ISSN: 0973-7510
E-ISSN: 2581-690X
Fish are often infected with different blood protozoa of the genera Babesiosoma, Trypanosoma, and Hemogrigarina. These blood parasites match the genera which infect the blood of mammals. A total of 120 samples from five different fish species (Barbus grypus, Barbus sharpyi, Liza abu, Carasobarbus luteus, and Aspius vorax) were collected from the Khazar River in Ninevah Governorate, Iraq at weekly intervals during the period from April 2022 to October 2022 for the detection of blood parasites and hematological analysis. Thin and thick Giemsa stained blood smears revealed significant infection with Trypanosoma, Babes iosoma, and Haemogregarina which were recorded in 32%, 16%, and 8% of Carasobarbus luteus samples, respectively; 30%, 15%, and 10% of B. sharpyi samples, respectively; and 23.3%, 16.7%, and 6.7% of B. grypus samples, respectively. No infection with Trypanosoma or Babesiosoma were recorded in L. Abu, and no infection with Babesiosoma or Haemogregarina were recorded in A. vorax. Hematological parameters of blood samples from infected fish showed a significant decrease in hemoglobin concentration, total red blood cells, and stacked cell size along with a significant increase in the number of inflammatory cells including lymphocytes, eosinophils, and basophils. This is the first study to identify blood parasites in fishes and to monitor the changes of hematological parameters in blood samples of infected fishes from the waters of Mosul, Iraq.
Trypanosoma, Babesiosoma, Haemogregarina, Fish, Hematological Parameters, Iraq
Fishes are considered as an important source of nutrition for humans and livestock as they are rich in protein, vitamins, minerals, and serve as a good source of omega-3 fatty acids.1,2 Like all animals, fishes can be infected with many parasites leading pathogenic diseases and economic losses due to decreases in the weight and nutritional value of infected fish.3 Blood protozoa, or haemaprotozoans, such as Trypanosoma, Babesiosoma, and Haemogregarina are of great interest to researchers due to their variety and pathogenicity in fishes. Babesiosoma parasitize both circulating erythrocytes and erythrocytes from reticuloendothelial tissues and, to complete their lifecycle, require leeches as an intermediate host. Trypanosoma are haemoflagelates that resemble the genus that infects the blood of mammals.4-6 Parasites in the blood stream can cause a range of symptoms from mild anemia, associated with low levels of parasitemia, to severe pathological changes, due to a heavy parasite burden, and serious illnesses that can result in anorexia, ascites, exophthalmia, with edema and splenomegaly, and even death.7,8
Haemogregarina protozoa have a sausage-like shape and are a malaria-like parasite of fish erythrocytes. Haemogregarina species are poorly studied parasitic sporozoans of the family Haemogregarinidae.9 The development of haemogregarines is complex and occurs through two types of hosts: blood-sucking invertebrates and vertebrates, including fish.10
Blood parasites are of veterinary interest because their host populations can be human food resources. Additionally, there is limited information available on these parasite in Iraq (7), thus the present study was designed to study and report the blood parasites of the genera Trypanosoma, Babesiosoma, and Haemogregarina parasitizing freshwater fishes in Mosul, Iraq. Further, their effects on the hematological profiles of infected fishes were analyzed.
Fishes
A total of 120 samples from five fish species (Barbus grypus, Barbus sharpyi, Liza abu, Carasobarbus luteus, and Aspius vorax) were collected from different locations in the Khazar River in Ninevah governorate at an at weekly intervals and brought to the Lab of Parasitological Research, Microbiological Department, College of Veterinary Medicine, University of Mosul, Iraq.
Blood sample collection
Blood samples were obtained from the caudal artery and heart of fish and thin and thick blood smears were immediately prepared. The smears were air dried, fixed in 95% methanol for five minutes, then stained with Giemsa. Prepared slides were examined under a microscope using a 100X oil immersion objective lens for the detection of blood parasites. Images were taken using a digital camera.11 To measure hematological parameters, approximately 1 mL of blood was placed in tubes with the anticoagulant EDTA. Hemoglobin (Hb) concentration, total red blood cells (TRBC), packed cell volume (PCV), mean cell volume (MCV), mean cell hemoglobin concentration (MCHC), and total leucocyte count (TLC) were measured. The percentage of each white blood cell type was calculated.12,13
Statistical analysis
Statistical analysis was performed using the Chi-square test and SigmaStat software V3 (Systat Software, San Jose, CA, USA).
