ISSN: 0973-7510
E-ISSN: 2581-690X
The dried biomass of Bacillus cereus RC1 and Kocuria kristinae RC3 were evaluated for their efficacy in decolorizing two azo dyes, Eriochrome Black T and Amido Black 10B, respectively in batch system. Further, the influence of the initial dye concentration, contact time, concentration of biomass, temperature and pH on the dye decolorization efficacy of the bacterial biomass was studied. The findings suggested the profound dye adsorption efficacy of the bacterial biomass towards the test dyes, in aqueous solution. In addition, the experimental findings also showed the effect of the process parameters on the extent of biosorption. The bacterial biomass of B. cereus RC1 (200mg/l) exhibited the highest dye uptake (EBT) of 72.66% in an aqueous solution of pH 7.0 (at 35°C) with an initial dye concentration of 45 mg/l with a contact time of 60min. In case of K. kristinae RC3 (300mg/l), the optimum decolorization (AB) of 91.27% was achieved at 35°C with an initial dye concentration of 15 mg/l and pH value of 7.0. The optimal contact time was found to be 90min. Thus, the biomass of B. cereus RC1 and K. kristinae RC3 may be employed to facilitate the treatment of dye contaminated water in an economical and eco-friendly way.
Decolorization, adsorption, Eriochrome black T, Amido black 10B, Biomass, Bacillus cereus.
Synthetic dyes are extensively employed in numerous sectors like pharmaceutical, textiles, cosmetics, food and paper industries. However, the improper discharge of these dyes, as a result of the inefficient dyeing process, is a menace to the natural ecosystem. The uncontrolled release of dye effluent in the aqueous environment affects the water quality by reducing dissolved oxygen concentration and penetration of sunlight. In addition, they are also lethal to the aquatic life forms as well as human health1. Some dyes and their degraded by-products also possess mutagenic and carcinogenic property2.
Among the synthetic dyes, the dyes containing azo function (N=N-) represents one of the most concerning dyes due to their high toxicity and carcinogenic nature3. The carcinogenic azo dye, Eriochrome Black T (EBT) is among the most widely employed textile dye commonly used for dyeing silk, wool, and nylon fibers. It is also employed as an indicator in the assessment of metal cations4. Another azo dye widely used in textile industries is Amido black 10B (AB). AB is used in the dyeing of natural (silk, wool, and cotton) as well as synthetic fibers like rayon and polyester. The dye is also used in staining of proteins in biochemical tests, in inks, paints, leather and plastics industries. AB is known to damage the human respiratory system and cause inflammation to skin and eyes5. Hence, development of cost-effective and eco-friendly adsorbent to effectively segregate and eliminate these dyes from industrial effluents has become a necessity in minimizing the pollution of water bodies and its toxic effects to humans as well as other life forms3.
Considering the problems associated with these dyes, numerous physical and chemical approaches including oxidation, ozonation, membrane filtration, coagulation are extensively studied for their ability to remove these dyes from wastewater. However, these techniques suffer from many constraints, such as high-costs and generation of unwanted toxic secondary pollutants6. Hence, in recent years the biological processes have received much attention as a better alternative for the remediation of dye-contaminated water bodies.
The process of biosorption is regarded as a promising way for the removal of recalcitrant pollutants (metals and dyes) from water bodies. Numerous organisms including algae, bacteria, fungi, and plants have been documented for their ability to remove and mineralize dyes. Biomaterials derived from fungi, algae, bacteria and agricultural by-products are known to preferably bind to various pollutants7. Numerous species of bacteria including Aeromonas1, Citrobacter8, Bacillus9, Klebsiella10 and Pseudomonas11 have exhibited substantial decolorization of textile dyes.
Microbial biomass has also been documented for their ability to accumulate a range of dyes and heavy metals. Based on the report of Aksu et al. (2006), the dried biomass of Rhizopus arrhizus was used as biosorbent to effectively remove Gemazol Turquise Blue-G reactive dye12. Though numerous studies have been executed on the dye decolorization potential of bacteria, reports on the use of non-pathogenic dried bacterial biomass are still limited. The advantages associated with the use of heat-treated bacterial biomass include less production time, inexpensive and ease in handling. Various researchers have used dead bacterial cells as an effective alternative to synthetic adsorbent of dyes. Busi et al. (2016) documented the efficient adsorption of azo dyes, Eriochrome black T, Acid Red 26 and Trypan blue using biomass (dried) of Aeromonas hydrophila RC113. In another report, Du et al. (2012) documented the ability of Pseudomonas sp. biomass in decolorizing Acid Black 17211. The biomass of Corynebacterium glutamicum was successfully employed as a biosorbent for the decolorization azo dye, Reactive Black 5 (RB5)14. The presence of carbonyl, amino, hydroxyl and phosphate groups imparts a negative charge to the cell wall of the bacteria. Consequently, the cationic environmental pollutants get adsorbed on the bacterial surface or taken up by either active or passive transport mechanisms7.
