ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed at assessing the correlations between gene expression of TNF-α, IFN-γ, TGF-β in peripheral lymphocytes from women suffering from repeated implantation failure before and after intravenous Intra-lipid (IL) therapy, and correlation between changes in gene expression with IL infusion and success rate of IVF cycles. Twenty-three women complaining of unexplained infertility without history of autoimmune disorders, or immunodeficient diseases were included. All women included aged <40 years, BMI <28 with history of recurrent IVF cycles failure, seeking medical advice for new IVF trial. All were average responders to induction of ovulation (≥5 oocytes in each cycle) with good quality embryos transferred to uterus at proper time. Included women received 200 ml of 10% IL slowly intravenous. Two venous blood samples were taken from all candidates, one before IL infusion and the second was at day of embryo transfer. The current study detected a significant reduction of expression in TNF-α and increased expression of TGF-β, while non-significant reduction in expression of IFN-γ after treatment. Significant associations between reduction of TNF-α, IFN-γ expression and positive clinical and ongoing pregnancy were observed, while increased TGF-β expression was associated with only positive ongoing pregnancy. In conclusion, IL therapy might have a positive impact on IVF pregnancy rates via alterations in peripheral cytokines expression mainly reduction of TNF-α mRNA expression and increased TGF-β mRNA expression.
Intralipid Therapy, IVF failure, IFN-γ, TNF-α, TGF-β
The occurrence and stabilization of pregnancy require coordination between the growing embryo and the receptive decidua. Failure of this process leads to many pregnancy complications including miscarriage, pre-eclampsia, fetal growth restriction, and pre-term birth.1
Cytokines are defined as structures with small molecular weights often secreted from leucocytes. The result of interaction between mother and fetus is maternal immunosuppression allowing development of the semi-allogeneic placental-fetal unit. Decidual cytokines play a vital role in maintenance of pregnancy also regulation of invasion of growing embryo and remodelling of spiral artery.2
Throughout the menstrual cycle, endometrium produces a diverse range of cytokines. During the establishment of pregnancy, these various cytokines play a vital role in preparation of the uterine environment for successful implantation of the growing embryo and creation of a functioning placenta.3
Altered decidual cytokines are involved in many complications of pregnancy including recurrent spontaneous miscarriage.4
Infertility is a problem all over the world., Infertility and IVF centres were only available in about one-quarter of the world’s nations in 2000. This number increased to nearly one-third of the world’s nations in 2005. But by the year 2010, when the International Federation of Fertility Societies (IFFS) survey was repeated, there was a marked increase in IVF services in the developing countries. The number of countries included in the survey was doubled.5
Recurrent IVF failure is commonly known as failure to acquire a pregnancy following two or more IVF cycles (including frozen embryos transfer FET), in which 5-10 good quality embryos were transferred into the uterine cavity.6
Because embryo(s) quality and endometrial receptivity are two major factors that believed to be the key points in implantation,7 several therapeutic approaches including immunomodulators have been described to improve the receptiveness of the endometrium. Immunological factors have been included in the pathogenesis of recurrent implantation failures (RIF) and different immunomodulatory agents and approaches have been used for the treatment of women with that problem.8
Intravenous fat emulsion (IVFE) is an admixture of fatty acids prepared to provide essential supplementation of fatty acids required for patients on parenteral nutrition (PN). Intravenous lipid emulsion (Intralipid (IL) infusions were usually used as adjuvant agents to supress and distract the maternal immune system during the window of implantation (WOI).9
IL was prescribed to produce a modulation effect on many immune cellular mechanisms, having positive effect on results of IVF cycles and pregnancy maintenance.10
The current study aimed at assessing the correlation between gene expression of (TNF-α, IFN-γ, TGF-β) in peripheral lymphocytes from women suffering from repeated IVF cycles failure before and after intravenous IL therapy, also assessing the correlation between change in gene expression with IL infusion and success rate of IVF cycles.
Subjects
This study was done on 23 women complaining of infertility with history of recurrent unexplained IVF cycles failure, in the period from January 2019 to June 2020 attending infertility unit at Ain Shams University maternity hospital.
