ISSN: 0973-7510
E-ISSN: 2581-690X
The ability of endophytic fungi to produce valuable bioactive compounds when surviving in medicinal herbs. Finding out endophytic fungi originated from Catharanthus roseus with antimicrobial, antioxidant and cytotoxicity activities is important for pharmaceutical development. The isolation was based on the morphology of fungi. Identification was performed by sequencing 18S rRNA that was compared with known genes using Blast search in the combination of phylogenic analysis by using Clustal W and PhyML in GenomeNet. For the antimicrobial test, the agar diffusion method was used. DPPH scavenging assay for the antioxidant activity determination by using spectrophotometry. The cytotoxicity test was carried out by Sulforhodamine B method. LC-MS was applied for predicting components. We isolated the Fusarium oxysporum F01 strain originated from Catharanthus roseus. The aqueous extracts showed inhibition against Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853 (15.00 ± 0.50 mm), Serratia marcescens ATCC 14756 (9.00 ± 0.87 mm), Vibrio parahaemolyticus ATCC 17802 (15.50 ± 0.87 mm), Escherichia coli ATCC 25922 (12.50 ± 0.50 mm). The antioxidant activity of the extract obtained from the supernatant was determined with an IC50 value of about 11 µg/mL. The extract also showed cytotoxicity effect on liver cancer cell line (HepG2) and breast cancer cell line (MCF-7) with the percentage of inhibition of 84.47 ± 3.18 and 94.69 ± 1.59, respectively. LC-MS was used to point out the presence of pratol, a melanogenesis agent. The study provided more interesting information about Fusarium oxysporum F01 isolated in Catharanthus roseus grown in Vietnam, contributing to pharmaceutical sources in the world.
Fusarium oxysporum F01, Catharanthus roseus, antimicrobial and antioxidant activity, cytotoxicity, LC-MS
Microorganisms produced many biological compounds such as bacteriocin, conjugated linoleic acid, gamma buryric acid, lactic acid from lactic acid bacteria1-4, prodigiosin production from Streptomyces coelicolor5, penicillin from fungi6. Defined as an organism growing, existing within the plant causing no harm to its host, the endophyte is known to generate no apparent symptoms of disease and some of natural plant compounds7. Endophytic fungi are symbiotically associated with their host plant, mimic their chemistry and synthesize the same natural products and bioactive compounds as these originated from their host, and thus are screened for the production of valuable compounds. There were Taxol and taxane production by endophytic Taxomyces andreanae isolated from pacific yew8. Endophytic Cladosporium from Boerhaavia diffusa Linn showed in-vitro antioxidant activity9.
There were many plants showing biological activities such as Plumbago indica L.10, Alpinia conchigera Griff11, Achillea millefolium Linn12, Murraya paniculata (L.) Bark13, Strobilanthes kunthianus14 and others. Catharanthus roseus or Vinca rosea, which is also commonly known as the Madagascar periwinkle or rose periwinkle is a plant species belongs to Apocynaceae family. It is used as an ornamental as well as medicinal plant. Catharanthus roseus is well known for production of copper nanoparticle15, antibacterial activities16, anti-cancerous agents such as vinca alkaloids which have effect on pain-relieving or contain anticancer properties. Vinblastine and vincristine of Catharanthus roseus have been developed and applied in anticancer drugs as prescriptions17. As a matter of fact, an endophyte called Fusarium oxysporum of rosy periwinkle plant, Catharanthus roseus was discovered to produce vincristine – an anticancer drug18-20. In term of Fusarium oxysporum, an ascomycete fungus of Nectriaceae family including many species that was discovered by Wollenweber and Reinking21. Fusarium oxysporum, which is dominant in active soil, can be harmless to plants or be a pathogenic to its host. In contrast, it is beneficial in form of endophytes – having a mutual relationship to plants, for example, some of these bioactive compounds have been found and extracted vincristine 18-20. Moreover, antibiotic resistance in microorganisms is increasing with a high rate nowadays such as Salmonella sp.22, Escherichia coli and Klebsiella23, Staphylococcus aureus24. Additionally, antioxidant activities that can help human in preventing some diseases were studied much on plant extracts25-28 whereas microbial extracts were not investigated in both the depth and width.