Due to simplicity in the process, low operating cost, high efficiency and environmental benign characteristics, adsorption is commonly adopted for remediation of an aqueous environment. However, the use of suitable adsorbent and process parameters is the key to achieve optimal removal of the pollutants4. This study was conducted to study the ability of dried biomass of B. cereus RC1 and K. kristinae RC3 in decolorizing two azo dyes, Eriochrome Black T and Amido Black 10B respectively. Further, the effect of varying environmental factors on the decolorization was studied to attain maximum dye removal from the aqueous solution.
The bacterial cultures, B. cereus RC1 and K. kristinae RC3 were isolated from water samples collected from textile effluent discharge located at Tirupur, Tamil Nadu, India15,16. The media components and the dyes used in the present study were procured from Hi-Media Laboratories Pvt. Ltd, Mumbai, India. The azo dyes, Eriochrome black T (EBT) and Amido black 10B (AB) were dissolved in distilled water to obtain the required concentration.
Bacterial growth and biomass yield
The bacterial cultures were cultivated in 250 ml of liquid medium containing peptone (10g/l), sucrose (20 g/l), KH2PO4 (1 g/l), MgSO4.7H2O (0.5 g/l) and NaNO3 (1 g/l). The flasks were incubated with shaking (at 150 rpm) at 37 °C and pH 7 for 24 h, The increase in cell density was recorded at a regular interval of 4 hrs, at 520 nm. The growth curves of bacterial isolates were generated and the corresponding biomass yield was determined13.
Preparation of biomass
After incubation for 24hrs, the culture broth of B. cereus RC1 and K. kristinae RC3 were subjected to centrifugation (6000 rpm) at 4°C for 10 min. The cell pellet was collected, rinsed thrice with 0.9% NaCl and oven-dried for 24 hrs at 80°C. The resulting dried biomass was employed for the subsequent assays17.
Dye adsorption study
The dye decolorization ability of B. cereus RC1 and K. kristinae RC3 dried biomass was executed in 250 ml conical flasks containing 100 mg/l of each dye, EBT and AB suspended in 100 ml of distilled water. After the incubation period, the optical density of the dye solution was measured at 523 nm and 620 nm for EBT and AB, respectively. The percentage decolorization for each treatment was calculated18.
Effect of different parameters on dye decolorization
The process parameters including dye concentration, adsorbent dose, biomass contact time, temperature and pH which may influence the dye adsorption capacity of the dried bacterial biomass were tested using batch experiments.
Effect of initial dye concentration
To investigate the effect of initial dye concentration on the adsorption efficacy of the bacterial biomass, the dried bacterial biomass (100 mg/ml) was dispensed in the dye solution containing a varying concentration of dye ranging from 10 – 100 mg/l. The medium was maintained at pH7.0 and incubated at 37°C in a shaking incubator (150 rpm). After 24 hrs of incubation, the dye concentration was determined19.
Effect of biomass concentration
To determine the effect of biomass dosage on the dye decolorization efficacy, the biomass ranging from 100 – 600 mg/ml was supplemented in the dye solution containing 100 mg/l of the test dye. After incubation of 24 h at 37°C, the decrease in the color intensity of the reaction mixture was measured spectrophotometrically20.
Effect of contact time
To determine the effect of contact time on the decolorization efficacy of the bacterial biomass, a volume of 100 mg/ml of the biomass was inoculated in distilled water supplemented with 100 mg/l of the dye. The medium was incubated in a shaker at 37°C for 150 min. The reduction in the dye concentration was recorded at every 30 min interval19.
Effect of temperature
The role of temperature on the decolorization process was determined based on the protocol of Siddhi & Disha (2017) with few modifications. The reaction mixture containing 100 mg/l each of the dye and the bacterial biomass was incubated at varying temperatures ranging from 30 – 60°C. The dye solution was maintained at pH7 and a temperature of 37°C. After 24 h of incubation, the % decolorization of the test dyes was assessed21.
