ISSN: 0973-7510
E-ISSN: 2581-690X
As sugarcane molasses is converted into ethanol, a sizable volume of effluent with high biochemical oxygen demand (BOD) and chemical oxygen demand(COD) is generated. This effluent contains melanoidin. Melanodin is a chemical broken down by certain bacteria and can produce peroxidases, phenoxidases, laccases, and mono- and dioxygenases. The primary function of these bacteria is to break down complex hydrocarbons, including aromatics such as coloring pigments. This study aimed to identify melanoidin-decolorizing microorganisms in natural resources that are thermally resistant and have the potential to be used in industrial-scale distillery treatment for effluent applications. A total of 55 distinct isolates were tested on a solid medium, including molasses pigments. Three thermotolerant bacterial isolates were identified as melanoidin-decolorizing agents: Bacillus nitratireducens (B2), Bacillus paramycoides (B1), and Brucellatritici (B3). These isolates under went additional optimization for decolorization under various physicochemical and nutritional conditions. At 40°C, B. nitratireducens (B2) exhibited the highest degree of decolorization (86%) among the three species while using 0.5% glucose(w/v), 0.5% peptone(w/v), 0.05% MgSO4, and 0.01% KH2PO4 at a pH of 6.0 over 40 h of incubation under static conditions. In submerged fermentation, the B2 strain of B.nitratireducens can withstand higher temperatures and requires only a small amount of carbon (0.5%, [w/v]) and nitrogen sources (0.5%, [w/v]). Therefore, it is feasible to use melanoidin on an industrial scale to decolorize distilled effluents.
Melanoidin, Spent Wash, Distillery Effluent, Physico-chemical
Molasses, bagasse, and fiber cake are by-products of the sugar industry; however, molasses is the most valuable. Molasses is highly valuable in practical applications because it can be used as an animal feed, biofertilizer, and in various fermentation processes.1,2 Molasses comprises approximately 48–50% sucrose. When molasses is used as the primary ingredient in ethanol production, spent wash is produced. This spent wash is dark brown in color and has a high biochemical oxygen demand (BOD) and chemical oxygen demand (COD), a low pH, and contains potentially dangerous chemicals such as phenols.2,3 Therefore, spent wash must undergo pretreatment before it can be safely disposed of in the environment because the dark hue is a problem. The main obstacle to waste remediation is the color of the distillery spent wash (DS), which includes approximately 2% by weight melanoidin pigment, a dark brown refractory pigment.1 By combining the carbonyl and amino groups of organic materials, the “Millard reaction” produces melanoidin, a naturally occurring browning polymer,4,5 and it has a strong relationship with the humic substances present in the natural world.
Because less sunlight can enter rivers, lakes, or lagoons due to the release of toxic distillery spent wash into the environment, aquatic life is harmed. This, in turn, has a negative impact on photosynthetic activity, and dissolved oxygen can be observed in these bodies of water.1 Dumping on land causes comparable harm because it decreases the pH and raises manganese (Mn) availability in the soil, inhibiting seed germination and plant development.6 Studies have examined several physicochemical and biological approaches for decolorizing distillery spent wash. Melanoidin can be removed using physicochemical treatments; however, these approaches require high reagent doses and create substantial sludge.7,8 Physical and chemical therapies can be used to eliminate melanoidin. Because of their low cost, ecological safety, and well-accepted treatment, biological techniques provide a promising option for the decolorization and bioremediation of DS and a less costly alternative to chemical-based breakdown procedures.9,8
Biological approaches offer promising options for decolorization and bioremediation of spent wash. Due to these benefits, biological procedures, such as bioadsorption and bio-degradation, provide a treatment alternative that is appealing and affordable when compared to chemical breakdown methods for the process of removing color from spent wash.10-15 Bacteria, fungi, yeasts, and cyanobacteria are examples of the numerous types of aerobic microorganisms that decolorize spent wash.16 Certain bacterial strains obtained from sewage and subsequently adapted to increase the concentration of distillery waste can reduce COD by 80% in approximately 4–5d without aeration. CO2, volatile acids, and biomass are the main byproducts following the breakdown process. Based on a previous17 investigation, raw molasses spent wash was more easily decolored by the marine fungus Flavodonflavus than by molasses wastewater that had undergone either anaerobic or aerobic treatment. The yeast Issatchenkia orientalis was isolated18 from fruit samples. After 1 week at 30°C, it was discovered that the yeast can decolorize melanoidin by 60% under aerobic conditions.
