ISSN: 0973-7510
E-ISSN: 2581-690X
Rice as a staple food for very large population suffers from various biotic and abiotic stresses. Among the biotic stresses, Blast, Bacterial leaf blight and Brown plant hopper (BPH) are considered to most potential threats that significantly affect the rice productivity. The use of chemical usage for controlling these diseases and pest attacks is not environmentally friendly and is expensive. Using Molecular Marker assisted backcross breeding program we have improved one of our hybrid parental line with 5 genes (xa13, Xa21, Pi54, Bph20 and Bph21). We have developed 15 near isogenic lines having similar agronomical characters as of recipient parent, they are promising for their direct induction in breeding program. Our results indicated that out of fifteen lines, Six lines i.e. GK 101-12, GK 101-15, GK 101-9, GK 101-5 and GK 101-2 out-performed in all the three locations tested, they had not only have very high level of resistance to BLB, Blast and BPH but also showed significantly higher yield compared to susceptible check as well as recipient parent. These lines may be used for improvement of our existing hybrid and also for developing of new hybrids with other CMS lines. These lines are also useful for developing new set of restorer lines through (R x R) and also useful for introgression of wide spectrum of resistance to the varieties/hybrid.
Bacterial Leaf Blight, Blast, Brown Plant Hopper, Introgression, Inoculation, Molecular Assisted Backcross Breeding
Rice (Oriza sativa L.) is a very important staple food for more than 85% population of Asia; it also plays a significant contribution to the people of Africa and Latin American countries. China and India are the world’s major rice producers, with about 203 and 164 million tonnes of paddy rice harvested, respectively in 2018. (World Rice Statistics, IRRI, www.ricestat.irri.org). Despite the significant increase in rice production over the past few decades as a result of advancements such as the introduction of the semi-dwarf gene sd1, hybrids, and improved cultivation management practices, it is still necessary to significantly boost production in order to keep up with the growing demand from the expanding global population.1,2 Among the various biotic and abiotic factors that substantially reduced the rice yield, biotic stresses viz., Rice Blast, Bacterial Leaf Blight and Brown plant hopper are the major contributors to reduce rice yield. Due to recent fast climate change, the severity of biotic pressures on rice production is increasing at an alarming rate.3
Bacterial leaf blight (BLB) disease is one of the most important bacterial disease caused by Xanthomonas oryzae pv. Oryzae (Xoo). It was first discovered in Japan in 1884.4 BLB is now widespread to varying degrees in different rice-growing regions of the world, including Africa, America, and Asia.5 Typically, up to 50–60% less yield is produced, and even the grains are not harvested.6,7 The disease can be primarily categorized into two distinct phases: the leaf blight phase and the “Kresek phase”.8 The bacteria enter plants through wounds or water pores found at the upper leaf margins. Lesions with undulating edges originate from the leaf tip, and as these water-soaked lesions expand, they change to a yellow hue, ultimately resulting in the demise of the plant.9
One of the most significant diseases that damage rice is blast, which is caused by the fungus Pyricularia oryzae L.10, 11 It is widely regarded as the most severe rice disease, posing significant risks to global cultivations. More than 85 countries have been affected by Blast. From seedling to harvest, it can affect the leaves, nodes, collar, panicles, and roots of rice plants. Blast disease caused varying degrees of yield losses across different countries. Specifically, Japan experienced losses of approximately 60%, Brazil 100%, India 7.5%, Korea 8%, China 14%, Philippines 67%, Vietnam 60%, Italy 24%, and Iran 50%.12 Commonly, symptoms of leaf blast include elongated lesions with a diamond shape, where the edges appear brown or reddish-brown, while the center appears grey or whitish.13
Among sucking insect pests, Brown Plant Hopper (BPH) caused by Nilaparvata lugens (Stal) is one of the important and very devastating pest of rice across Asia, that is capable of causing yield losses of up to 60% in epidemic conditions14,15 induces Hopper burn by sucking sap from the xylem and phloem tissues through its feeding process. The BPH acts as a vector for viral diseases such as grassy stunt virus and ragged stunt virus,16 indirectly leading to additional damage.
The three-line hybrid system is the primary method for producing hybrid rice, which entails utilizing a cytoplasmic male sterile (CMS) line, a corresponding isonuclear maintainer line, and a genetically diverse restorer line. Breeding techniques can more easily improve restorer lines compared to sterile lines since sterility is not a factor to consider in the former. The restorers were subsequently evaluated through artificial inoculation. The use of chemical pesticides, bactericides and fungicides are not only hazardous to environment but it also increases the cost of cultivation. Developing host plant resistance is the most effective strategy for managing diseases and insects. By breeding rice varieties with multiple genes that confer resistance to both diseases and insects, the spectrum of resistance can be expanded, leading to increased durability of resistance in the resulting varieties. Therefore, this study employed the MAS technique to develop new restorers that possess resistance to biotic stress.