Effect of pH
To study the effect of different pH on the decolorization efficacy of the bacterial biomass, the reaction mixture containing 100 mg/l of each test dye were prepared separately. The dye solution was adjusted to different pH ranging from 5- 9 using 1N HCl or 1N NaOH. A volume of 100 mg/l of the bacterial biomass was supplemented into the dye solution and the reaction mixture was incubated overnight at 36°C in a shaker22. The concentration of dyes, before and after the adsorption was recorded by recording the change in optical density at 523 nm for EBT and 620 nm for AB. All the assays were conducted in triplicate.
Bacterial growth and biomass yield
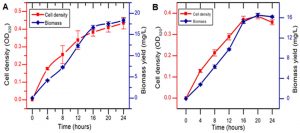
As depicted in the growth curve of Fig. 1, a gradual increase in the cell density and biomass yield in both the bacteria, B. cereus RC1 and K. kristinae RC3 were observed with increase in incubation time. After 24 hrs of incubation, the optical density of RC1 and RC3 were found to be 0.43 ± 0.032 and 0.35 ± 0.01, respectively. Further, the total bacterial biomass concentration extracted from the broth culture of RC1 and RC3 on 24 hrs of incubation were quantified to be 18.35 ± 0.47 mg and 16.42 ± 0.18 mg, respectively (Fig. 1).
Fig. 1. Bacterial growth and biomass yield of B. cereus RC1 (A) and K. kristinae RC3 (B) recorded for a period of 24 hrs.
Effects of different process parameters on dye decolorization efficacy
Effect of dye dosage and biomass concentration
A gradual increase in percentage adsorption by the biomass of B. cereus RC1 could be observed with the increase in the concentration of EBT from 20–45 mg/l which was followed by a steady decrease with the further increase in dye concentration up to 90mg/l (Fig. 2A). However, in the case of K. kristinae RC3, a gradual decrease in the % biosorption of AB with the corresponding increase in the dye concentration was observed. The highest % biosorption of 64.38 ± 2.65% and 88.76 ± 2.36% by RC1 and RC3 were observed at 45mg/l and 15mg/l dye concentration, respectively.
The effect of varying biomass concentration of both the bacteria on the removal of the dye, EBT and AB is shown in Fig. 2B. At the dye concentration of 100mg/l, there was not much change on the percent decolorization with an increase in biosorbent concentration for both B. cereus RC1 and K. kristinae RC3. However, the percent removal of the EBT by B. cereus RC1 was notably lower as compared to percentage decolorization of AB by K. kristinae RC3 as presented in Fig. 2b. The maximum percentage removal of EBT by 72.66 ± 3.62% was achieved at the biomass concentration of 200mg/l whereas the maximum percentage removal of AB by 89.07 ± 2.81% was obtained 300mg/l of biomass (Fig. 2).
Fig. 2. (A) Effect of dye concentration on decolorization percentage of dyes EBT and AB by B. cereus RC1 and K. kristinae RC3, respectively. (B) Effect of biomass on decolorization percentage of dyes EBT and AB by B. cereus RC1 and K. kristinae RC3, respectively.
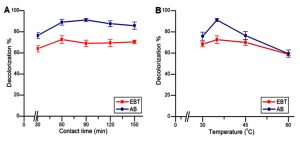
Fig. 3. (A) Effect of contact time on decolorization percentage of dyes EBT and AB by B. cereus RC1 and K. kristinae RC3, respectively. (B) Effect of temperature on decolorization percentage of dyes EBT and AB by B. cereus RC1 and K. kristinae RC3, respectively.
Effect of contact time and temperature
As depicted in Fig. 3A, a gradual increase in the percent of decolorization of both EBT and AB was observed with increase in contact time, which was followed by a slight decrease up to 150min of incubation. In the case of EBT, the maximum decolorization percent of 72.66 ± 3.62% was observed at a contact time of 60 min which decreased gradually from 90 to 150 min. Similarly, in the case of AB, the maximum decolorization was attained at a contact time of 90 min with a decolorization percent of 91.27 ± 1.40% and then gradually decreases to 85.85 ± 3.36% at 150 min, as shown in Fig. 3A.
Fig. 3B represents the effect of temperature on the biosorption of EBT and AB at the biosorbent dosage of 100mg/l. With the gradual increase in temperature from 30°C to 35°C, a significant increase in the percent of decolorization for both the dyes was achieved. At 35°C, EBT and AB exhibited the highest percentage decolorization of 72.66 ± 3.62% and 91.27 ± 1.40%, respectively. However, the dye removal capacity gradually decreased with increase in temperature (Fig. 2B). At 60°C, the percentage biosorption of the dyes, EBT and AB were found to be 58.97 ± 1.37% and 59.48 ± 3.42%, respectively.