In this study, we aimed to isolate natural strains with a high capacity to develop at higher temperatures without the need for simple sugars. In addition, a large percentage of melanoidin losing its color was observed and recorded.
Distillery spent wash (DSW), which included molasses, was obtained from the Masodha distillery in Ayodhya, India, where it was stored in a sterile environment and included molasses. After removing the suspended particles from the spent wash through centrifuging at 10,000 rpm for 15 min before use, the spent wash was stored at 4°C.1 After filtration through Whatman No. 1 filter paper, the spent wash from the distillery was diluted in distilled water. Based on a study,19 numerous physico-chemical parameters, such as color, odor, BOD, COD, total dissolved solids (TDS), pH, total sugar, phosphorous, sulfates, and calcium, were analyzed, and standard methods were used for the assessment of water and wastewater.
Isolation, screening, and identification of melanoidin-decolorizing bacteria
Soil samples were collected from the Masodha Sugarcane Distillery in Faizabad, India. Melanoidin-decolorizing bacteria were identified and cultured from these samples. These bacteria were cultivated on GPYE medium for 24–48 h. The original pH was changed to 6.0, and the culture medium included 0.05% MgSO4.12H2O, 0.01% KH2PO4, 0.1% yeast extract, and 0.5% glucose. The medium had an optical density (OD) of 3.5 at 475 nm, and the effluent had an OD of 3.5 at 475 nm. Agar-based Petri dishes were used for 103–106 serial dilutions of 1 g of soil to identify molasses-decolorizing bacteria. This procedure is repeated several times. The plates were then allowed to rest in an incubator for 24–48 h at 35 ± 2°C and 45 ± 2°C. The impact of decolorization was not able after 24–48 h of incubation. Isolates that showed a greater degree of melanoidin decolorization were selected for further study. After 15 d of cultivation on the same medium in slants at 4°C, these isolates were transferred to a new culture. CytoGene Laboratories in Lucknow, India, identified both species and genera in the cultures.
Inoculum preparation
To create a cell suspension, 1 mL of the culture that had been cultivated for 24 h was inoculated into 50 mL of basal broth. Once the active exponential phase of the culture was reached, the inoculation was heated to 35°C for 24 h. Then, 5 x 10 culture-forming units (CFU)/mL were placed in a conical flask and incubated under steady conditions. By comparing the OD readings obtained at 475 nm with a reading recorded with the instrument in blank mode, an ultraviolet (UV)spectrophotometer was used to calculate the quantitative decolorization value.
Decolorization assay
The baseline broth medium was incubated with the melanoidin-decolorizing microbial isolates for the required period, then centrifuged at 10,000 rpm for 10 min. After centrifugation, the absorbance of the supernatant was measured at a wavelength matching the maximum melanoidin absorbance (Amax).10 The decolorization yield is described as the percentage reduction in the absorbance measured at 475 nm compared to the absorbance measured at the same wavelength at the beginning of the process. A medium that was not infected served as a control. The complete test was performed three times, and the results were compared with those of the control group. The following equation was used to represent the degree to which each isolate successfully removed color from the sample:
% Decolorization = [ Initial absorbance -Final absorbance / Initial absorbance ] x 100
Optimization of decolorization conditions
Physical parameter selection for melanoidin decolourization
A basal medium was selected for melanoidin decolorization. The temperatures used for the process were 25, 30, 35, 40, 45, and 50°C, and the incubation periods were 8, 16, 24, 32, 40, and 48h. The pH of the medium, which was initially 6.0, was changed to a value that was either higher or lower, depending on the circumstances, by adding either 1N NaOH or 1N HCl. Subsequently, the basal medium was inoculated with a 0.5% (v/v) inoculum of microbial isolates with a population of 5 x 10 CFU/mL and incubated at different pH values (4.0, 5.0, 6.0, and 7.0) to optimize melanoidin decolorization.
Selection of nutritional parameter for melanoidin decolorization
Fructose, glucose, sucrose, lactose, and other carbon sources were individually added to the base medium at a concentration of 0.5% (w/v). After inoculation with 0.5% (v/v) microbial cultures alone, the medium was incubated for 24 h at the respective optimal temperatures and pH levels for decolorization. The best sugar source was evaluated at concentrations of 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6% (w/v) to further optimize melanoidin decolorization.