Therefore, the study isolated Fusarium oxysprorum 01 from Catharanthus roseus collected in Binh Thuan (Vietnam). The strain was used to prove an excellent endophyte for many valuable substances. The antimicrobial assay was carried out to present the power of Fusarium oxysporum F01 isolated from Catharanthus roseus against Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Serratia marcescens ATCC 14756, Vibrio parahaemolyticus ATCC 17802, Escherichia coli ATCC 25922 when using agar diffusion method and screened antioxidant activity and cytotoxicity. Moreover, the production of valuable compounds in a specific amount was also screened by liquid chromatography-mass spectrum (LC-MS). The results contributed to the evidence of endophytic fungi showing activities when having a mutual life with medicinal herbs.
Specimens
Catharanthus roseus trunk was collected in Binh Thuan (Vietnam).
Pathogenic bacteria including Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Serratia marcescens ATCC 14756, Vibrio parahaemolyticus ATCC 17802, Escherichia coli ATCC 25922 were purchased from American typed culture Collection (USA).
Cancer cell lines (MCF-7 and Hep-G2) were purchased from Sigma (USA).
Chemicals and reagents
Culture media and medium components for media including Potato dextrose broth (PDB), Potato dextrose agar (PDA), Luria-Bertani (LB) broth, Yeast Extract, Malt Extract were purchased from Bio Basic (Canada).
1,1-diphenyl-2-picrylhydrazyl (DPPH) and Sulforhodamine B (SRB) were purchased from Sigma (USA).
Antibiotics were purchased from Institute of Drug Quality Control (Ho Chi Minh City, Vietnam).
DNA isolation kit was purchased from Qiagen (Germany).
Solvents were purchased from Merck (USA).
Other analytical chemicals were purchased from Thermo Fisher Scientific (USA).
Isolation and Identification
The trunk (100g) of Catharanthus roseus collected in Binh Thuan (Vietnam). Immediately, it was cut into small pieces, cultured in potato dextrose broth (PDB) in 5 days. The culture was spread on potato dextrose agar (PDA) to select the single colonies with mycelium. The colony was used to culture in PDB in 5 days for DNA extraction using DNA extraction kit. After checking the purity and concentration of DNA, DNA was used for sequencing. The sequence was compared to well-known strains in gene bank using Blast search29. The sequence was aligned with the related sequence and reconstructed phylogenetic tree using the function “build” of the Environment for Tree Exploration (ETE3) v3.1.130 as implemented on the GenomeNet. ML tree was inferred using PhyML v20160115 ran with model and parameters according to Guidon et al31.
Cultivation for antimicrobial activity
Fusarium oxysporum F01 isolated from Catharanthus roseus in Vietnam was cultured in modified yeast extract- malt extract (YEME) as medium for the fermentation process in 14 days old cultures. The culture of Fusarium oxysporum F01 were centrifuged at 10,000 rpm for 10 minutes at 25°C to separate the mycelia from the culture broth as pellet and supernatant. The supernatant was concentrated and fractionated with distilled water in an appropriate proportion for further analysis.
For the preparation crude pellet extract, the pellet (1 g) was sonicated in water before centrifugation. The crude pellet extract was collected for furthur analysis.
Antimicrobial activity test
Pathogenic organisms for testing were Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Serratia marcescens ATCC 14756, Vibrio parahaemolyticus ATCC 17802, Escherichia coli ATCC 25922. From stock, the pathogens were inoculated and cultured in LB broth until OD600 of 0.5 was achieved for further tests. Antibiotics including ampicillin, ciprofloxacin, gentamycin were used as control.
Agar well diffusion technique was used to evaluate the antimicrobial activity of aqueous extracts. As described by previous study32, the LB agar plate was inoculated by spreading 20 μL of pathogen culture onto the agar plate by sterile swabs, then four 5-mm diameter holes were aseptically punched by sterile tips and then 80 μL extracts from supernatant and intracellular extract obt from pellet extracts was introduced to the wells. Antibiotics were used as positive controls whereas the negative control was modified YEME. The agar plates were incubated under 37°C and such antimicrobial agents diffused into the agar and inhibit the growth of microbes. After 10 – 12 hour incubation, the inhibition zones around the wells were measured. Table 1 showed the reference and the concentration of antibiotics corresponding to type of organisms.
Table (1):
Organisms with corresponding positive controls.
