ISSN: 0973-7510
E-ISSN: 2581-690X
Itraconazole is now being used as the first line drug for dermatophytosis. Complete clinical and mycological cure are not achieved in some cases. The Super Bioavailable (SB) formulation is being marketed as a better formulation of drug in terms of bioavailability. To compare the efficacy and safety of SB and conventional Itraconazole in treatment of dermatophytosis. We compared the efficacy and safety of conventional itraconazole 100 mg twice daily with SB itraconazole 50 mg twice daily in dermatophytosis for two weeks. A convenient sample size of 30 was taken in each group. There was no significant difference in clinical parameters like erythema, scaling, number of papules between the two groups. Change in mean haemoglobin, total leucocyte count, platelet count, liver enzymes SGOT and SGPT and ALP did not differ significantly between the groups. There was no significant difference in the change in KOH status between the groups. There was no significant difference in clinical and mycological clearance between the conventional and the Super Bioavailable itraconazole at the end of two weeks in case of dermatophytosis of glabrous skin.
India, Conventional Itraconazole, Dermatophytosis, Super Bioavailable Itraconazole
Itraconazole is a commonly used drug for dermatophytosis. However, it is recently seen that it fails to achieve clinical and mycological cure in some cases. One of the hypothesized reasons attributed is less bioavailability of the drug and its absorption being influenced by food intake and gastric pH.1 The Super Bioavailable (SB) formulation is believed to have better bioavailability and its absorption is not affected by food intake.1 Hence, a better clinical and microbiological outcome is expected with SB itraconazole formulation. Hence, this trial was undertaken to compare the efficacy of these two formulations of Itraconazole in treatment of dermatophytosis.
Aims and Objectives
Primary objective of the study was to compare the efficacy of Itraconazole 100mg dose with SB Itraconazole 50mg, both administered twice daily, at the end of two weeks in dermatophytosis. The secondary objective was to compare the safety of either formulation.
This was a comparative randomised observer-blinded pilot study. It has an exploratory framework with an allocation ratio of 1:1. A sample size of 60 was calculated considering a minimum number of patients to be 30 in each group for a pilot study. These 60 patients were assigned to 2 groups of 30 each using a computer-generated random allocation. Patients in Group A received oral conventional Itraconazole 100mg BID with food and the patients in Group B received Oral SB Itraconazole 50mg BID irrespective of food intake for 2 weeks. Both drugs were supplied by Glenmark Pharmaceuticals Ltd, India. Topical clotrimazole cream (Candid®) and levocetirizine 5mg daily (Teczine®) was prescribed in both the groups. Prior to starting the study, Institutional Ethics committee approval was taken, and the trial was registered with the Clinical Trial Registry of India (No. CTRI/2021/11/038062)
Inclusion criteria
1. All the patients with dermatophytosis beyond 12 years of age.
2. All patients with skin scrapping positive for fungal hyphae on KOH test.
Exclusion criteria
1. Patients with prior use of systemic/topical antifungals in last 1 month.
2. Pregnancy and lactation.
3. Deranged hepatic enzymes (More than 2 times the upper normal limit), renal or hematological profile at baseline.
4. History of Diabetes
5. History of cardiac disease (ventricular dysfunction, congestive heart failure)
6. Patients with onychomycosis.
7. Immunocompromised or patients on chemotherapy.
8. Previous hypersensitivity to any azole or imidazole compound.
9. Patient on any drug that is known to affect the bioavailability of itraconazole or with drug interaction with Itraconazole.
The study was done among the patients visiting the outpatient department of our tertiary care centre from December 2021 to February 2022. The patients underwent baseline investigations of complete blood counts, liver function test and KOH mount from the most active site. All 3 investigations were repeated at the follow-up visit at 2 weeks. The clinical data regarding 4 parameters including body surface area in terms of palm areas involved, scaling, erythema, peripheral papules graded on a scale of (0-3) were kept at the baseline and at 2 weeks follow-up by an independent dermatologist who was blinded to the intervention (Observer blinding).
Both the drug formulations dispatched were enclosed in similar looking packets and handed over to the patients. The patients were instructed to return the empty strips of the blister packet on the follow-up visit.