In the second experiment, various organic and inorganic nitrogen sources were added to the basic medium at a concentration of 0.5% (w/v). The nitrogen sources included yeast extract, beef extract, ammonium sulfate, peptone, and ammonium nitrate. An inoculum at a concentration of 0.5% (v/v) and 5 x 10 CFU/mL was injected into a culture of actively developing individual bacteria. Subsequently, the most efficient nitrogen supply was optimized at several concentrations for melanoidin decolorization. The concentrations used were 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6% (w/v).
Statistical analysis
All experiments were performed in triplicate, representing the data as the average of three measurements. Using Microsoft Excel, the standard deviation (SD) of each trial outcome was determined. In addition, different factors such as pH, temperature, media composition, and incubation period are required to effectively decolorize melanoidin.
Several physicochemical parameters such as color, smell, pH, COD, BOD, TDS, total sugars, sulfates, phosphorus, and calcium were studied, and the results are shown in Table.
Table:
Physico-chemical characteristics of the distillery effluent
No. |
Parameters |
Characteristics of distillery waste (mg/L) |
|---|---|---|
1 |
Temperature (°C) |
82 °C |
2 |
Odor |
Similar to that of molasses |
3 |
Color |
Dark brown |
4 |
pH |
4.2 |
5 |
Alkalinity |
1360 |
6 |
Hardness |
2390 |
7 |
Total solids |
87900 |
8 |
Total dissolved solids |
81800 |
9 |
Total suspended solids |
5965 |
10 |
Dissolved oxygen |
0 |
11 |
BOD |
47000 |
12 |
COD |
104230 |
13 |
Total nitrogen |
1645 |
14 |
Ammonia nitrogen |
433 |
15 |
Nitrate nitrogen |
960 |
16 |
Phosphorus |
165 |
17 |
Potassium |
8770 |
18 |
Sodium |
215 |
19 |
Chloride |
4848 |
20 |
Calcium |
1819 |
21 |
Sulphate |
1738 |
Isolation, screening, and identification of melanoidin-decolorizing bacteria
Isolates from the soil of the Masodha distillery in Ayodhya that showed the potential to decolorize were isolated on basal agar medium using qualitative criteria. The colonies were cultivated on molasses agar at a pH of 6.0 for 24–48 h at a temperature of 40°C, and the isolates with colonies with a larger clear zone were selected. Isolates that were efficient at decolorization were determined to have a clear zone that was >1 cm in diameter and surrounded the colony (data not shown).
For the secondary screening, a quantitative approach was used using a melanoidin broth medium, including molasses wastewater diluted with distilled water to an OD of 3.5. The medium included 0.01%, 0.05%, MgSO4.12H2O; K2HPO4; 0.5%, 0.1%, yeast extract, and glucose with an initial plating level of 6.0. Each individual isolate was injected with 50 mL of medium in a 250-mL flask and incubated at 25 and 50°C for 48 h to identify thermotolerant melanoidin-decolorizing bacteria. Three bacterial isolates showed a greater degree of decolorization than the other bacterial isolates. These isolates of bacteria were investigated for increased decolorization under a variety of physicochemical and nutritional conditions. The three bacterial strains isolated from the cultures were identified as B. paramycoides (B1), B. nitratireducens (B2), and B. tritici (B3) by the CytoGene Lab in Lucknow, India.
Optimization of different physico-chemical and nutritional parameters for melanoidin decolorization
Effect of temperature on melanoidin decolorization
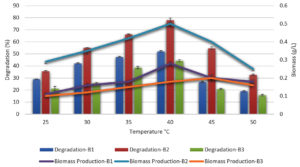
The effects of various temperatures on melanoidin decolorization by three unique bacterial strains at various physicochemical and nutritional levels were examined. The temperature ranged 35–50°C. When compared to that in B. paramycoides (B1) (53%), which showed the lowest thermotolerance at 40°C, and B. tritici (B3) (45%), which exhibited the lowest thermotolerance at 35°C, B. nitratireducens (B2)had the highest decolorization (78%) at 40°C, thus demonstrating the optimal capacity to tolerate high temperatures (Figure 1).