Test organisms |
Positive control |
Concentration (µg/mL) |
|---|---|---|
Staphylococcus aureus |
Gentamycin |
64 |
Pseudomonas aeruginosa |
Ciprofloxacin |
2 |
Serratia marcescens |
Ciprofloxacin |
4 |
Vibrio parahaemolyticus |
Ampicillin |
32 |
Escherichia coli |
Ampicillin |
128 |
Similarly, the SE samples were used to screen for antimicrobial activity. The antimicrobial compounds diffused into the agar and inhibited microbial growth. Consequently, the inhibition growth zones were measured.
Antioxidant by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging test. The radical scavenging activity of different concentration of fungal crude extracts was determined by using DPPH assay33. DPPH (1,1-diphenyl-2-picrylhydrazyl) solution was prepared by dissolving DPPH with methanol. The DPPH solution was kept out of light. The blank was set up with 189 µL of methanol along with 9 µL of DPPH. Ascorbic acid was used as standard sample which was prepared by diluting stock solution with distilled water into different concentrations (1, 2, 3, 4, 5, 6 µg/mL). The working solution of extracts was prepared with different concentrations (1, 2.5, 5, 7.5, 10, 12.5 µg/mL) with distilled water. Then, 9 µL DPPH solution was mixed with 180 µL of standard solution or diluted extracts. All mixtures were incubated in the dark at room temperature for 30 minutes. The absorbance of the mixture was then recorded at 517 nm. The experiment was triplicate.
Cytotoxicity test
Sulforhodamine B (SRB) assay was a colorimetric method to determine sensitivity and cytotoxicity of a substance34. The anionic dye binds electrostatically with the positive-charged parts of the proteins. The amount of binding dye correlates with the number of total cellular proteins. The cells were cultured to attain 70-80% coverage of culture flasks and passed into 96-well plate with a density of 104 cells per well. The plates were incubated at 37°C in 5% CO2 for 24 hours. Next, the medium of sample was added into the wells and incubated at 37°C in 5% CO2 for 48 hours. Then, the cells were fixed with trichloroacetic acid (TCA) and stained with SRB (0.2%) and left at room temperature (25°C) for 5 to 20 minutes. After SRB was discarded, the cells were washed gently with acetic acid (1%) for four times. Let the cells air-dry at room temperature (25°C) from 12 to 24 hours. The results were obtained by loading 200 μL tris base into each well, then shaking for 10 minutes until dissolved completely for measuring the optical density (OD) at 492 nm and 620 nm. In the test, the positive control was camptothecin (0.07 µg/mL) and the negative control was DMEM (0.25%).
Liquid Chromatography – Mass Spectroscopy (LC-MS)
The predicted compounds containing in samples were determined by LC-MS. Begin with the ionization, the ions were separated according to their mass to charge (m/z) ratio; followed by the transfer of ions to mass analyzer. Then, the spectra were recorded in the positive ionization mode.
Statistical analysis
In antimicrobial assay, data were reported and presented as mean ± standard deviation. The statistic was analyzed by SPSS software and Microsoft Excel, especially Two-way ANOVA was used for checking significant difference between two factors: stains and treatments; then Tukey test was applied as Post Hoc test for multiple comparisons of groups on treatment factor.
Identification of fungi
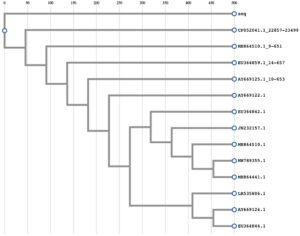
By using Blast search, the strain had 100% identity to Fusarium oxysporum strain F-H.6.5-030318-02 by comparing its 18S, 5.8S, 28S ribosomal RNA sequences to partial gene of 18S ribosomal RNA at the internal transcribed spacer 1, and complete sequence of 5.8S ribosomal RNA gene with the internal transcribed spacer 2. The sequence was deposited in DNA Data Bank of Japan (DDBJ) with accession number (LC645215). The sequence was aligned with the sequences of other Fusarium oxysporum strains (Fig. 1). Moreover, after phylogeny analysis using neighbor joining evaluation, the strain was closed to Fusarium oxysporum strains (Fig. 2). As a result, Fusarium oxysporum 01 originated from Catharanthus roseus was identified.