All 30 patients in each group were assessed. There were no dropouts from the study.
The statistical analysis was done using SPSS version 26.0 and P value <0.05 was considered significant at 95% confidence interval. The continuous variable was analyzed as mean ± standard deviation (SD) and the categorical variable were assessed as frequency and percentage. Paired t-test was used to find the association of variables between the either group and at baseline and follow-up. Chi-square test was used to find out association of categorical variables in either group.
The mean age of patients in Group A was 35.07±14.44 years and in Group B was 34.43±11.75 years and was comparable between the 2 groups. The gender distribution between the groups was also comparable at baseline (P=0.293).
The mean duration of disease in Group A was 9.40±9.53 months and in Group B was 7.36±10.04 months with no statistically significant difference between the 2 groups. (p=0.397) [Table 1]. Tinea cruris was the predominant form in either group [Table 2].
Table (1):
Demographic distribution data
| Group A | Group B | P-Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age (in years) | 35.07±14.44 | 34.43±11.75 | 0.836 |
| Gender | |||
| Female | 14(46.67%) | 10(33.33%) | 0.293 |
| Male | 16(53.33%) | 20(66.67%) | |
| Duration of disease (in months) | 9.40±9.53 | 7.36±10.04 | 0.397 |
Table (2):
Frequency of disease variability in group A and group B
| Types of Tinea | Group A | Group B |
|---|---|---|
| n (%) | n (%) | |
| T. Cruris | 9(30%) | 11(36.67%) |
| T. Corporis | 2(6.67%) | 3(10%) |
| T. Cruris/corporis | 17(56.67%) | 15(50%) |
| T. Cruris/Faciei | 1(3.33%) | 1(3.33%) |
| T. Cruris/corporis/Faciei | 1(3.33%) | 0(0%) |
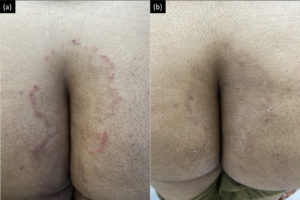
Clinically, the mean body surface area (BSA) involved at baseline in Group A and B was 7.52±7.17 and 10.00±7.81, respectively with no statistically significant difference (p= 0.217). At follow-up, the mean BSA involved was 3.55±5.08 and 5.87±5.70 for Group A and B, with no statistically significant difference (p=0.134). [Figure 1 and 2]
Figure 1. (A) Pre-treatment and (B) Post treatment photograph of tinea corporis post 14 days SB itraconazole
Figure 2. (A) Pre-treatment and (B) Post treatment photograph of tinea corporis post 14 days conventional itraconazole
The mean erythema at baseline was 1.47±0.81 and 1.73±0.74, in Group A and B, respectively. At follow-up, it was 0.40±0.56 and 0.63±0.72 for either group with no statistically significant difference (p=0.199) [Table 3].
Table (3):
Baseline and follow-up treatment analysis by statistic between group A and B
| Group A | Group B | ||
|---|---|---|---|
| P-value | |||
| BSA | |||
| Baseline | 7.52±7.17 | 10.00±7.81 | 0.217 |
| Follow-up | 3.55±5.08 | 5.87±5.70 | 0.134 |
| Erythema | |||
| Baseline | 1.47±0.81 | 1.73±0.74 | 0.223 |
| Follow-up | 0.40±0.56 | 0.63±0.72 | 0.199 |
| Scaling | |||
| Baseline | 1.83±0.74 | 1.73±0.58 | 0.586 |
| Follow-up | 0.87±0.63 | 1.00±0.58 | 0.380 |
| Papules | |||
| Baseline | 1.63±0.808 | 1.67±0.48 | 0.839 |
| Follow-up | 0.37±0.62 | 0.57±0.73 | 0.312 |
| SGOT | |||
| Baseline | 29.8±23.02 | 26.11±11.52 | 0.418 |
| Follow-up | 27.17±10.9 | 26.88±12.63 | 0.922 |
| SGPT | |||
| Baseline | 28.74±16.31 | 26.15±15.13 | 0.496 |
| Follow-up | 29.0±13.42 | 28.17±15.94 | 0.826 |
| ALP | |||
| Baseline | 113.52±42.91 | 104.94±43.38 | 0.326 |
| Follow-up | 114.73±44.62 | 109.72±37.91 | 0.601 |
| Hb | |||
| Baseline | 12.81±1.58 | 13.87±1.33 | 0.014 |
| Follow-up | 12.71±1.59 | 13.7±1.33 | 0.026 |
| TLC | |||
| Baseline | 8807.33±2961.36 | 10838.33±12021.05 | 0.396 |
| Follow-up | 8161.33±2727.15 | 7980.00±1298.72 | 0.711 |
| Platelets | |||
| Baseline | 252.57±82.97 | 249.67±60.43 | 0.875 |
| Follow-up | 235.60±80.68 | 235.00±51.04 | 0.973 |
| KOH- negative Patient (After treatment) | 16(53.33%) | 13(43.33%) | 0.354 |
The mean scaling at baseline for Group A and B was 1.83±0.74 and 1.73±0.58, with no difference statistically (p=0.586). At follow-up, scaling in either group was 0.87±0.63 and 1.00±0.58, with no statistically significant difference (p=0.380) [Table 3].