Effect of the incubation period on melanoidin decolorization
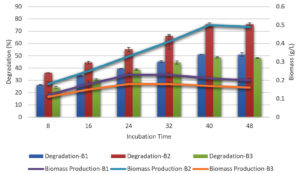
The incubation duration was set for decolorization shortly after the liquid medium temperature was optimized for melanoidin decolorization. The procedures were performed simultaneously. B. nitratireducens (B2) showed 76% decolorization of the sample after incubation for 40 h. No correlation exists between the incubation time and the degree of decolorization (Figure 2). B. paramycoides (B1) showed the lowest degree of decolorization (51%) after 40 h of incubation, whereas B. tritici (B3) exhibited the highest degree of decolorization (49%) after the same amount of time. As a result, B. nitratireducens (B2) had greater levels of decolorization when compared to that in the other strains.
Figure 2. Effect of different incubation periods with biomass production on melanoidin decolorization
Effect of pH on melanoidin decolorization
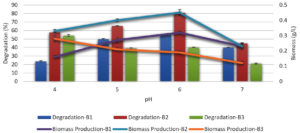
At the ideal temperature for bacterial growth and the length of incubation time required, a range of pH values (4.0–7.0) was tested for their ability to decolorize melanoidin. At pH 6.0, B. nitratireducens (B2)showed a higher degree of decolorization (81%). B. paramycoides (B1) exhibited the highest degree of decolorization (40%) at pH 6.0 (Figure 3), whereas B. tritici (B3) showed the lowest degree of decolorization (58%). Additionally, a change in the pH of the medium, either increasing or decreasing, had a diminishing effect on decolorization.
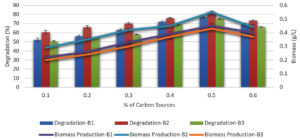
Effect of carbon sources on melanoidin decolorization
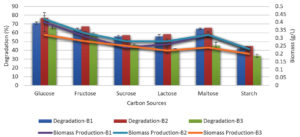
Different carbon sources such as fructose, sucrose, glucose, and lactose were evaluated individually at a concentration of 0.5 % in the basal medium at the optimal temperature, incubation duration, and pH to determine how they affected the ability of the bacteria to decolorize melanoidin.
Fructose and glucose were the two most efficient carbon sources for melanoidin decolorization. B. nitratireducens (B2) was observed to have higher levels of decolorization (78 %), whereas fructose and sucrose were observed to promote decolorization. B. paramycoides (B1) and B. tritici (B3) exhibited decolorization levels of 71 and 67 %, respectively, when glucose was observed (Figure 4).
Effect of glucose concentration on melanoidin decolorization
The ability of different glucose concentrations to decolorize melanoidin was examined separately from other factors using the same growth conditions used to evaluate carbon sources (ranging from 0.1–0.6% glucose in the medium) showed in Figure 5. B. nitratireducens (B2) decolorized 83% at a glucose concentration of 0.5%; however, B. paramycoides (B1) and B. tritici (B3) decolorized 78% and 76%, respectively, at the same glucose concentration.
Figure 5. Effect of different glucose concentrations with biomass production on melanoidin decolorization
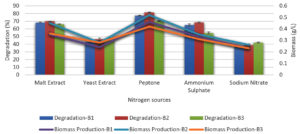
Effect of nitrogen sources on melanoidin decolorization
At a concentration of 0.5% in the basal media, the bacteria were able to decolorize melanoidin by inorganic and organic nitrogen sources such as yeast extract, beef extract, peptone, ammonium sulfate, and ammonium nitrate (Figure 6). This allowed the bacteria to break down the structure of the melanoidin molecule, resulting in the removal of melanoidin. The bacteria were able to decolorize approximately the same amount of melanoidin after the medium was modified with peptone; however, the decolorization percentage did not increase with other nitrogen sources. Peptone decolorized B. nitratireducens (B2) by 81%, whereas B.p aramycoides (B1) and B. tritici (B3) decolorized only 77% and 70%, respectively.
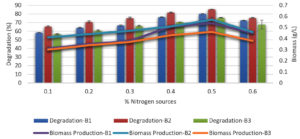
Effect of peptone concentration on melanoidin decolorization
Under the same growth conditions used for assessing nitrogen sources, various concentrations of peptone in the medium (0.1, 0.2, 0.3, 0.4, and 0.5%) were evaluated for their capacity to decolorize melanoidin. These concentrations ranged from 0.1 to 0.5%. B. nitratireducens (B2) demonstrated superior decolorization (86%) at 0.5% peptone compared to that in B. paramycoides (B1) and B. tritici (B3), which showed 80% and 76% decolorization, respectively, and decolorization decreased as the concentration increased, as did the biomass level (Figure 7).
