.29 A study in India noted melioidosis in 8% of children, primarily at local lesion sites.30 Research in Southeast Asia indicates that children more frequently exhibit local manifestations such as skin lesions or lymphadenopathy, ranging from 46%-63%.31 Geographically, the distribution of melioidosis cases in this study extended beyond North Sumatra; one pediatric patient with a burn lesion infection originated from Aceh Province, specifically in Aceh Singkil District, and was referred to Universitas Sumatera Utara Hospital for surgical treatment. The other eight cases were from North Sumatra Province (Figure 3). Transmission of B. pseudomallei typically occurs through unprotected occupational exposure to soil or water, ingestion of contaminated water or food, and inhalation.30 North Sumatra, with a predominant traditional farming community, poses a higher risk for melioidosis. Additionally, its proximity to Thailand and Malaysia, countries with high incidences of melioidosis, increases the risk of cross-border transmission, particularly for travelers.
A high index of suspicion is essential for diagnosing melioidosis in non-endemic regions. Clinicians should consider melioidosis in patients presenting with fever who have any of the following: history of residence or travel to endemic areas; occupational or recreational exposure to soil or water potentially harboring B. pseudomallei, including military personnel; or risk factors like diabetes mellitus or kidney disease.31 Early engagement with clinical microbiology laboratories is crucial when investigating potential cases to enhance the recognition of significant pathogens in mixed cultures. Moreover, B. pseudomallei is categorized as a hazard group 3 biological agent, requiring stringent containment measures during handling. Employing selective agar is recommended to improve culture sensitivity from non-sterile clinical samples.21
In Thailand, the management of melioidosis is informed by a series of clinical trials spanning the last 25 years and involves a dual-phase treatment approach: an acute phase aimed at mitigating life-threatening sepsis, followed by an eradication phase designed to eliminate residual bacteria and reduce recurrence risk.25 The acute phase employs ceftazidime, with carbapenem reserved for severe cases or when initial treatment fails, and amoxicillin/clavulanic acid (co-amoxiclav) as a secondary option. The eradication phase typically involves trimethoprim/sulfamethoxazole or co-amoxiclav as alternatives, with treatment customization based on the patient’s clinical manifestations and response.26,32
Diagnostic investigations for melioidosis depend on the resources available, which are often limited in regions where the disease is endemic. Supporting laboratory tests include those for acute renal failure, liver function abnormalities, anemia, and coagulation disorders, which are prevalent in severe cases of melioidosis.4 All patients should undergo chest X-ray imaging, which might show localized alveolar patch infiltrates, diffuse bilateral alveolar effusions, or multiple nodular lesions indicative of hematogenous spread. Differentiating upper lobe infections from pulmonary TB can be challenging due to similarities in clinical and radiological presentations. Conditions such as empyema or pulmonary abscess are well-documented, warranting repeated chest radiographs for patients with respiratory involvement. Arterial blood gases are recommended for those with pulmonary issues or sepsis. Comprehensive imaging such as ultrasound, CT scans, or other modalities should be used to check for abscesses in organs like the liver and spleen and to assess potential prostate involvement.9
Currently, there is no vaccine for melioidosis; thus, preventive measures are crucial. Even individuals who have previously had melioidosis can be reinfected if exposed again. The diverse clinical presentations make it challenging to differentiate melioidosis from other bacterial infections, acute or chronic. Confirming a diagnosis relies on proper specimen collection, culture, and isolation of B. pseudomallei, with culture remaining the diagnostic gold standard.
Although diagnosing melioidosis is complex, this study highlights the significant finding of B. pseudomallei in Indonesia, particularly in North Sumatra Province and Nanggroe Aceh Darussalam. This highlights the urgency for heightened awareness within the medical community to ensure accurate melioidosis diagnoses. ASA, a selective medium, is recommended for routine use in microbiology laboratories for diagnosing suspected melioidosis cases, particularly in tropical countries like Indonesia.
ACKNOWLEDGMENTS
The authors would like to thank the Research Institute of Universitas Sumatera Utara for providing funds from TALENTA USU 2018, and the director, doctors, nurses, and clinical microbiology laboratory assistants at the Universitas Sumatera Utara Hospital, who have contributed to the sample collection process in this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RLK conceptualized the study. RLK and MH designed the study. AWS and RLK performed data collection. MH performed data interpretation. RLK, AWS, MH and CG wrote the manuscript. CG reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported by the Research Institute of Universitas Sumatera Utara through TALENTA USU 2018 Scheme of Research Development of the University of Sumatera Utara Hospital (PPRSU) with contract number: 72/2.3.1/PPM/KP-TALENTA USU/2018.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Health Research Ethical Committee of the Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia, vide reference number 231/TGL/KEPK/FKUSU/RSUPHAM/2018.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Hantrakun V, Kongyu S, Klaytong P, et al. Clinical epidemiology of 7126 melioidosis patients in Thailand and the implications for a national notifiable diseases surveillance system. Open Forum Infect Dis. 2019;6(12):ofz498.