Success of IVF cycle was defined as a positive pregnancy test, while negative pregnancy test two weeks after embryo (s) transfer indicates failure of cycle, clinical pregnancy is confirmed by radiological (Ultrasonic) findings of pregnancy (intrauterine embryonic sac, positive fetal pole, positive fetal echo).
All the included women had unexplained infertility without history of autoimmune disorders. Infertility was properly diagnosed in couples who had regular unprotected marital relations for at least 1 year without pregnancy, all patient’s data were managed confidentially.
Informed consents were obtained from participating women, and the study was approved by the Local Ethics Committee at Faculty of Medicine Ain Shams University, (FAMASU M D 201/2018) and in consonance with “The World Medical Association’s Code of Ethics (Declaration of Helsinki).
All included women received induction of ovulation before ovum pick up using long luteal protocol with LHRH agonist (triptorelin 0.1mg/ml) daily injection starting from day 18 of previous cycle till the day of ovulation trigger.
During induction of ovulation, we used Human Menopausal Gonadotropin (HMG) (dose ranging 150-300 IU) according to women response observed by transvaginal ultrasound examination and serum Estradiol (E2) level. It takes 12-16 days for complete follicular maturation.
Trigger of ovulation done by Human Chorionic Gonadotropin (HCG) 10000 IU single injection; Ovum pick up done about 36 hours from trigger of ovulation.
Intracytoplasmic Sperm Injection (ICSI) done to collected oocytes, fertilization and zygotes cleavage were observed in triphasic incubators using Life Global® culture media supporting embryologic development till embryo transfer. Embryo transfer done at day 5 (blastocyst stage) to all included women.
All enrolled women received intralipid emulsion infusion therapy with start of induction of ovulation, they received 200 ml of 10% IL intravenous slowly with good observation and monitor for vital data during infusion.
Inclusion criteria
All women included in the study were diagnosed with unexplained infertility (age <40 years, BMI<28) with history of In-Vitro Fertilization & Embryo transfer (IVF-ET) cycles and recurrent implantation failure, seeking medical advice for new IVF-ET cycle. All women were average responders to ovulation induction (≥5 oocytes were collected in each cycle) with good quality embryos transferred to uterus at proper time regarding embryologic stage in previous cycles.
Exclusion criteria
Women with history of poor embryos quality in previous trials, chronic endometriosis, poor responders to ovulation induction with low embryos number, (two or less embryos), abnormal semen analysis of husband were excluded.
Methods
The steps in the current study were summarized as follows:
- Full medical history and clinical data were obtained from participating women.
- Collection of specimens and processing, PBMCs isolation by Ficoll-Paque PLUS density gradient centrifugation.
- RNA isolation
- cDNA Synthesis.
- Real-time qPCR using SYBR Green.
- Calculation of Relative Quantification (RQ) (relative expression)
- Data analysis
Complete medical history and clinical data
Detailed history taking, clinical and ultrasonic examination and full hormonal profiles (FSH, LH, prolactin, TSH) were done.
Sample collection and processing
Two blood samples from 23 women were collected in EDTA containing tubes: First sample before Intralipid infusion (before start of induction of ovulation), second sample at day of embryo(s) transfer (ET) (14 to 21 days after first sample and Intralipid infusion).
Freshly isolated peripheral blood mononuclear cells PBMCs from the infertile women blood were stored in liquid nitrogen to extract the RNA for studying of gene expression using real-time polymerase chain reaction (RT-PCR).