B. nitratireducens (B2) was determined to be superior to B. paramycoides (B1) and B. tritici (B3) with regard to melanoidin decolorization. Different strains of microorganisms have varying degrees of melanoidin decolorization capacity. Melanoidin may break down quicker due to the constitutive and induced properties of certain microbial enzymes. The metabolic processes and regulatory systems of these microorganisms are diverse.
In this study, B. nitratireducens was able to function at temperatures of 35–40°C without impairing its exponential growth phase, which is considered to be the primary factor in increased melanoidin decolorization. Previous studies 20,21 reported that greater biomass could be obtained within 24–48 h with rapid decolorization at temperatures ranging from 25 to 40°C. Another research group22 reported a reduction in the decolorizing activity when the temperature was increased to 45°C. Thus, higher temperatures were hypothesized to kill cells or inactivate enzymes responsible for decolorization more effectively. Based on previous studies, the decolorization of melanoidin by certain bacteria is caused by the enzymatic activity of the bacteria, which includes oxidases, ligninases, and peroxidases. According to a previous study,8 the capacity of a bacterial consortium (Pseudomonas spp. and Proteus mirabilis) to decolorize was negatively impacted by an increase in temperature to >40°C, which was optimal at lower temperatures ranging 20–37°C. When the temperature was >45°C, the enzymatic activity of the microbes was impaired, similar to that of peroxidases. Therefore, it may be hypothesized that the temperature at which melanoidin is most effectively decolorized depends on the various microbial strains and their genetic diversity, as these strains have been isolated from a broad climatic setting.
Melanoidin decolorization by various microorganisms has been shown to occur within a short period of incubation with optimal growth.23-26 However, this procedure is more timely when conducted with filamentous fungi.27 Numerous bacteria thrive under submerged conditions. According to a previous study8, the progression of time during which effluent decolorization was researched paralleled the expansion of the consortium. At the peak of development, enzyme synthesis reaches its highest level, and these enzymes are responsible for the ability of the microorganism to bleach melanoidin. According to another study,28 maximum growth limits melanoidin decolorization because of the creation of additional metabolites or enzymes by microbes as a feedback inhibitory mechanism that occurs throughout the metabolic process. In this study, bacterial strains demonstrated maximal decolorization in a relatively short amount of time, namely 24–48 h of incubation, in comparison to fungi, which required a longer period.
According to a previous study,29 the enzymes created by microbes during the process of melanoidin decolorization are only effective in acidic environments. This was determined when melanoidin decolorization was tested at a pH ranging from 3.0 to 7.0. Based on studies,30,21 when the pH is high, melanoidin polymerizes and nutrients are utilized more effectively, decreasing color. The fact that considerable decolorization was achieved within an ideal range of 5.0–6.0 demonstrates that pH plays an important role in the process of color removal. In this study, bacteria decolorized most effectively at pH ranges of 6.0–7.0. When soil samples were used as the inoculum instead of isolated organisms, the results were in agreement.30,1,20
These findings have been published in three separate papers. Melanoidin decolorization was inhibited by enzyme synthesis at and below the optimal pH, leading to a lower rate of melanoidin decolorization. Because of the proteinaceous nature of enzymes, certain proteins are generally denatured at higher or lower pH values. However, microorganisms require a specific pH for the development and functioning of their enzymes.