Fig. 1. Multiple sequence alignment. (EU364859.1:14-657: Fusarium oxysporum strain F-X.1.7-030520-12; AY669122.1: Fusarium oxysporum strain F-W.6.2-030304; AY669125.1:10-653: Fusarium oxysporum strain F-X.1.7-030520-12; EU364842.1: Fusarium oxysporum strain F-H.6.5-030318-02; JN232157.1: Fusarium oxysporum isolate 152; LR535806.1: Fusarium oxysporum 247; EU364844.1: Fusarium oxysporum strain F-H.6.5-030318-01; CP052041.1:22857-23499: Fusarium oxysporum Fo47 chromosome IV; MH864510.1: Fusarium oxysporum strain CBS 127297; MH864441.1: Fusarium oxysporum strain CBS 127149; MH864510.1:9-651: Fusarium oxysporum strain CBS 127297; AY669124.1: Fusarium oxysporum strain F-H.6.5-030318-J1; MW789355.1: Fusarium oxysporum isolate F3-2018; EU364842.1: Fusarium oxysporum strain F-H.6.5-030318-02; JN232157.1: Fusarium oxysporum isolate 152; AY669122.1: Fusarium oxysporum strain F-W.6.2-030304; LR535806.1: Fusarium oxysporum 247; seq: the sequence for isolated strain).
Fig. 2. Phylogeny analysis. (EU364859.1:14-657: Fusarium oxysporum strain F-X.1.7-030520-12; AY669122.1: Fusarium oxysporum strain F-W.6.2-030304; AY669125.1:10-653: Fusarium oxysporum strain F-X.1.7-030520-12; EU364842.1: Fusarium oxysporum strain F-H.6.5-030318-02; JN232157.1: Fusarium oxysporum isolate 152; LR535806.1: Fusarium oxysporum 247; EU364844.1: Fusarium oxysporum strain F-H.6.5-030318-01; CP052041.1:22857-23499: Fusarium oxysporum Fo47 chromosome IV; MH864510.1: Fusarium oxysporum strain CBS 127297; MH864441.1: Fusarium oxysporum strain CBS 127149; MH864510.1:9-651: Fusarium oxysporum strain CBS 127297; AY669124.1: Fusarium oxysporum strain F-H.6.5-030318-J1; MW789355.1: Fusarium oxysporum isolate F3-2018; EU364842.1: Fusarium oxysporum strain F-H.6.5-030318-02; JN232157.1: Fusarium oxysporum isolate 152; AY669122.1: Fusarium oxysporum strain F-W.6.2-030304; LR535806.1: Fusarium oxysporum 247; seq: the sequence for isolated strain).
Antimicrobial activity
The antimicrobial activity of Fusarium oxysporum F01 on pathogens was demonstrated in Table 2. As shown in Table 2, the extract collected from supernatant of culture had effect on five organisms. The extract had the strongest inhibitory effect on Staphylococcus aureus (17.8 ± 0.58 mm) than Vibrio parahaemolyticus (15.50 ± 0.8 mm) and Serratia marcescens (9.00 ± 0.87 mm) and Escherichia coli (12.50 ± 0.50 mm) Pseudomonas aeruginosa (15.00 ± 0.50 mm). Specially, when the effect against Staphylococcus aureus was compared to Gentamycin (64 µg/mL), the extract gave inhibition zone that was not significant to gentamycin by statistical analysis. This fungus could be an ideal source of antimicrobial agent with high potency in Staphylococcus aureus treatment while Staphylococcus aureus is resistant to antibiotic highly.
Table (2):
Inhibition zone diameter of water extracts to organisms.
Test organisms |
Positive control |
Aqueous extract |
|---|---|---|
Staphylococcus aureus |
18.50 ± 0.50 |
17.83 ± 0.58 |
Pseudomonas aeruginosa |
30.33 ± 0.58 |
15.00 ± 0.50 |
Serratia marcescens |
40.33 ± 0.29 |
9.00 ± 0.87 |
Vibrio parahaemolyticus |
44.50 ± 0.50 |
15.50 ± 0.87 |
Escherichia coli |
22.33 ± 0.58 |
12.50 ± 0.50 |
For Pseudomonas aeruginosa, Serratia marcescens, Vibrio parahaemolyticus and Escherichia coli, the activities against these strains were not as strong as antibiotics used as control samples. However, the results showed the extract could contain many potential agents. To improve the activity, the concentration of extract should be increase. Moreover, all the extracts obtained from pellet did not show antimicrobial activity. To predict which compound in the pellet originated extract, LC-MS was presented in Fig. 3. The molecular mass ([M+ H]+) was 269.0748. The detection showed pratol in the extract. Pratol is an melanogenesis35. Therefore, the pellet extract did not show activities tested in the study.