PBMCs were isolated and kept in liquid nitrogen until RNA was extracted. Ficoll-Paque PLUS density gradient centrifugation was employed to isolate PBMCs (peripheral blood monocytes).11 (Ficoll-Paque PLUS and PBS buffer used in room temperature). Blood was diluted 1:1 in PBS, and the contents were blended by carefully inverting the vial. Each of the 50 mL Eppendorf conical tubes received fifteen (15) mL of Ficoll-Paque. Using a serological pipette at the lowest speed setting, Ficoll-Paque was overlaid with the blood/PBS combination. The sample was centrifuged at 400 x g and 20 °C for 30 minutes in the selected swing-out rotor, with acceleration/deceleration rates of 9/0 or 9/3, respectively, at the setting “at specified rpm.” After the centrifugation was completed, the samples were carefully removed from the centrifuge to avoid phases mixing. A sterile serological pipette was used to carefully aspirate two thirds (2/3) of the top layer (containing the plasma and platelets) until the interphase (containing the mononuclear cells) was reached. The entire lymphocyte layer was sucked using an Eppendorf pipette, with the volume kept to a minimum, and transferred to a new tube. Three volumes of PBS were added to the lymphocyte layer and gently mixed by pipetting up and down. The supernatant was discarded after centrifugation at 100 x g and 20 °C for 10 minutes. Steps 3 and 4 were carried out once more. The cell pellet was re-suspended in a medium that may be used in further experiments.
RNA isolation using The GeneJET™ RNA Purification Kit
cDNA Synthesis
Reverse transcription of extracted RNA into cDNA using: AMV Reverse Transcriptase, Promega, and Madison.VVI, USA (Catalog No.: M5101).
Real-time qPCR using SYBR Green I
Real-time qPCR amplification and analysis using an Applied Biosystem with software version 3.1 (StepOne™, USA) were performed. The qPCR assay with the primer sets were optimized at the annealing temperature.
Sequences of primers used for real-time polymerase chain reaction were as follows:
IFN-γ: CTAATTATTCGGTAACTGACTTGA, ACAGTT CAGCCATCACTTGGA
TNF-α: GCCTGCTGCACTTTGGAGTG, TCGGGGTT CGAGAAGATGAT
TGF-β: AAATTGAGGGCTTTCGCCTTA, TGAACCCGT TGATGTCCACTT
Estimation of Relative Quantification (RQ) (relative expression)
The relative quantitation was estimated in accordance with Applied Bio system (TaqMan® Gene Expression Assays) software using the following equation:
∆ Ct = Ct gene test – Ct endogenous control (Catalog NO.: 4331182)
∆∆Ct = ∆Ct sample1 – ∆Ct calibrator.
RQ = Relative quantification = 2-∆∆Ct.
The RQ is the fold change compared to the calibrator (untreated sample).
Data analysis
The data was analysed utilizing IBM Statistical Program for Social Science (SPSS) version 25.0. To convey quantitative data, the mean and standard deviation were utilised. The McNemar’s Test was employed for quantitative analysis of the Q-PCR results, and p-values less than 0.05 were regarded to demonstrate statistical significance.
In addition, ΔCT values were used to compare gene expression in the samples, and 2–ΔΔCT was used for relative gene expression analysis.
Initial Characteristics of enrolled women as shown in Table 1, age (25-37 years), BMI (16.69 – 29.1 Kg/m2), with 2-5 previous failed IVF cycles. Basal serum hormonal profiles (FSH, LH, Prolactin and TSH) in enrolled women were within normal ranges as shown in Table 2.
Table (1):
Initial characteristics of enrolled women.
Age (years) Range Mean ± SD |
25 – 37 30.87 ± 3.81 |
BMI (kg/m2) Range Mean ± SD |
16.69 – 29.10 31.15 ± 3.72 |
No. of Previous Failed IVF/ICSI Cycles Range Median (IQR) |
2 – 5 3 (3 – 4) |
SD standard deviation – BMI body mass index.
IQR interquartile range.
Data presented as range, mean ± SD; or range, median (IQR).
Table (2):
Basal serum hormonal profile (FSH, LH, Prolactin and TSH) in enrolled women.
Serum FSH (IU/L) Range Mean ± SD |
3.2 – 8.7 5.23 ± 1.83 |
Serum LH (IU/L) Range Mean ± SD |
2.7 – 8.5 5.99 ± 1.93 |
Serum Prolactin (ng/ml) Range Mean ± SD |
13.3 – 17.9 15.98 ± 1.38 |
Serum TSH (IU/L) Range Mean ± SD |
0.8 – 3.2 1.99 ± 0.74 |
FSH follicle stimulating hormone – LH luteinizing hormone.