In this study, both glucose and fructose as carbon sources resulted in the highest amount of melanoidin decolorization at a concentration of 0.5% glucose. However, 0.1% glucose displayed the same decolorization capability as 0.5% glucose. This effect may be explained by organisms using readily available carbon sources provided in the medium during the early stages of growth. After this step, the spent wash, a complex carbon source, starts to degrade.11 According to a previous study,10 Aspergillus fumigatusG-2-6 used glucose as the preferred carbon source for maximum degradation or decolorization of melanoidin. This conclusion was based on the findings of previous studies. Increases in glucose concentration led to an increase in mycelial biomass; however, there was no discernible difference in the quantity of decolorization. Based on a study,31 melanoidin decolorization or degradation by Cariolus sp. No. 20 occurs through an intracellular enzyme that requires active oxygen and sugars. This enzyme converts glucose to gluconic acid and was later named sorbose oxidase.32,33 Presumably, a considerable amount of sugar in the medium had an inhibitory effect on enzymes such aslaccase, which inhibits lignolytic activity, and peroxidase, which inhibits oxidation activity, resulting in a reduction of melanoidin decolorization or degradation.17,34,35,21,36 Bacillus subtilis, Bacillus cereus, and Pseudomonas spp. were identified as the three most likely melanoidin-decolorizing thermotolerant microbial isolates. These microbial isolates were further optimized for melanoi dindecolorization using various physicochemical and nutritional parameters. This research was published in 2012.37 Of these three microorganisms, B.subtilis demonstrated the highest level of decolorization (85%), when incubated at 45°C with(w/v) 0.1% glucose,0.1% peptone, 0.05% MgSO4, 0.01% KH2PO4, and pH 6 for 24 h under static conditions. Decolorization of melanoidin was improved using a variety of nitrogen sources. Peptone at a concentration of 0.1% resulted in the greatest levels of melanoidin decolorization compared to other organic and inorganic nitrogen sources. Peptone is the nitrogen source that is most successful in melanoidin decolorization research.32,10,23,24,20 Throughout the secondary phase of the metabolic growth process, a research group33 discovered that peptone causes enzymatic systems to catalyze the breakdown of lignin and lignin-like compounds. Lignin peroxidase, known as ligninase or manganese-dependent peroxidase (MnP), is produced and secreted in response to nutrient deficits such as a lack of carbon and nitrogen resources. Due to excessive nitrogen supplementation, which restricts development, there was no substantial decolorization, even at high compound concentrations. This was observed when modest peptone concentrations were used as the nitrogen source to decolorize the melanoidin pigment present in the waste wash. This was because the waste wash contained melanoidin pigment, and it was discovered that neither static nor agitated bacterial cultures had any effect on decolorization efficiency with regard to optimized parameters of sugar beetroot molasses vinasse decolorization.38 Optimal doses of glucose and yeast extract are crucial for the decolorization of sugar beetroot molasses vinasse by Lactobacillus plantarum MiLAB393.39 In textile industries, the most common complex, reactive blue 222, is considered difficult to degrade on an industrial scale. However, physicochemical methods are both impractical and ineffective. In this study, a unique facultative indigenous bacterium, Enterobacter sp., was observed in industrial waste. Using the design of experiments (DOE) technique to optimize the physical and chemical parameters, the dye degradation efficiency increased to >90%. Our research showed that the bioaugmentation of Enterobacter sp., CU2004, combined with the optimal conditions of pH 8 at 30–37°C with 133–249 ppm dye concentration, lactose as a carbon source, and yeast extract as a nitrogen source, can effectively decolorize the complex industrial dye RB222 (>90%). The successful design space establishment highlights that the optimized process is scalable and can be expanded to an industrial scale. This isolate is a viable candidate for bioremediation because of its expansive potential in building a cost-effective, efficient, and scalable microbial dye-degrading process.40
Over the past few decades, the use of microorganisms for bioremediation has gained increasing attention. Multiple microorganisms, including bacteria and fungi, exhibit a promising ability to decolorize melanoidin-containing wastewater from the distillation industry. Furthermore, a better understanding of the microbial activity required for melanoidin degradation would help increase the overall effectiveness of this therapeutic system. Identifying the melanoidin end product is crucial throughout the deterioration process. In the future, the genetic makeup of isolates may be altered to increase their decolorization potency.41 The yeast strain Candida tropicalis has the efficient capacity to decolorize complex melanoidin compounds in the presence of minute quantities of carbon and nitrogen sources throughout a 32 h incubation period at 35°C and 5 pH. Through an effective and environmentally friendly decolorizing melanoidin pigment, this strain can potentially minimize environmental pollution.42
B. nitratireducens (B2) is a bacterial strain that can decolorize melanoidin across broad temperature and pH ranges. Additionally, this strain requires only a small amount of carbon and nitrogen sources to grow and requires only a brief incubation period. As a result, this strain is promising on an industrial scale for the efficient and cost-effective treatment of distillery effluents.
ACKNOWLEDGMENTS
The authors would like to extend their gratitude to the administration of Dr. Rammanohar Lohia Avadh University for enabling them to carry out the study by providing the required resources.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RS conceptualized the study and designed the experiment. AP performed the experiments. RS and RG analyzed the data. AP drafted the manuscript. RS and RG reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
The authors (Ranjan Singh and Rajeeva Gaur) would like to express their gratitude to the Department of Higher Education, Government of Uttar Pradesh, Lucknow, Uttar Pradesh, India, which funded the “Research and Development of State Universities of Uttar Pradesh” project number (47-2021/606/77-4-2021-4/56/2020) and (108/2021/2588/77-4-2021-4(28)/2021), respectively. In particular, the authors would like to thank the “Research and Development of State Universities of Uttar Pradesh” plan.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Pazouki M, Shayegan J, Afshari A. Screening of Microorganisms for Decolorization of Treated Distillery Wastewater. Iranian Journal of Science & Technology, Transaction B, Engineering, 2008; 32(B1):53-60.