Antioxidant by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging
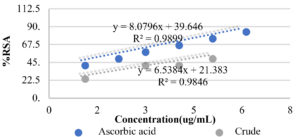
The evaluation of the antioxidant activity of the extract was determined by measuring its scavenging ability against DPPH in the stable radical assay. Based on the curve of ascorbic acid (y = 8.0796x + 39.646, r² = 0.9899), the antioxidant activities of the extract prepared with different concentrations were determined. All the inhibitory percentage in antioxidant assay built up the straight curve (y = 8.0796x + 39.646, r² = 0.9899) (Fig. 4). It meant that the obtained results were relative and reliable. The IC50 of crude extract is 11.03 µg/mL that was higher than ascorbic acid (IC50 = 8 µg/mL). The crude extract showed the weaker antioxidant activity than ascorbic acid.
Cytotoxicity activities
The samples were prepared at the concentration of 50 mg/mL for cytotoxicity test on MCF-7 and Hep-G2 cells. All the samples had cytotoxicity on these cell line (Table 3). In the study, we couldn’t detect vincristine and vinblastine in Fusarium oxysporum F01 by LC-MS. The cytotoxicity was caused by others compounds in Fusarium oxysporum F01.
Table (3):
Cytotoxicity (% inhibition) of the crude extract Fusarium oxysporum.
Cancer cell lines |
Fungal extract |
Camptothecin (Positive control) |
DMEM medium (Negative control) |
|---|---|---|---|
Hep G2 |
84.47 ± 3.18 |
98.15 ± 2.05 |
0 |
MCF-7 |
94.69 ± 1.59 |
97.62 ± 2.80 |
0 |
The biological activities were due to this strain had symbiotic effects with Catharanthus roseus which had well-known activities. Fusarium oxysporum originated from environment commonly causes diseases, however, Fusarium oxysporum F01 showed biological activities such as antimicrobial and antioxidant activities together with cytotoxicity. The study brings out the importance of microoganisms showing pathogenic or beneficial effects depending on their host.
BLAST search is the tool for comparing biological sequence information. A BLAST search help to identify database sequences which are similarity to the unknown sequence. In the study, Fusarium oxysporum F01 had partial sequence of 18S, 5.8S and 28S rRNA with 100% identity to the gene information of strain F-H.6.5-030318-02. the reconstruction, analysis, and visualization of phylogenetic trees and multiple sequence alignments were analyzed simplify by ETE that supports in comparative genomics and phylogenetics. In the combination of phylogeny analysis, Fusarium oxysporum F01 originated from Catharanthus roseus was identified obviously.
Table 2 showed the antimicrobial activity of Fusarium oxysporum F01 extract collected from supernatant of culture on five organisms. The extract showed inhibition against the growth of Staphylococcus aureus, Vibrio parahaemolyticus, Serratia marcescens and Escherichia coli and Pseudomonas aeruginosa in different manners, leading to the different effects. Interestingly, the potency of the extract was not significant to gentamycin (64 µg/mL) by statistical analysis when inhibited Staphylococcus aureus growth. Therefore. Exploiting this strain metabolites to treat these bacteria is important to inhibit the tested bacteria that had risks of resistance such as Staphylococcus aureus. The extract obtained from cultural supernatant showed the weaker cytotoxicity activity than ascorbic acid due to it contained the mixture of antioxidant agents and non-antioxidant agents that interfere the activity. However, the antioxidant agent existed in the extract after antioxidant activity screening. Moreover, the extract at the concentration of 50 mg/mL for cytotoxicity test on MCF-7 and Hep-G2 cells. By the comparison to the controls, the extract gave the significantly lower activity than camptothecin on HepG2, but insignificant difference from camptothecin on MCF-7. In the study, we couldn’t detect vincristine and vinblastine in Fusarium oxysporum F01 by LC-MS analysis due to low concentration or the unsuitable cultivation. The cytotoxicity was caused by others compounds in Fusarium oxysporum F01. The pellet extract could not show strong antimicrobial and antioxidant activities in the study. However, the pellet extract contained pratol that is a melanogenesis. Obviously, Fusarium oxysporum F01 isolated from Catharanthus roseus showed the benefit activities, strengthening that symbiotic endophyte could produce useful activities when it may be harmful when living in the other sources.