TSH thyroid stimulating hormone.
Data presented as range, mean ± SD.
Table (3):
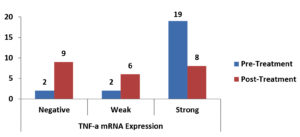
Paired differences between pre-and post-Treatment TNF-α mRNA expression in enrolled women.
| Post-Treatment TNF-α mRNA Expression |
P | |||
|---|---|---|---|---|
| Negative | Weak | Strong | ||
| Pre-Treatment TNF-α mRNA Expression Negative Weak Strong |
0 (0%) 0 (0%) 9 (39.1%) |
1 (4.3%) 0 (0%) 5 (21.7%) |
1 (4.3%) 2 (8.7%) 5 (21.7%) |
0.034 S |
TNF-α tumor necrosis factor-alpha – mRNA messenger ribonucleic acid
Data presented as frequency (percentage)
Analysis using McNemar’s Test.
S significant.
Table (4):
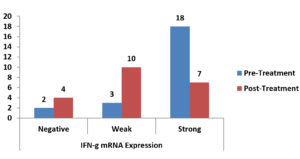
Paired differences between pre-and post-treatment IFN-γ mRNA expression in enrolled women.
| Post-Treatment IFN-γ mRNA Expression |
P | |||
|---|---|---|---|---|
| Negative | Weak | Strong | ||
| Pre-Treatment IFN-γ mRNA Expression Negative Weak Strong |
1 (4.3%) 0 (0%) 3 (13%) |
0 (0%) 1 (4.3%) 9 (39.1%) |
1 (4.3%) 2 (8.7%) 6 (26.1%) |
0.065 NS |
IFN-γ interferon-gamma – mRNA messenger ribonucleic acid
Data presented as frequency (percentage).
Analysis using McNemar’s Test.
NS non-significant
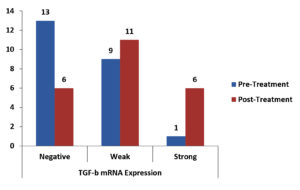
There was a statistically significant reduction in the expression of TNF-α mRNA as shown in Table 3, Fig. 1, There was a non-significant reduction of expression in IFN-γ mRNA as shown in Table 4, Fig. 2, finally, there was a significant increase in the expression of TGF-β mRNA after treatment in enrolled women as shown in Table 5, Fig. 3.
Table (5):
Paired differences between pre-and post-treatment TGF-β mRNA expression in enrolled women.
| Post-Treatment TGF-β mRNA Expression |
P | |||
|---|---|---|---|---|
| Negative | Weak | Strong | ||
| Pre-Treatment TGF-β mRNA Expression Negative Weak Strong |
4 (17.4%) 2 (8.7%) 0 (0%) |
8 (34.8%) 3 (13%) 0 (0%) |
1 (4.3%) 4 (17.4%) 1 (4.3%) |
0.035 S |
TGF-β transforming growth factor-beta – mRNA messenger ribonucleic acid
Data presented as frequency (percentage)
Analysis using McNemar’s Test.
S significant.
Fig. 1. Bar-Chart showing difference between pre-and post-Treatment TNF-α mRNA expression in enrolled women.
Fig. 2. Bar-Chart showing difference between pre-and post-Treatment IFN-γ mRNA expression in enrolled women.
Fig. 3. Bar-Chart showing Difference between Pre-and Post-Treatment TGF-β mRNA Expression in Enrolled women.
Details of IVF cycles in enrolled women are shown in Table 6 regarding number of oocytes picked up, rates of fertilization, and how many embryos transferred. Of the included 23 women, 12 women (52.2%) failed to get pregnant, 11 (47.8%) had positive biochemical pregnancy, 8 (34.8%) had positive clinical pregnancy, and 6 (26.1%) had positive ongoing clinical pregnancy. These outcomes were statistically significant (p = 0.001, 0.006, and 0.029, respectively); and clinically significant (NNT = 2, 3 and 4, respectively as in Table 7.