- Dahiya J, Singh D, Nigam P. Decolourisation of synthetic and spent wash melanoidins using the white-rot fungus Phanerochaete chrysosporium JAG-40. Bioresource Technology. 2001;78(1):95-98.
Crossref - Sen KY, Hussin MH, Baidurah S. Biosynthesis of poly (3- hydroxyl butyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal Agric Biotechnol. 2019;17:51-59.
Crossref - Wedzicha BL, Kaputo MT. Melanoidins from glucose and glycine: composition, characteristics and reactivity towards sulphite ion. Food Chemistry. 1992;43(5): 359-367.
Crossref - Fujita M, Ike M, Kawagoshi Y, Miyata N. Biotreatment of persistent substances using effective microorganisms. Water Sci Technol. 2000;42(12):93-106.
Crossref - Agrawal CS, Pandey GS. Soil pollution by spent wash discharge: depletion of manganese (II) and impairment of its oxidation. J Environ Biol. 1994;15(1):49-53.
- Pena M, Coca M, Gonzalez G, Rioja R, Garcia MT. Chemical oxidation of wastewater from molasses fermentation with ozone. Chemosphere. 2003;51(9):893-900.
Crossref - Mohana S, Desai C, Madamwar D. Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Bioresour Technol. 2007;98(2):333-339.
Crossref - Moosvi S, Keharia H, Madamwar D. Decolourization of textile dye Reactive Violet 5 by a newly isolated bacterial consortium RVM 11.1. World J Microbiol Biotechnol. 2005;21:667-672.
Crossref - Ohmomo S, Kainuma M, Kamimura K, Sirianuntapiboon S, Aoshima I, Atthasampunna P. Adsorption of melanoidin to the mycelia of Aspergillus oryzae Y-2-32. Agricultural and Biological Chemistry. 1988;52(2):381-386.
Crossref - Kumar V, Leela Wati, FitzGibbon F, et al. Bioremediation and decolorization of anaerobically digested distillery spent wash. Biotechnol Lett. 1997;19(4):311-314.
- Kumar P, Chandra R. Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresource Technology. 2006;97(16):2096-2102.
Crossref - Marta P, Cosovic B, Lee C. Copper complexing properties of melanoidins and marine humic material. Sci Total Environ. 2006;366(1):310-319.
Crossref - Pant D, Adholeya A. Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol. 2007;98(12):2321-2334.
Crossref - Nwuche CO, Ugoji EO. Effects of heavy metal pollution on the soil microbial activity. Int J Environ Sci Technol. 2008;5(3):409-414.
Crossref - Nwuche CO, Ugoji EO. Effect of co-existing plant species on soil microbial activity under heavy metal stress. Int J Environ Sci Technol. 2010;7:697-704.
Crossref - Raghukumar C, Rivonkar G. Decolorization of molasses spent wash by the white-rot fungus Flavodonflavus, isolated from a marine habitat. Appl Microbiol Biotechnol. 2001;55(4):510-514.
Crossref - Tondee T, Sirianutapiboon S. Screening of melanoidin decolorization activity in yeast strain. Int Conf Environ. 2006; 99:5511-9.
Crossref - Eaton AD, Franson MAH. Standard Methods for the Examination of Water and Wastewater. 21st Edn., American Public Health Association, Washington, 2005 DC., ISBN: 0875530478.
- Ravikumar R, Vasanthi NS, Saravanan K. Single factorial experimental design for decolorizing anaerobically treated distillery spent wash using cladosporium cladosporioides. Int J Environ Sci Technol. 2011;8:97-106.
Crossref - Jiranuntipon S, Chareonpornwattana S, Damronglerd S, Albasi C, Delia ML. Decolorization of synthetic melanoidins-containing wastewater by a bacterial consortium. J Ind Microbiol Biotechnol. 2008;35(11):1313-1321.
Crossref - Cetin D, Donmez G. Decolorization of reactive dyes by mixed cultures isolated from textile effluent under anaerobic conditions. Enzyme Microb Technol. 2006;38(7):926-930.