The mean papules at baseline were 1.63±0.808 and 1.67±0.48, in Group A and B, respectively. At follow-up, it was 0.37±0.62 and 0.57±0.73 for either group with no significant difference statistically (p=0.312) [Table 3].
There was no difference in the percentage of patients’ achieving mycological clearance (Negative for fungal elements on KOH) between the groups on follow-up visit (p=0.354).
The mean values of haematological parameters and hepatic enzymes at baseline and follow-up for either group have been tabulated in Table 3. There was no statistically significant difference in the change of mean haemoglobin, total leucocyte count, platelet count, SGOT, SGPT and alkaline phosphatase for either group as indicated in Table 4. Adverse effects like nausea, drug rash or congestive heart failure were not seen in any of our patients.
Table (4):
Differentiate group A and B parameters (Hb, Platelet, TLC, SGOT, SGPT, ALP) through pair t-test
Testing Parameters |
Difference between group A and B (Mean±SD) |
P-value |
|---|---|---|
Differentiate Hb (in baseline- follow-up) |
-0.07±1.04 |
0.703 |
Differentiate TLC (in baseline-follow up) |
-2212.33±12260.98 |
0.331 |
Differentiate Platelet (in baseline-follow-up) |
2.30±59.16 |
0.833 |
Differentiate SGOP (in baseline- follow-up) |
2.89±18.73 |
0.405 |
Differentiate SGPT (in baseline- follow-up) |
1.77±12.93 |
0.460 |
Differentiate ALP (in baseline- follow-up) |
3.56±32.72 |
0.556 |
A drug has to undergo various challenging steps like design and clinical validation by various phases of clinical trial before it gets approved for marketing.2,3 Most of the lead candidate drugs do hardly satisfy all ideal drug parameters like potency, toxicity, solubility, metabolisms profiles and as a result, some of the them are withdrawn in different clinical trial stages or even removed from market.3,4 Overall, each drug profile from lead candidate selection to clinical trial and post-marketing/ patients’ satisfaction play a major role in long-term existence in market.5 After toxicity, pharmacokinetics is one of the crucial parameters in clinical trial validation stages towards the selection of lead candidate drug in a mainstream therapeutic application.6,7 It defines absorption, distribution, metabolism, and excretion/toxicity profiles of a drug after administration/consumption.7 It is one of the essential profiles for dose (mg/kg) preparation and assessing the safety and effectiveness of drugs in an individual patient. Thus, most drugs are also withdrawn from the market or further undergo improvement of poor bioavailability and pharmacokinetic profiles through different formulations or drug-delivery platforms.4,7 Similarly, the present study also compared the efficacy of the commonly used systemic antifungal drug, itraconazole, and with newly formulated Super-Bioavailable (SB)-itraconazole for the treatment of dermatophytosis in respect to clinical efficacy.8,9 As we know, conventional itraconazole has some adverse pharmacokinetics profiles as its dissolution depends on gastric acid, which means pH dependency, and fluctuates in absorption; as a result, it showed inter and intra-patent variability due to poor bioavailability so require multiple dose administration.1,8 Currently introduced newer formulation, the SB-itraconazole, claims to have improved bioavailability, no food interaction and less inter-subject variability.10
Generally, itraconazole directly targets the skin and serum level tissue interactions.11 Potent formulations with higher bioavailability drug like SB-itraconazole is preferred. Several studies have highlighted conventional itraconazole has poor bioavailability and produces lesser potency. A study by Lindsay et al. confirmed that an equivalent dose of 200 mg SB-itraconazole achieved a lesser intra-patient variation (35%) than conventional-itraconazole (60%).12 As a result, SB-itraconazole reduces intra-patient variation as it can interact in serum and tissue levels during treatment due to higher bioavailability.12 Similarly, Yun et al also observed that conventional-itraconazole had an increased bioavailability after intake of bread and milk while decreased after taking rice meal.13 Co-administration of itraconazole with acidic beverages (cola) is known to increase its absorption.14