Table (6):
IVF/ICSI cycle outcomes in enrolled women.
No. of Oocytes Retrieved Range Median (IQR) |
9 – 17 13 (11 – 15) |
Fertilization Rate Range Mean ± SD |
0.56 – 0.82 0.69 ± 0.09 |
No. of Embryos Transferred 2 3 |
11 (47.8%) 12 (52.2%) |
SD standard deviation
IQR interquartile range
Data presented as range, median (IQR); range, mean ± SD; or frequency (percentage)
Table (7):
Pregnancy outcome in enrolled women.
Rate |
P |
NNT |
|
|---|---|---|---|
Positive Biochemical Pregnancy |
11 (47.8%) |
0.001 S |
2 |
Positive Clinical Pregnancy |
8 (34.8%) |
0.006 S |
3 |
Positive Ongoing Clinical Pregnancy |
6 (26.1%) |
0.029 S |
4 |
Data presented as frequency (percentage)
Analysis using chi-squared test (in comparison to previous IVF/ICSI failure)
NNT number needed to treat.
S significant
Table (8):
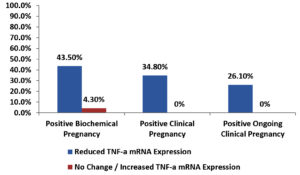
Association between positive pregnancy outcomes and change in TNF-α mRNA expression in enrolled women.
| Change in TNF-α mRNA Expression | P | ||
|---|---|---|---|
| Reduced | No Change / Increased | ||
| Positive Pregnancy Outcome Biochemical Clinical Ongoing Clinical |
10 (43.5%) 8 (34.8%) 6 (26.1%) |
1 (4.3%) 0 (0%) 0 (0%) |
0.219 (NS) 0.016 (S) 0.004 (S) |
Data presented as frequency (percentage)
Analysis using McNemar’s Test.
NS non-significant – S significant
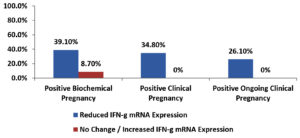
Among the enrolled women, there was a significant association between reduced TNF-α mRNA expression and each of positive clinical and positive ongoing clinical pregnancy outcomes also there was a non-significant association between reduced TNF-α mRNA expression and positive biochemical pregnancy outcome as shown in Table 8 and Fig. 4.
Fig. 4. Bar-Chart showing association between positive pregnancy outcomes and change in TNF-α mRNA expression in enrolled women.
Current study showed a significant association between reduced IFN-γ mRNA expression and each of positive clinical and positive ongoing clinical pregnancy outcomes while non-significant association between reduced IFN-γ mRNA expression and positive biochemical pregnancy outcome as shown in Table 9 and Fig. 5.
Table (9):
Association between positive pregnancy outcomes and change in IFN-γ mRNA expression in enrolled women.
| Change in IFN-γ mRNA Expression | P | ||
|---|---|---|---|
| Reduced | No Change / Increased | ||
| Positive Pregnancy Outcome Biochemical Clinical Ongoing Clinical |
9 (39.1%) 8 (34.8%) 6 (26.1%) |
2 (8.7%) 0 (0%) 0 (0%) |
0.453 (NS) 0.031 (S) 0.008 (S) |
IFN-γ interferon-gamma – mRNA messenger ribonucleic acid
Data presented as frequency (percentage)
Analysis using McNemar’s Test.
NS non-significant – S significant.
Fig. 5. Bar-Chart showing Association between positive pregnancy outcomes and change in IFN-γ mRNA expression in enrolled women.
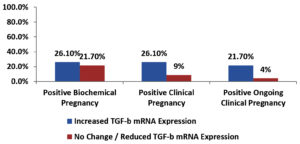
Among the enrolled women, there was a significant association between increased TGF-β mRNA expression and positive ongoing clinical pregnancy outcome, but there was a non-significant association between increased TGF-β mRNA expression and each of positive biochemical and clinical pregnancy outcomes shown in Table 10 and Fig. 6.