Crossref - Sirianuntapiboon S, Zohsalam P, Ohmomo S. Decolorization of molasses wastewater by Citeromyces sp. WR-43-6. Process Biochemistry. 2004;39(8):917-924.
Crossref - Suntud S, Phothilangka P, Ohmomo S. Decolorization of molasses wastewater by a strain No. BP103 of acetogenic bacteria. Bioresour Technol. 2004;92(1):31-39.
Crossref - Chavan MN, Kulkarni MV, Zope VP, Mahulikar P. Microbial degradation of melanoidins in distillery spent wash by an indigenous isolate. Indian J Biotechnol. 2006;5(3):416-421.
- Sanroman MA, Herva FJD, Dominguez A, Barrio, Longo MA. Dye decolourization by newly isolated thermophilic microorganisms. Chem Engin Trans. 2010;20:151-156.
- Kim SJ, Shoda M. Decolorization of molasses and a dye by a newly isolated strain of the fungus Geotrichum candidum Dec 1. Biotechnol Bioengin. 1999;62(1):114-119.
Crossref - Jadhav JP, Phugare SS, Dhanve RS, Jadhav SB. Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation. 2010;21:453-463.
Crossref - Seyis I, Subasioglu T. Screening of different fungi for decolorization of molasses. Braz J Microbiol. 2009;40(1):61-65.
Crossref - Adikane HV, Dange MN, Selvakumari K. Optimization of anaerobically digested distillery molasses spent wash decolorization using soil as inoculum in the absence of additional carbon and nitrogen source. Bioresource Technology. 2006;97(16):2131-2135.
Crossref - Tiwari S, Gaur R, Rai P, Tripathi A. Decolorization of distillery effluent by thermotolerant Bacillus subtilis. Am J Appl Sci. 2012;9(6):798-806.
Crossref - Miyata N, Mori T, Iwahori K, Fujita M. Microbial decolorization of melanoidin-containing wastewaters: combined use of activated sludge and the fungus Coriolus hirsutus. J Biosci Bioeng. 2000;89(2):145-150.
Crossref - D’Souza DT, Tiwari R, Sah AK, Raghukumar C. Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes. Enzyme Microbial Technol. 2006;38(3-4):504-511.
Crossref - Guimaraes C, Porto P, Oliveira R, Mota M. Continuous decolourization of a sugar refinery wastewater in a modified rotating biological contactor with Phanerochaete chrysosporium immobilized on polyurethane foam disks. Process Biochemistry. 2005;40(2):535-540.
Crossref - Pant D, Singh A, Satyawali Y, Gupta RK. Effect of carbon and nitrogen source amendment on synthetic dyes decolourizing efficiency of white-rot fungus, Phanerochaete chrysosporium. J Environ Biol. 2008;29(1):79-84.
- Zhao YC, Yi Xy, Zhang M, Liu L, Ma WJ. Fundamental study of degradation of dichlorodiphenyltrichloroethane in soil by laccase from white rot fungi. Int J Environ Sci Technol. 2010;7:359-366.
Crossref - Kent KT, Schultz EE, Connors WJ, Lorenz LF. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol. 1978;117:277-285.
Crossref - Krzywonos M, Chalupniak A, Zabochnicka- Swiatek M. Decolorization of beet molasses vinasse by Bacillus megaterium ATCC 14581. Bioremediation Journal. 2017;21(2):81-88.
Crossref - Wilk M, Krzywonos M. Distillery wastewater decolorization by Lactobacillus plantarum MiLAB393. Archives of Environmental Protection. 2020;46(1):76-84.
- Lakshamaiah VV, Nag A, Gotekar S, More S, Jayanna SK. Sustainable biodegradation of textile dye reactive blue 222 by the novel strain Enterobacter CU2004, isolated from the industrial waste: A design of experiment based optimization study and characterisation of metabolites. J Appl Biol Biotechnol. 2023:1-11.
Crossref - Patel A, Singh R, Verma T, Gaur R. Challenges of Distillery Effluent Treatment and its Bioremediation Using Microorganism: A Review. Current World Environment. 2023;18(2), 446-461.
Crossref - Patel A, Singh R, Verma T, Shukla V, Gaur R. Distillery Effluent Melanoidin Decolorization Induced by a Yeast Strain Candida tropicalis (Y-2). Journal of Ecophysiology and Occupational Health, 2023; 23(3): 141-152.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.