Effect of pH
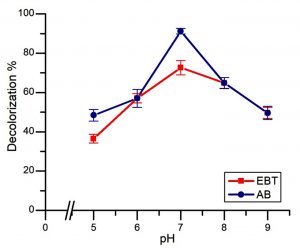
As presented in Fig. 4, a significant variation in the percentage decolorization was seen at different pH. There was a steady increase in the percent removal of EBT from 36.5 ± 2.18% at pH5 to 72.66 ± 3.62% at pH 7. This was followed by a decrease in percentage decolorization by 49.61 ± 2.18% at pH9. Similarly, in the case of AB, the increase in pH from 5 to 7 was accompanied by an increase in percentage removal from 48.45 ± 2.97% to 91.27 ± 1.40%, respectively at a dye concentration of 100 mg/l. The maximum decolorization of 72.66 ± 3.62% and 91.27 ± 1.40% for EBT and AB respectively, was observed at pH 7.
The dried bacterial biomass of B. cereus RC1 and K. kristinae RC3 were successfully employed for the removal of EBT and AB from aqueous solution. These bacterial isolates have been reported for their decolorization potential in live conditions at different physiochemical conditions and results were further confirmed by response surface methodology15,16. The ability of bacterial biomass to interact with dyes is governed by various factors. Some of the most important factors influencing the performance of biological dye removal are the chemical structures of the dyes, type of biomass, process conditions including dye concentration, biomass dosage, pH, temperature and contact time7. Hence, the adsorption studies were performed under different process parameters to achieve maximum decolorization. Both the test bacteria showed significant dye decolorizing efficacy in vitro. However, the percentage removal of EBT by B. cereus RC1 was found to be considerably lower as compared to the percentage removal of AB by K. kristinae RC3.
The bioadsorption efficacy is directly governed by the dye concentration and the availability of binding sites for the dye molecules on the adsorbent surface. Hence, the unavailability of adsorption sites usually decreases the decolorization efficiency23. The initial dye dosage significantly affects the biosorption efficacy of the dyes. A gradual increase in percentage decolorization of EBT with the increase in concentration was followed by a steady decrease with the further increase in dye concentration from 45 mg/l. However, in the case of K. kristinae RC3, a continuous decrease in the percentage biosorption of AB was observed with increasing dye concentration from 15 mg/l. Hence, 45 mg/l and 15 mg/l of EBT and AB were determined to be the maximum dye dosage that could be adsorbed on 100mg/l of the test bacterial biomass. The decrease in the dye decolorization efficacy with the subsequent increase in dye dosage might have resulted due to the saturation of biomass surface and unavailability of adsorption sites. Wang et al. (2013) presented the dye absorption ability of Bacillus species YZU1. The effect of varying concentration of Reactive Black 5 on the overall decolorization efficacy was also documented. At a concentration of 50 mg/l of Reactive Black 5, 90% decolorization was observed within 60 h of incubation while at 300 mg/l the time required was 120 h9.
From an economic point of view, optimization of biosorbent dosage is required to obtain a high rate of dye elimination with low biosorbent dose. Usually, the efficiency of dye decolorization in an aqueous medium increases with the corresponding increase in the adsorbent dosage. This results due to the availability of more surface area and binding sites for the dye molecules23. The optimum biomass concentration resulting in the maximum adsorption of 100mg/l of dye was found to be 200mg/l and 300mg/l for B. cereus RC1 and K. kristinae RC3, respectively. The equilibrium state observed in case of both bacteria strains indicated that further increase in biomass dosage did not exert much effect on the absorption of the dyes. According to the report of Nacera & Aicha (2006), the increase in the biosorption of methylene blue onto the biomass of Streptomyces rimosus corresponds to the increase in the quantity of the bacterial biomass used in the batch experiment20.
Contact time is also a major factor that regulates the adsorption of dissolved substance onto the biomass surface, thereby influencing the dye decolorization process. In the case of EBT, a rapid biosorption of the dye was seen at the initial 60 min of exposure to the biomass. Thereafter, the rate of biosorption gradually reached equilibrium at 90 min. Similarly, in the case of AB, the maximum decolorization was attained at a contact time of 90 min. The high degree of adsorption indicates the affinity of the bacterial biomass for the test dyes. In a similar report, on supplementation of the Rhizopus arrhizus biomass, a rapid decolorization of dye was observed within 15–20 min of exposure; thereafter the process of adsorption was less significant. Within a contact time of 30 and 60 min, respectively, the maximum percentage decolorization of Congo red dye and Fast Red A was attained19.