From a drug characteristic comparison point of view between conventional itraconazole and SB-itraconazole, the non-pellet formulation with pH-dependent hypromellose phthalate (HPMCP) formulation in SB-itraconazole is able to enhance the bioavailability, higher intestinal absorption, and target drug release, while the conventional-itraconazole is a pellet formulation with hydroxypropyl methylcellulose (HPMC) shows lower bioavailability, restricted to the stomach and no target drug delivery.1,8 However, the highlighted advantages such as significant higher potency against dermatophytosis, serum level expression, large set inter-individual variations, hepatic saturation of SB-itraconazole is more potential than conventional-itraconazole are still unclear as different studies observed more controversial outputs.1,10 Thus, more investigations are needed to clarify the controversial statement. In our study, we have found that there is no significant difference was observed between both treatment groups.
Based on clinical resolution of symptoms to differentiate effectiveness and safety in dermatophytosis, Manjunath et al. in their clinical trial found SB-itraconazole as a potent therapeutic choice to control dermatophytosis with 84.61% of patients achieving mycological cure within four weeks of treatment. In our study also, we found 43.33% patients achieved mycological cure.15 This difference could be due to the shorter duration of therapy in our study. In another retrospective study, the authors concluded that SB-itraconazole was more effective with similar safety profiles when compared with conventional itraconazole in the treatment of dermatophytosis.16 Mahajan et al, in their retrospective analysis evaluated effectiveness of conventional Itraconazole (100mg twice daily), the authors found out that 70% of patients who did not respond to topical monotherapy, responded with complete clearance when given in combination with itraconazole. The standard duration of therapy for dermatophytosis is 1-2 weeks, however a longer duration of treatment is necessary to achieve complete cure and prevent recurrence.17 In another retrospective clinical data assessment done by Ghate et al, to assess effectiveness of 50mg of SB-itraconazole for dermatophytosis, the authors reported complete clinical cure in 51% of the patients at end of 4 weeks while 46% patients had more than 50% improvement in their clinical symptoms. The authors concluded that SB-Itraconazole is an effective alternate for new, chronic as well as recurrent cases of dermatophytosis.18 However, we did not find any better clinical or mycological clearance with SB itraconazole as compared to conventional itraconazole.
Limitation
- A short duration of course of treatment that is 2 weeks and no follow-up for any recurrence was the major limitation of our study.
- Topical drug was used in addition to the Itraconazole capsules.
- Serum Itraconazole estimation could not be done due to unavailability of resources.
- Fungal culture and sensitivity could not be done due to limited resources.
Super Bioavailable itraconazole has better pharmacokinetic profile than conventional itraconazole. This may not reflect in the form of better efficacy and safety than in the treatment of dermatophytosis. Multiple other factors might have a role to play in the lack of adequate clinical response of dermatophytosis to itraconazole. We could not find any better efficacy or safety of SB itraconazole as compared to conventional itraconazole. Prospective trials with larger sample size and longer follow-ups are the need of the hour to consolidate the findings of the study.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
LM, BSTPS and IA conceptualized the study. IA and ND performed literature review and drafted the manuscript. LM, BSTPS, IA and BRK reviewed and edited the manuscript. BRK approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by Institutional Ethics Committee, Institute of Medical Sciences (IMS) and Sum Hospital with letter number Ref.no./DRI/IMS.SH/SOA/2021/098
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Sardana K, Khurana A, Gupta A. Parameters that determine dissolution and efficacy of itraconazole and its relevance to recalcitrant dermatophytoses. Expert Rev Clin Pharmacol. 2019;12(5):443-452.