Table (10):
Association between positive pregnancy outcomes and change in TGF-β mRNA expression in enrolled women.
| Change in TGF-β mRNA Expression | P | ||
|---|---|---|---|
| Increased | No Change / Reduced | ||
| Positive Pregnancy Outcome Biochemical Clinical Ongoing Clinical |
6 (26.1%) 6 (26.1%) 5 (21.7%) |
5 (21.7%) 2 (8.7%) 1 (4.3%) |
0.774 (NS) 0.180 (NS) 0.039 (S) |
TGF-β transforming growth factor-beta – mRNA messenger ribonucleic acid
Data presented as frequency (percentage)
Analysis using McNemar’s Test.
NS non-significant – S significant.
Previous studies demonstrated an association between a group of infertility patterns and immune system dysfunction. Several investigations have found that cellular immunity effector pathways play a key role in the pathogenesis of unexplained infertility and poor pregnancy outcomes.12 Furthermore, it has been suggested that successful pregnancy is typically associated with a predominance of anti-inflammatory mediators while an increased inflammatory immune response is associated with recurrent pregnancy loss (RPL) and multiple implantation failures, an imbalance between regulatory and effector cells has been postulated as a possible cause of poor pregnancy outcomes and a variety of other pregnancy-related diseases.13
In concordance to current results, many studies reported a negative correlation between TNF-α and INF-γ higher rate of expression and successful IVF outcome, and positive correlation between TGF-β expression and pregnancy outcome.14-18 Current study showed that before treatment there were increased TNF-α mRNA expression (82.6%), IFN-γ mRNA expression (78.3%), and decreased expression of TGF-β in 56.5% of enrolled women. On the other hand, Banja, and colleagues, found different results regarding immune mediators expression,19 this may be explained by presence of other mediators that play important roles in occurrence and maintenance of pregnancy.
These contradictory findings underlined the necessity of immunological and inflammatory responses being regulated at the time of fertilisation and implantation for IVF and pregnancy success.15 Current findings backed up this theory, showing that inflammatory responses were elevated while regulatory responses were downregulated in women who had failed IVF attempts.
Exposure of infertile women to intralipid altered immune response and expression of certain mediators that positively affected the outcome of IVF cycles as the following: There was a significant reduced mRNA expression of TNF-α after treatment in enrolled women (p value= 0.034), same results were reported by Gornikiewicz and colleagues regarding reduced TNF-α expression after immunomodulatory therapy.16
There was a non-significant reduction in mRNA expression of IFN-γ after treatment in enrolled women (p value= 0.065) while other studies reported significant expression reduction of IFN-γ after intralipid and immunomodulatory therapy17,18 this difference might be due longer time required to detect alteration of IFN-γ expression after therapy.
There was a significant increase in mRNA expression of TGF-β after treatment in enrolled women (p.value = 0.035), Joseph Eldor published results of his study regarding increased production of TGF-β in women with multiple sclerosis and trigeminal neuralgia after intralipid therapy as immunomodulation, moreover, Pcurrentakbari and his colleagues published same results of increased TGFβ in women with infertility related diseases treated with immunomodulators,20,21 this reflect role of TGF-β in pregnancy outcome.