Another important factor affecting the biosorption process is the temperature. High temperature increases the surface active sites and kinetic energy of the dye molecules, subsequently resulting in enhanced biosorption23. However, the high temperatures are also reported to physically damage the biosorbent. In case of passive or physical adsorption, the dye molecules interact with the biomass via a weak bond. In such a case, the exposure to high temperature might break the bonds which subsequently lead to desorption of the dye. In this study, a significantly lower percentage of decolorization was seen with an increase as well as a decrease in temperature from 35°C. Hence, for both the dyes, EBT and AB the optimum temperature for dye removal was determined to be 35°C. Similarly in another report, the temperature of the dye solution was found to significantly affect the biosorption capacity of Corynebacterium glutamicum biomass. In support of the present finding, 35°C was determined to be the most favored temperature for the biosorption of Reactive Black 5 by C. glutamicum in aqueous solution14.
The dye removal efficacy of a bio-sorbent is also significantly governed by the pH of the environment. The protonated dried biomass of C. glutamicum has been earlier used as a biosorbent for the removal of Reactive Red 424. Generally, a solution with high pH facilitates adsorption of cationic dye as compared to anionic ones. In contrast, anionic dyes are adsorbed more efficiently at low pH23. As the pH decreases, the H+ ion concentration increases; therefore cell surface holds H+ ions rather than other cations25. For both EBT and AB, the optimum pH required for maximum decolorization was determined as pH 7. The percentage decolorization was found to decrease at pH lower than and higher than pH 7 suggesting that the dye-loaded biomass could be regenerated at pH>7 or <7. In a similar study, Won et al. (2005) reported the impact of pH on the dye removal efficacy of the biomass of C. glutamicum. The dried biomass showed an increase in dye uptake with a decrease in pH and a negligible dye uptake was observed at pH >724.
The present study is one of the very few communications on the utilization of dried bacteria biomass for the decolorization of dyes in aqueous solution. The findings also represent the initial report on the removal of two azo dyes, EBT and AB in batch systems using dried B. cereus RC1 and K. kristinae RC3 as a biosorbent. However, the affinity of dried biomass of K. kristinae RC3 towards AB was found to be relatively stronger to the binding of B. cereus RC1 biomass for EBT. Further, it was also found that the variation in the initial dye dose, adsorbent dose, contact time, temperature and pH significantly influenced the dye uptake efficiency of the biomass. The bacterial biomass of B. cereus RC1 and K. kristinae RC3 exhibited the highest dye uptake of 72.66% and 91.27%, at 35°C and pH7. Hence, the bacterial biomass might be employed as an eco-friendly and cost-effective approach for the remediation of water bodies contaminated with azo dyes.
ACKNOWLEDGMENTS
The authors acknowledged Kalasalingam Academy of Research and Education for providing the research facility.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors made a substantial contribution to the work and approved the final manuscript.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
- Chen KC, Wu JY, Liou DJ,Hwang SC. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol., 2003; 101(1): 57-68.
Crossref - Imran M, Crowley DE, Khalid A, Hussain S, Mumtaz MW, Arshad M. Microbial biotechnology for decolorization of textile wastewaters. Rev. Environ. Sci. Bio/Technol., 2015; 14(1): 73-92.
Crossref - Saratale RG, Gandhi SS, Purankar MV, Kurade MB, Govindwar SP, Oh SE, Saratale GD. Decolorization and detoxification of sulfonatedazo dye C.I. Remazol Red and textile effluent by isolated Lysinibacillus sp. RGS. J. Biosci. Bioeng., 2013; 115(6): 658-667.
Crossref - Khalid A, Zubair M, Ihsanullah A. Comparative study on the adsorption of eriochrome black T dye from aqueous solution on graphene and acid-modified graphene. Arab J. Sci. Eng., 2018; 43(5): 2167–2179.
Crossref - Angelova R, Baldikova E, Pospiskova K, Safarikova M, Safarik I. Magnetically modified sheaths of Leptothrix sp. as an adsorbent for Amido black 10B removal. J. Magn. Magn. Mater., 2017; 427: 314– 319.
Crossref - Khan R, Bhawana P, Fulekar M. Microbial decolorization and degradation of synthetic dyes: a review. Rev. Environ. Sci. Bio., 2013; 12: 75–97.