Crossref - Roemer T, Krysan DJ. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014;4(5):a019703.
Crossref - Surur AS, Fekadu A, Makonnen E, Hailu A. Challenges and Opportunities for Drug Discovery in Developing Countries: The Example of Cutaneous Leishmaniasis. ACS Med Chem Lett. 2020;11(11):2058-2062.
Crossref - Craveiro NS, Lopes BS, Tomas L, Almeida SF. Drug Withdrawal Due to Safety: A Review of the Data Supporting Withdrawal Decision. Curr Drug Saf. 2020;15(1):4-12.
Crossref - Potter M, Donnelly JP. The role of itraconazole in preventing and treating systemic fungal infections in immunocompromised patients. Acta Haematol. 2004;111(3):175-180.
Crossref - Ruiz-Garcia A, Bermejo M, Moss A, Casabo VG. Pharmacokinetics in drug discovery. J Pharm Sci. 2008;97(2):654-690.
Crossref - Reichel A, Lienau P. Pharmacokinetics in Drug Discovery: An Exposure-Centred Approach to Optimising and Predicting Drug Efficacy and Safety. Handb Exp Pharmacol. 2016;232:235-260.
Crossref - Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59(9):5681-5696.
Crossref - Mouton JW, van Peer A, de Beule K, Van Vliet A, Donnelly JP, Soons PA. Pharmacokinetics of itraconazole and hydroxyitraconazole in healthy subjects after single and multiple doses of a novel formulation. Antimicrob Agents Chemother. 2006;50(12):4096-4102.
Crossref - Sardana K, Mathachan SR. Super Bioavailable Itraconazole and Its Place and Relevance in Recalcitrant Dermatophytosis: Revisiting Skin Levels of Itraconazole and Minimum Inhibitory Concentration Data. Indian Dermatol Online J. 2021;12(1):1-5.
Crossref - Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J. 2016;7(2):77-86.
Crossref - Lindsay J, Sandaradura I, Wong K, et al. Serum levels, safety and tolerability of new formulation SUBA-itraconazole prophylaxis in patients with haematological malignancy or undergoing allogeneic stem cell transplantation. J Antimicrob Chemother. 2017;72(12):3414-3419.
Crossref - Yun HY, Baek MS, Park IS, Choi BK, Kwon KI. Comparative analysis of the effects of rice and bread meals on bioavailability of itraconazole using NONMEM in healthy volunteers. Eur J Clin Pharmacol. 2006;62(12):1033-1039.
Crossref - Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur J Clin Pharmacol. 1997;52(3):235-237.
Crossref - Shenoy M, Dhoot D, Mahajan H, Barkate H. An Open-Label, Randomized, Double-Arm Clinical Trial to Compare the Effectiveness and Safety of Super Bioavailable Itraconazole Capsules and Itraconazole Capsules in the Management of Dermatophytosis in India. Clin Cosmet Investig Dermatol. 2021;14:1367-1376.
Crossref - Mahajan H, Dhoot D, Deshmukh G, Barkate H. Comparative clinical effectiveness and safety of super bioavailable itraconazole and conventional itraconazole in management of dermatophytosis: a retrospective data analysis. Int J Res Dermatol. 2021;7(3):388-394.
Crossref - Mahajan H, Dhoot D, Barkate H. Clinical assessment of itraconazole in dermatophytosis (CLEAR Study): a retrospective evaluation. Int J Sci Stud. 2020;8:1-5.
- Ghate S, Dhoot D, Mahajan H, Barkate H. Clinical assessment of super bioavailable Itraconazole 50 mg in dermatophytosis (Clear 50). IP Indian J Clin Exp Dermatol. 2021;7(2):125-129.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.