Extract from cultural supernatant of Fusarium oxysporum F01 had inhibitory activities against Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Serratia marcescens ATCC 14756, Vibrio parahaemolyticus ATCC 17802, Escherichia coli ATCC 25922. Fusarium oxysporum F01 will contribute to develop antimicrobial activities against Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Vibrio parahaemolyticus and Escherichia coli when these strains were resistant to antibiotics besides antioxidant and cytotoxicity activities. The pellet extract was screened to predict pratol used for melanogenesis prevention although it did not show antimicrobial and antioxidant activities. The mechanisms and purified compounds will be exploited to understand well and apply for future.
ACKNOWLEDGMENTS
We would like to thank to Vietnam National University, Ho Chi Minh City (VNU-HCM) for the support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NHKT proposal investigator, ideas, design experiment. NHKT, NTTT, DTTV and HDL performed the experiment. NHKT, NTTT, DTTV and HDL performed the data analysis. NPQA design figures and tables. NHKT, NTTT and NPQA wrote the manuscript. All authors read and approved the manuscript for final publication.
FUNDING
This research is funded by Vietnam National University, Ho Chi Minh City (VNU-HCM) under grant number C2019-28-02.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
- Nghe D, Nguyen T. Characterization of antimicrobial activities of Pediococcus pentosaceus Vtcc-B-601. J Appl Pharm Sci. 2014;4(5):61-64.
Crossref - Anh NPQ, Yen TTH, Vinh DTT, Tien TP, Dung NA, Khue NH. Study on the ability of producing of conjugated Linoleic acid of Lactobacillus fermentum A01 isolated from human digestive tract. Res J Pharm Technol. 2021;14(3):1319-1322.
Crossref - Thien Phuc C, Thanh Vinh DT, Khoa My T, et al. Study on conditions for Gama buryric acid (GABA) production in Lactobacillus fermentum A01 isolated from human. Res J Pharm Technol. 2021;14(4):2188-2190.
Crossref - Nguyen HHH, Nguyen DA, Nguyen TKH. Biological activities of poly (Lactic acid) polymer produced from Lactobacillus rhamnosus PN04. Res J Pharm Technol. 2018;11(7):3057-3062.
Crossref - Thu TTM, Vinh DTT, Dung NA, Tu NHK. Effect of lactic acid produced by lactic acid bacteria on prodigiosin production from streptomyces coelicolor. Res J Pharm Technol. 2021;14(4):1953-1956.
Crossref - Vo G, Nguyen D, Nguyen T. Improvement of antibiotic production in fungi. Res J Pharm Technol. 2018;11(7):3227-3233.
Crossref - Prerana V, Kilingar NV. Enhanced production of an Anti-Cancer pigment from Bacillus endophyticus JUPR15: Single factor system Vs RSM. Res J Pharm Technol. 2021;14(1):153-161.
Crossref - Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260(5105):214-216.
Crossref - Sundar RDV, Anitha KPGU, Arunachalam S. In vitro Antioxidant and Phytochemical analysis of crude extracts of endophytic fungi (Cladosporium sp) from Boerhaavia diffusa Linn. Res J Pharm Technol. 2021;14(1):202-206.
Crossref - Dibyajyoti S, Swati P. Cytotoxic Activity of Methanolic Extract of Plumbago indica L. (Family: Plumbaginaceae). Asian J Pharm Tech. 2012;2(2):59-61
- Dibyajyoti S, Swati P. Cytotoxic Activity of Methanolic Extract of Alpinia conchigera Griff (Family: Zingiberaceae). Asian J Pharm Res. 2012;2(2):86-88.
- Subramanian G, Meyyanathan SN, Gowramma B, Palanisamy DS. Liquid chromatography-mass spectrometric method for simultaneous estimation of apigenin and luteolin from Achillea millefolium Linn. Asian J Res Chem. 2016;9(12):629-632.