Crossref - Vijayaraghavan K,Yun YS. Bacterial biosorbents and biosorption. Biotechnol. Adv., 2008; 26(3): 266-291.
Crossref - Wang HJ, Su Q, Zheng XW, Tian Y, Xiong XJ, Zheng TL. Bacterial decolorization and degradation of the reactive dye Reactive Red 180 by Citrobacter sp. CK3. Int. Biodeterior. Biodegradation, 2009; 63: 395–399.
Crossref - Wang ZW, Liang JS, Liang Y. Decolorization of Reactive Black 5 by a newly isolated bacterium Bacillus sp. YZU1. Int. Biodeterior Biodegradation, 2013; 76: 41-48.
Crossref - Zablocka-Godlewska E, Przystas W, Grabinska-Sota E. Dye Decolourisation Using Two Klebsiella Strains. Water Air Soil Pollut, 2015; 226: 2249.
Crossref - Du LN, Wang B, Li G, Wang S, Crowley DE, Zhao YH. Biosorption of the metal-complex dye Acid Black 172 by live and heat-treated biomass of Pseudomonas sp. strain DY1: kinetics and sorption mechanisms. J. Hazard Mater., 2012; 205-206: 47-54.
Crossref - Aksu Z, Cagatay SS. Investigation of biosorption of Gemazol Turquise Blue-G reactive dye by dried Rhizopus arrhizus in batch and continuous systems. Separ. Purif. Tech., 2006; 48(1): 24-35.
Crossref - Busi S, Chatterjee R, Rajkumari J, Hnamte S. Ecofriendly biosorption of dyes and metals by bacterial biomass of Aeromonas hydrophila RC1. J. Environ. Biol., 2016; 37(2): 267-274.
- Vijayaraghavan K,Yun YS.Utilization of fermentation waste (Corynebacterium glutamicum) for biosorption of Reactive Black 5 from aqueous solution. J. Hazard Mater., 2007; 141(1): 45-52.
Crossref - Uppala R, Sundar K, Muthukumaran A. Response Surface Methodology Mediated Optimization of Textile Azo Dye, Eriochrome Black T Decolorization by Bacillus cereus RC1. Desalin. WATER Treat, 2017; 81: 242–251.
Crossref - Uppala R, Sundar K, Muthukumaran A. Response Surface Methodology Mediated Optimization of Decolorization of Azo Dye Amido Black 10B by Kocuria kristinae RC3. Int. J. Environ. Sci. Technol., 2018; 1–12.
Crossref - Guendouz S, Khellaf N, Zerdaoui M. et al. Biosorption of synthetic dyes (Direct Red 89 and Reactive Green 12) as an ecological refining step in textile effluent treatment Environ. Sci. Pollut. Res., 2013; 20: 3822.
Crossref - Ali NF, El-Mohamedy RSR. Microbial decolourization of textile waste water. J. Saudi Chem. Soc., 2012; 216: 117-123.
Crossref - Salvi NA, Chattopadhyay S. Biosorption of Azo dyes by spent Rhizopus arrhizus biomass. Appl. Water Sci., 2017; 7(6): 3041–3054.
Crossref - Nacera Y, Aicha B. Equilibrium and kinetic modeling of methylene blue biosorption by pretreated dead Streptomyces rimosus: effect of temperature. Chem. Eng. J., 2006; 119(2-3): 121-125.
Crossref - Siddhi MI, Disha D. Bioremediation of Azo Dye: Eriochrome Black T by the novel organism Bacillus lentus. Biosci. Discov., 2017; 8(4): 771-775.
- Lalnunhlimi S, Krishnaswamy V. Decolorization of azo dyes (Direct Blue 151 and Direct Red 31) by moderately alkaliphilic bacterial consortium. Braz. J. Microbiol., 2016; 47(1): 39-46.
Crossref - Seow TW, Lim CK. Removal of dye by adsorption: a review. Int. J. Appl. Eng. Res., 2016; 11(4): 2675–2679.
- Won SW, Choi SB, S, YY. Interaction between protonated waste biomass of Corynebacterium glutamicum and anionic dye Reactive Red 4. Colloids Surf. A Physicochem Eng. Asp., 2005; 262: 175-180.
Crossref - Argun YA, Karacali A, Calisir U, Kilinc N, Irak H. Biosorption method and biosorbents for dye removal from industrial wastewater: a review. Int J. Adv. Res., 2017; 5(8): 707-714.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.