Crossref - Rattanavong S, Wuthiekanu V, Langla S, et al. Randomized soil survey of the distribution of Burkholderia pseudomallei in rice fields in Laos. Appl Environ Microbiol. 2011;77(2):532-536.
Crossref - Mohan A, Podin Y, Liew DW, et al. Fine-needle aspiration to improve diagnosis of melioidosis of the head and neck in children: a study from Sarawak, Malaysia. BMC Infect Dis. 2021;21(1):1069.
Crossref - Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: Insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4(4):272-282.
Crossref - Dance DAB. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 2000;74(2-3):159.
Crossref - Cheng AC, O’brien M, Jacups SP, Anstey NM, Currie BJ. C-reactive protein in the diagnosis of melioidosis. Am J Trop Med Hyg. 2004;70(5):580-582.
Crossref - White NJ, Chaowagul W, Wuthiekanun V, Dance DAB, Wattanagoon Y, Pitakwatchara N. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;2(8665):697-701.
Crossref - Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8(2):225-230.
Crossref - Cheng AC, Currie BJ. Melioidosis: Epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18(2):383-416.
Crossref - Limmathurotsakul D, Golding N, Dance DAB, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1(1):1-13.
Crossref - Tauran PM, Wahyunie S, Saad F, et al. Emergence of melioidosis in Indonesia and today’s challenges. Trop Med Infect Dis. 2018;3(1):1160-1163.
Crossref - Anggraini D, Siregar FM, Rosdiana D, et al. Epidemiology and genetic diversity of Burkholderia pseudomallei from Riau Province, Indonesia. PLoS Negl Trop Dis. 2024;18(5):e0012195.
Crossref - Peacock SJ, Schweizer HP, Dance DAB, et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008;14(7):e2.
Crossref - Estrada-de los SP, Vinuesa, P, Martinez-Aguilar L, Hirsch AM, Caballero-Mellado J. Phylogenetic analysis of Burkholderia species by multilocus sequence analysis. Curr Microbiol. 2013;67(1):51-60.
Crossref - Ashdown LR. An improved screening technique for isolating Pseudomonas pseudomallei from clinical specimens. Pathology. 1979;11(2):293-297.
Crossref - Chantratita N, Wuthiekanun V, Boonbumrung K, et al. Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei. J Bacteriol. 2007;189(3):807-817.
Crossref - Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035-1044.
Crossref - Hsueh PR, Teng, LJ, Lee, LN, et al. Melioidosis: An emerging infection in Taiwan? Emerg Infect Dis. 2001;7(3):428-433.
Crossref - MORU. Standard Operating Procedure (SOP) isolation of Burkholderia pseudomallei from clinical samples. Mahidol-Oxford Tropical Medicine Research Unit (MORU). 2015:1-10.
- Meumann EM, Cheng AC, Ward L, Currie BJ. Clinical features and epidemiology of melioidosis pneumonia: Results from a 21-year study and review of the literature. Clin Infect Dis. 2012;54(3):362-369.
Crossref - Peacock SJ, Chieng G, Cheng AC, et al. Comparison of Ashdown’s medium, Burkholderia cepacia medium, and Burkholderia pseudomallei selective agar for clinical isolation of Burkholderia pseudomallei. J Clin Microbiol. 2005;43(10):5359-5361.
Crossref - Bart J, Currie-Sharon J, Peacock, Peter AR, Vandamme. Manual of clinical microbiology, 10th Ed. ASM Press, Washington DC. 2015
- Benoit TJ, Blaney DD, Doker TJ, et al. A review of melioidosis cases in the Americas. Am J Trop Med Hyg. 2015;93(6):1134-1139.
Crossref - Mukhopadhyay C, Eshwara VK, Kini P, Bhat V. Pediatric melioidosis in Southern India. Indian Pediatr. 2015;52(8):711-712.
- Dance D. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents. 2014;43(4):310-318.
Crossref - Cohen J, Powderly WG, Opal SM. Infectious Disease, 4th Ed. Elsevier, USA. 2017.
- Tan SY. Tuberculosis and melioidosis at distinct sites occurring simultaneously. Case Rep Infect Dis. 2020;2020(1):1-4.
Crossref - Nandi T, Tan P. Less is more: Burkholderia pseudomallei and chronic melioidosis. mBio. 2013;4(5):e00709-13.
Crossref - Foong YW, Tan NW, Chong CY, Thoon KC, Tee NWS, Koh MJA. Melioidosis in children: A Retrospective study. Int J Dermatol. 2015;54(8):929-938.
Crossref - Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82(6):1113-1117.
Crossref - Limmathurotsakul D, Peacock SJ. Melioidosis: A Clinical overview. Br Med Bull. 2011;99(1):125-139.
Crossref - Inglis TJJ. The treatment of melioidosis. Pharmaceuticals (Basel) 2010;3(5):1296-1303.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.