Crossref - Nowaz RN, Mostafizur MR, Farhad MH. Free Radical Scavenging Activity and Cytotoxic Potential of Crude Extractives of Murraya paniculata (L.) Bark. Res J Pharmacogn Phytochem. 2012;4(1):18-22
- Balasubramaniam G, Sekar M, Badami S. In-vitro Antioxidant and Cytotoxic properties of Strobilanthes kunthianus. Res J Pharm Technol. 2021;14(5):2522-2528.
Crossref - Chauhan R, Patel C, Panigrahi J. Greener approach for copper nanoparticles synthesis from Catharanthus roseus and Azadirachta indica leaf extract and their antibacterial and antioxidant activities. Asian J Res Pharm Sci. 2018;8(2):81-90.
Crossref - Safhi MM, Ali M, Sivakumar SM, Jabeen A, Manohar YN. Antibacterial studies of catharanthus roseus of jazan province against the selected bacterial strains. Res J Pharm Technol. 2013;6(4):403-405
- Zhang LB, Gou LH, Zeng SV. Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Chin Tradit Herb Drugs. 2000;31(11):805-807
- Tung CY, Yang DB, Gou M. A Preliminary study on the condition of the culture an isolate of endophytic fungus producing vincristine. J Chuxiong Norm Univ. 2002;6:39-41
- Kour A, Shawl AS, Rehman S, et al. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J Microbiol Biotechnol. 2008;24(7):1115-1121.
Crossref - Shams KA, Nazif NM, Abdel Azim NS, et al. Isolation and characterization of antineoplastic alkaloids from Catharanthus roseus L. Don. cultivated in Egypt. African J Tradit Complement Altern Med. 2009;6(2):118-122.
Crossref - Wollenweber HW, Reinking OA. Die Fusarien, ihre Beschreibung, Schadwirkung und Bekämpfung. Paul Parey;1935
- Kanth SV, Aparna M, Sumit S, Jabez OW. Isolation and characterization of drug resistant Salmonella typhi from sewage water. Res J Pharm Technol. 2015;8(2):167-171.
Crossref - Abbas HA, Kadry AA, Shaker GH, Goda RM. Resistance of Escherichia coli and Klebsiella pneumoniae isolated from different Sources to β-lactam Antibiotics. Res J Pharm Technol. 2017;10(2):589-591.
Crossref - Bharadwaj, Gopinath P. Detection of inducible clindamycin resistance among clinical isolates of Staphylococcus aureus. Res J Pharm Technol. 2018;11(9):3990-3992.
Crossref - Sarkar D, Debnath S, Bose SK. Phytochemical analysis and assessment of Antioxidant properties of black tea extract obtained from Camellia sinensis. Res J Pharm Technol. 2020;13(10):4539-4544.
Crossref - Keshri V, Dutt KR. Inhibitory effect of phenolic and flavonoidal content of H. indicum root extract on 1, 1-diphenyl-2-picrylhydrazyl radicals. Res J Pharm Technol. 2021;14(1):235-238.
Crossref - Mohamed SH, Mohamed WS, Shaheen MNF, Elmahdy EM, Mabrouk MI. Cytotoxicity, Antibiotic Combination and Antiviral Activity of Papain Enzyme: In vitro study. Asian J Res Pharm Sci. 2020;10(1):6-10.
Crossref - Soni A, Femida P, Sharma P. In-vitro cytotoxic activity of plant saponin extracts on breast cancer cell-line. Res J Pharmacogn Phytochem. 2017;9(1):17-22.
Crossref - Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389-3402.
Crossref - Huerta-Cepas J, Serra F, Bork P. ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Mol Biol Evol. 2016;33(6):1635-1638.
Crossref - Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307-321.
Crossref - Rajesh T, Venkatanagaraju E, Goli D, Basha SJ. Evaluation of antimicrobial activity of different herbal plant extracts. Int J Pharm Sci Res. 2014;5(4):1460-1468.
Crossref - Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Technol. 1995;28(1):25-30.
Crossref - Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107-1112.
Crossref - Chung YC, Kim S, Kim JH, et al. Pratol, an O-methylated flavone, induces melanogenesis in B16F10 melanoma cells via p-p38 and p-JNK upregulation. Molecules. 2017;22(10):1704.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.