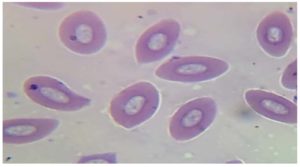
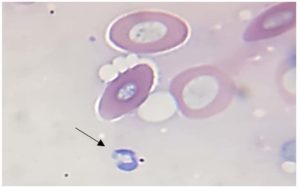
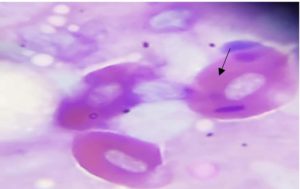
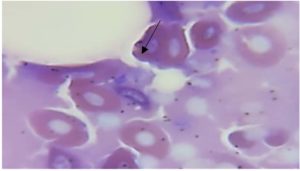
Parasitological examination of thin and thick blood smears stained with Giemsa revealed that fishes were infected with three genera of blood parasites (Trypanosoma, Babesiosoma, and Haemogregarina) as shown in Figures 1, 2, 3, and 4. Of the 30 examined of B. grypus fish, 7 (23.3%) were infected with Trypanosoma, 5 (16.7%) were infected with Babesiosoma, and 2 (6.7%) were infected with Haemogregarina. Of the 20 examined B. sharpyi, the infection rates with Trypanosoma, Babesiosoma, and Haemogregarina were 6 (30%), 3 (15%), and 2 (10%) respectively. No infections with Trypanosoma or Babesiosoma were recorded in the 25 examined L. abu, but 1 (4%) was infected with Haemogregarina. In the 25 examined C. luteus fish, 8 (32%), 4 (16%) and 2 (8%) were infected with Trypanosoma, Babesiosoma, and Haemogregarina, respectively. Of the 20 Aspius vorax fish examined, 3 (15%) were infected with Trypanosoma, but no Babesiosoma or Haemogregarina infections were recorded (Table 1).
Table (1):
Prevalence of blood parasites according to species of fish.
| Species of fish | No. of fish examined | Trypanosoma | Babesiosoma | Haemogregarina | |||
|---|---|---|---|---|---|---|---|
| No. of fish infected | Prevalence % |
No. of fish infected | Prevalence % |
No.of fish infected | Prevalence % |
||
| Barbus grypus | 30 | 7 | 23.3a | 5 | 16.7a | 2 | 6.7a |
| Barbus sharpyi | 20 | 6 | 30a | 3 | 15a | 2 | 10a |
| Liza abu | 25 | 0 | 0 | 0 | 0 | 1 | 4a |
| Carasobarbus luteus | 25 | 8 | 32a | 4 | 16a | 2 | 8a |
| Aspius vorax | 20 | 3 | 15b | 0 | 0 | 0 | 0 |
Different letters vertically indicate significant differences at p≤0.05
Figure 3. Haemogregarina parasite in the erythrocytes of fish blood smears stained with Giemsa stain
Blood Hb concentrations, TRBC, PCV, MCV, MCHC and TLC all decreased significantly in fish infected with blood parasites, while the number of inflammatory cells (eosinophils, lymphocytes and basophils) were significantly increased in infected fish as shown in Tables 2 and 3.
Table (2):
Hematologic parameters of infected fishes with blood parasites.