Of the included 23 women, 11 (47.8%) had positive biochemical pregnancy, 8 (34.8%) had positive clinical pregnancy, and 6 (26.1%) had positive ongoing clinical pregnancy. These outcomes were statistically significant (p = 0.001, 0.006, and 0.029, respectively); and clinically significant (NNT = 2, 3 and 4, respectively), Kilian Vomstein published that around 50% of cases of idiopathic recurrent pregnancy loss, also the European Society of Reproduction and Embryology (ESHRE) guidelines published 2018 report that supported the role of immunological factors as a cause of RPL and IVF failures.6,22
Intralipid infusion therapy played an important role in improving outcome in women with reproductive failures, that was reported by study done by Coulam,23 and other authors,24 among the enrolled women in current study, There was a significant association between reduced TNF-α mRNA expression and each of positive clinical and positive ongoing clinical pregnancy outcomes, however, there was a non-significant association between reduced TNF-α mRNA expression and positive biochemical pregnancy outcome which was supported by a study published by Kwak-Kim and colleagues.25
Current study showed a significant association between reduced IFN-γ mRNA expression and each of positive clinical and positive ongoing clinical pregnancy outcomes, however a non-significant association between reduced IFN-γ mRNA expression and positive biochemical pregnancy outcome which was also observed in a study published by Tomassetti and colleagues.26
Among the enrolled women, there was a significant association between increased TGF-β mRNA expression and positive ongoing clinical pregnancy outcome, however, there was a non-significant association between increased TGF-β mRNA expression and each of positive biochemical and clinical pregnancy outcomes, this goes in accordance with Perucci L.O. and colleagues.27 Nonetheless, this increase was not significant and was not sufficient to modulate immune profile in some women with recurrent implantation failure. While Min-zhi Gao and others published no association between TGF-β increased level and pregnancy outcome in women undergoing IVF cycles.28 This discrepancy may have been due to a difference in response to different immune modulators and this possibility should be investigated further.
In conclusion, results of the current study indicate that IVF cycles failure was associated with abnormal cytokines expressions (increased TNF-α, IFN-γ and decreased TGF-β). Also, it was revealed that IL had a positive impact on IVF outcomes via alterations in the peripheral cytokines expression mainly reduction of TNF-α and increased TGF-β mRNA expression. Although, many studies supported the role of IFN-γ mRNA expression and its important role as an inflammatory mediator on poor pregnancy outcome, however, this was statistically non-significant in current study. Meanwhile, a significant association between reduced IFN-γ mRNA expression and each of positive clinical and positive ongoing clinical pregnancy outcomes was observed in the present study.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MAF and IKAH contributed to samples collection and acquisition of patients’ data. MAF performed examination of the patients and diagnosed the cases. IKAH contributed to all laboratory work with analysis and interpretation of data. OIA, WKZ, NNSE and IKAH contributed to drafting the article. OIA, WKZ and NNSE contributed to revising the draft critically for important intellectual and scientific content. MAF revised the clinical content. All authors read and approved the final manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Faculty of Medicine, Ain Shams University, Cairo, Egypt with reference number FMASU MD 201/2018.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Lash GE, Ernerudh J. Decidual cytokines and pregnancy complications: focus on spontaneous miscarriage. J Reprod Immunol. 2015;108:83-89.
Crossref - Kajihara T, Brosens JJ, Ishihara O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol. 2013;46(2):61-68.
Crossref - Kammerer U, Wolff M, Markert UR. Immunology of human endometrium. Immunobiology. 2004;209(7):569-574.
Crossref - Pitman H, Innes BA, Robson SC, Bulmer JN, Lash GE. Altered expression of interleukin-6, interleukin-8 and their receptors in decidua of women with sporadic miscarriage. Human Reproduction. 2013;28(8):2075-2086.
Crossref - Jones Jr. HW, Cooke I, Kempers R, Brinsden P, Saunders D. International Federation of Fertility Societies Surveillance: preface. Fertil Steril. 2010;95(2):491.
Crossref - Cimadomo D, Craciunas L, Vermeulen N, Vomstein K, Toth B. Definition, diagnostic and therapeutic options in recurrent implantation failure: an international survey of clinicians and embryologists. Human Reproduction. 2021;(36(2):305-317.
Crossref - Governini L, Luongo FP, Haxhiu A, Piomboni P, Luddi A. Main actors behind the endometrial receptivity and successful implantation. Tissue and Cell. 2021;73:101656.
Crossref - Timeva T, Shterev A, Kyurkchiev S. Recurrent implantation failure: the role of the endometrium. J Reprod Infertil. 2014;15(4):173-183. PMCID: PMC4227974
- Vanek VW, Seidner DL, Allen P, et al. A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 2012;27(2):150-192.