| Parameters | Mean ± standard error | |
|---|---|---|
| Control fishes | Infected fishes | |
| RBC x 106 microliter | 1.2 ± 0.05 | 0.6±0.2 |
| Hb g/100 ml | 8.2±0.2 | 5.5 ± 2.31 |
| PCV % | 30 ± 0.79 | 25.62 ± 5.62 |
| MCV % | 260.9 ± 16.83 | 450.75 ± 11.30 |
| MCHC g/100 ml | 70.8± 2.80 | 120.06 ± 50.21 |
| TLC x 103 microliter | 36.0±6.0 | 90.85 ± 75.35 |
Table (3):
The percentage of leukocyte cells of fishes infected with blood parasites
| Cell type x 103 ml | Mean ± standard error | |
|---|---|---|
| Control fishes | Infected fishes | |
| Neutrophils % | 8.21±0.12 | 2.54±0.02 |
| Lymphocyte % | 40.81±0.25 | 60.65±0.18 |
| Eosinophil % | 8.93±0.05 | 11.51±0.07 |
| Basophils % | 1.05±0.03 | 1.20±0.05 |
| Monocytes % | 20.81±0.18 | 20.05±0.14 |
In this study, three blood parasite genera (Trypanosoma, Babesiosoma, and Haemogregarina) were recorded in different species of fish. The highest infection rates were observed in C. luteus, B. sharpyi, and B. grypus which may have been due to pollution, habitat preferences, or the availability of intermediate hosts for the parasites. 14 Trypanosoma infection was observed in 23.3%, 30%, and 32% of B. grypus, B. sharpyi, and C. luters samples, respectively. The results of this study were similar to those obtained by Jarallah6 who found that Trypanosoma infection rate in fish was 41.4%.
The high prevalence of Babesiosoma infection in fish observed here is in agreement with Shahi et al.15 who reported a Babesiosoma infection rate of 16.6% in fish. The observed mixed infection with Trypanosoma and Babesiosoma has also been previously described.16 The prevalence of blood parasites in fish are often the result of interactions with leeches.17 The infection rate of Haemogregarina was low compared to a previous report18 that recorded a Haemogregarina infection rate of 14.9 %.
Hematological parameters revealed a decrease in red blood cell count, hemoglobin value, and packed cell volume in infected fishes, which are conditions that often lead to anemia.19 Parasitic infection causes stress that leads to the release of catecholamine which can mobilize RBCs from the spleen. Entry of fluid into the leukocyte cellular compartment has also been shown to increase in infected fishes. The results reported here align with these prior reports.20,21 Another study indicated that Trypanosoma causes damage to the hematopoietic tissues, vascular system, and kidneys. Fish with a heavy infection have sunken eyes and become lethargic, anemic, and emaciated with damage to the excretory part of the kidney, causing osmoregulatory problems.22
While the infection rate with Trypanosoma was lower in A. vorax (15%), which is similar to the infection rate obtained by Jarallah6 (12.28%), in this case, the low infection rate may have been due to improved host immunity. Leukocytes are considered to be the first line of defense for the immune system6; surveying the changes in the number and distribution of leukocytes is a good indicator to the presence of infection. It has been previously reported,23 and this study confirmed, that leukocytes are directly related with an increase in parasitemia, especially lymphocytes and eosinophils, during Trypanosoma infection in fishes.
To the best of our knowledge, this is the first study investigating the prevalence of blood parasites in fishes of Mosul, Iraq. The evaluation of blood parameters could be used to determine the health status of fish, establish the degree of damage to tissues, and determine the severity of the infection. This study has reported the presence of three blood parasites: Trypanosoma, Babesiosoma, and Haemogregarina. It is recommended that further research on fish parasites be performed to investigate the occurrence of parasites in aquatic systems and provide information on the potential hazards to fish health. This is especially important with the growing demand for fish as a source of animal protein for humans and livestock.
ACKNOWLEDGMENTS
The authors would like to thank College of Veterinary Medicine, University of Mosul, Iraq, for their effort and support given to the current study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Al-Gburi NM. Detection and pathogenicity of Listeria monocytogenes in common carp (Cyprinus carpio) fish in Baghdad. Iraqi J of Vet Sci. 2020;34(2):311-316.
Crossref - Yusni E, Frantika C, Batubara AS. Detection of endoparasites in mackerel tuna (Euthynnus affinis) in north Sumatra province, Indonesia. Iraqi J Vet Sci. 2022;36(2):519-524.
Crossref - Altaee AF. Endoparasites of the fresh water fish Liza abu in Mosul, Iraq. Iraqi J Vet Sci. 2008;22(1):25-29.
Crossref - WOO, PTK. Diplomonadida (Phylum: Parabasalia), Kinetoplastea (Phylum Euglenozoa). In: WOO PTK. (ed.): Fish diseases and disorders, vol. 1: Protozoan and metazoan infections. 2nd ed. CABI, Wallingford. 2006:46-114.
- Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonosis: status and issues. Int J Parasitol. 2005;35(11-12):1233-1254.
Crossref - Jarallah HM. Study of Haemoflagellates trypanosoma Sp. Infection in some fish of Iraq marshes and relationship of leukocytes with inflammatory response. IOP Conf Series: Earth and Environmental Science. 2021;877(012011).
Crossref - Al-Niaeemi BH, Dawood MH. Biomarkering metabolic activities of the tapeworm Khawia armeniaca (Cholodkovsky, 1915) in association to its fish host Barbus grypus (Hekle, 1843). Iraqi J Vet Sci. 2021;35(1):169-176.
Crossref - Islam AK, Woo PTK. Trypanosoma danilowskyi in Carassius auratus: the nature of the protective immunity in recovered gold fish. J Parasitol. 1991;77(2):258-262. https://pubmed.ncbi.nlm.nih.gov/2010858/
- Gupta DK, Gupta N, Gangwar R. Two new species of Trypanosoma from freshwater fish (Heteropnueutes fossilis and Channa punctaus) from Bareilly India. J Par Dis. 2006;30(1):58-63. https://www.researchgate.net/publication/311900151
- Khamnueva TR, Baldanova DR. The first record of haemogregarine infection in fish of Lake Baikal. Parazitologiya. 2016;50(1):92-95.
- Dacie JV, Lewis SM. Practical Haematology. Churchill Livingstone, London. 9th ed. 2001.
- Rosenblatt JE. Laboratory diagnosis of infections due to blood and tissue parasites. Clin Infect Dis. 2009;49(7):1103-1108.
Crossref - Lark TD, Donaldson MR, Drenner SM et al. The efficacy of field techniques for obtaining and storing blood samples from fishes. J Fish Biol. 2011;79(5):1322–1333.
Crossref - Khan RA, Bareett M, Murphy J. Blood parasites of fish from the north western Atlantic Ocean. Can J Zool. 1979;58(11):770-781.
Crossref - Shahi N, Yousuf AR, Rather MI Ahmed F. First report of blood parasites in fishes from Kashmir and their effect on the hematological profile. Open Vet Journal. 2013;3(2):89-95.
- Pereira GR, Soares P, Gomes MQ et al. Are fish paratenic natural hosts of the caiman haemoparasite Hepatozoon caimani? Parasitol Res. 2013;113(1):39–45.
Crossref - Porter A, Vinall HF. A protozoan parasite (Ichthyosporidium sp.) of the neon fish Hyphessobrycon innesi. J Zool. 2009;126(3):397–402.
Crossref - Khamnueva TR., Baldanova, DR. Blood parasites of fish of the Baikal basin. Erfosch Boil Res. Mongolia Halle. 2021;14:311-315. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1262&context=biolmongol
- Eyiwumi FA, Augustine O, Ovie AK. The hematological parameters of catfish (Clariasgariepinus) fed fish feeds with replaced premix using Moringa leaf meal (MLM). Madridge J Aquac Res Dev. 2018;2(1):35–39.
Crossref - Mazur OE. Leukocyte composition of pronephros of Leocottus kesslerii infected with hemoflagellates of the genus Trypanosoma (Kinetoplastea: Trypanosomatida) IOP Conf. Series: Earth and Environmental Science. 2021;908(12):1-6.
Crossref - Mani SR, Kandhasamy P, Vijayan K, Palanichamy M, Jacob JP, Kandhasamy S. Haematological parameters of Cyprinus carpio with reference to probiotic feed: A machine learning approach. The Israeli Journal of Aquaculture. 2021;73(1425477):1-10.
Crossref - Lom J, Dykova I. Protozoan parasites of fishes. Developments in Aquaculture and Fisheries Science. 1992;26:280.
- Ahmed MS, Shafiq K, Ali H, Ollevier F. Pathogenic effects associated with Trypanosoma danilewskyi strain FCC 1 infection in juvenile common carp, Cyprinus carpiol. J Animal Plant Sci. 2011;21(4)800–806.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.