Crossref - Harrity C, Shkrobot L, Walsh D. ART implantation failure and miscarriage in women with elevated intracellular cytokine ratios: response to immune support therapy. Fertil Res Pract. 2018;4:7.
Crossreef - Corkum CP, Ings DP, Burgess C, Karwowska S, Kroll W, Michalak T. Immune cell subsets and their gene expression profiles from human isolated PBMC isolated by Vacutainer Cell Preparation Tube (CPT™) and standard density gradient. BMC Immunol. 2015;16:48.
Crossref - Hayakawa S, Karasaki-Suzuki M, Itoh T, et al. Effects of paternal lymphocyte immunization on peripheral Th1/Th2 balance and TCR V beta and V gamma repertoire usage of women with recurrent spontaneous abortions. Am J Reprod Immunol. 2000;43(2):10715.
Crossref - Shufaro Y, Schenker JG. Implantation failure, etiology, diagnosis and treatment. Int J Infertil Fetal Med. 2011;2(1):1-7.
Crossref - Ticconi C, Pietropolli A, Di Simone N, Piccione E, Fazleabas A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int J Mol Sci. 2019;20(21):5332.
Crossref - Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ Regulatory T Cells and T Helper 1, T Helper 2, and T Helper 17 Cells in Human Early Pregnancy Decidua. Biol Reprod. 2010; 82(4):698-705.
Crossref - Spittler A, Reissner CM, Oehler R. Immunomodulatory effects of glycine on LPS-treated monocytes: reduced TNF-α production and accelerated IL-10 expression. The FASEB Jcurrentnal The FASEB J. 1999;13(3):563-571.
Crossref - Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49(3):397-412.
Crossref - Meng L, Lin J, Chen L, et al. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch Gynecol Obstet 2016;294(1):29-39.
Crossref - Banja QM, Mutlk T, Jassam SA, Al-araji MK, Al-kafaji QA. Correlation between interleukin 17 and transforming growth factor beta with habitual abortion in women infected with cytomegalovirus. Int J Adv Res. 2018;6(Mar):232-240.
Crossref - Eldor J. Intralipid Infusion for Myelin Sheath Repair in Multiple Sclerosis and Trigeminal Neuralgia. J Health Sci Devl. 2018;1:25-42.
- Pourakbari R, Ahmadi H, Yousefi M, Aghebati-Maleki L. Cell therapy in female infertility-related diseases: Emphasis on recurrent miscarriage and repeated implantation failure. Life sciences. 2020;258:118181.
Crossref - EShRE Guideline Group on RPL, Atik RB, Christiansen OB, Elson J, et al. ESHRE guideline: Recurrent pregnancy loss. Hum Reprod Open. 2018;18(2):hoy004.
Crossref - Coulam CB. Intralipid treatment for women with reproductive failures. A J Reproductive Immunol. 2021;85(4):e13290.
Crossref - Placais L, Kolanska K, Kraiem YB, et al. Intralipid therapy for unexplained recurrent miscarriage and implantation failure: Case-series and literature review. Eur J Obstet Gynecol ReprodBiol. 2020;252:100-104.
Crossref - Kwak-Kim J, Park JC, Ahn HK, Kim JW, Gilman-sachs A. Immunological modes of pregnancy loss. Am J Reprod Immunol. 2010;63(6):611-623.
Crossref - Tomassetti C, Meuleman C, Pexsters A, et al. Endometriosis, recurrent miscarriage, and implantation failure: is there an immunological link? Reprod Biomed Online. 2006;13(1):58-64.
Crossref - Perucci LO, Gomes KB, Freitas LG, et al. Soluble endoglin, transforming growth factor-Beta 1 and soluble tumor necrosis factor alpha receptors in different clinical manifestations of preeclampsia. PloS one. 2014;9(5):e97632.
Crossref - Gao MZ, Zhao XM, Lin Y, Sun ZG, Zhang HQ. Effects of EG-VEGF, VEGF and TGF-β1 on pregnancy outcome in women undergoing IVF-ET treatment. J Assist Reprod Genet. 2012;29(10):1091-1096.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.