The experimental material was GK 4602 hybrid restorer line, i.e. GK 4602 R, one of the Ganga Kaveri Seed Company’s top hybrid; this hybrid is susceptible to Bacterial Leaf Blast (BLB), Blast (BL) and Brown Plant Hopper (BPH). Since the hybrid have a good agronomical attributes and popular amongst the farmer, therefore it was a candidate variety for introgression of resistance to biotic stresses viz., BL, BLB and BPH. Biotechnology team along with rice breeding team has introgressed BLB genes viz., xa13, Xa21, the line thus generated named GK4602 BLB and this was the experimental material.
The improved GK4602 BLB (which introgressed the BLB genes viz., xa13 and Xa21 from donor IRBB60) used in this investigation as a recipient parent and two donors viz., NLR 145 (donor for Pi54 blast gene) and IR 71033 (donor for Bph20 & Bph21 genes) for Blast and BPH.
The parental material recipient variety Improved GK 4602 BLB and donor parents NLR 145 and IR 71033 were planted in crossing block for generating F1s that later used for back crossing. For making the crosses the experimental material was planted in three staggered sowing dates for optimized the synchronization of flowering of the lines to get sufficient amount of F1 seeds.
The breeding scheme followed using two sets of crosses; in the first set the cross was made between the GK 4602BB improved and IR 71033, and in other set this recipient was crossed with NLR 145.
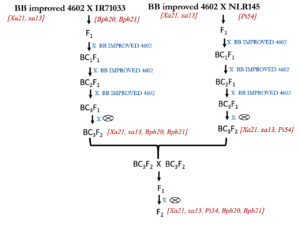
Two sets of the crosses were made between donor and recipient parents as per the crossing scheme mentioned in Figure 1.
Figure 1. Molecular marker-assisted stacking of xa13, Xa21, Pi54, Bph20 & Bph21 genes into an elite cultivar GK 4602 through marker-assisted backcrossing (MABB). The experiment was conducted during Kharif and Rabi of 2016 – 2019
The crosses thus generated were sown in crossing block. The F1 from both the sets were backcrossed with recipient parent and from each cross 5000 seed were generated. The foreground selection was done from BC1F1 in batches; the heterozygous plants were selected for further back crosses, the resultant BC1F1 of positive plants were sown along with the recipient parent and this processed were continue till BC3F1, along with the foreground selection, the background selection was also made. The plants that show the phenotypic similarity with the recipient parent are positive were advanced from BC2F1. In BC3F1, these positive plants (having all the introgressed genes) and similar to the recipient parent were selfed to produced BC3F2 from both the sets, these two sets of seed was planted in breeding block and they were intercrossed, the F1 thus generated was advance to F2, the five gene positive plants through markers were selected that showed the recipient parent plant characters. The recovery of genome of recipient parent was 99% for all the morphological traits. Primers used for foreground and background selection were mentioned in Table 1 and Table 2.
Table (1):
Foreground primers used and their sequences
Gene |
Primer |
F |
R |
Reference |
|---|---|---|---|---|
Xa21 |
pTA 248 |
AGACGCGGAAGGGTGGTTCCCGGA |
AGACGCGGTAATCGAAGATGAAA |
Ronald et al.17 |
xa13 |
xa13 prom |
GGCCATGGCTCAGTGTTTAT |
GAGCTCCAGCTCTCCAAATG |
Sundaram et al.18 |
Xa4 |
MP1 and MP2 |
ATCGATCGATCTTCACGAGG |
TGCTATAAAAGGCATTCGG |
Ma et al.19 |
Pi1 |
RM5926 |
ATATACTGTAGGTCCATCCA |
AGATAGTATAGCGTAGCAGC |
Fjellstrom et al.20 |
Pi54 |
Pi54 mas |
CAATCTCCAAAGTTTTCAGG |
GCTTCAATCACTGCTAGACC |
Ramkumar et al.21 |
Bph 20 |
BP-20-2 |
AACCAAAGTTGGTAACGAGAGC |
CGCAATCTATTAGACACCGTTC |
Rahman et al.22 |
Bph 21 |
B121 |
CGTCGTACATTCTGAAATGGAG |
GGACATGGAGATGGTGGAGA |
Rahman et al.22 |
Table (2):
Background polymorphic primers used for BLB, BLAST and BPH
Chr. Loc |
BB Polymorphic Primers |
Blast Polymorphic Primers |
BPH Polymorphic Primers |
|---|---|---|---|
1 |
RM(1,151,246, 212) |
RM(1, 151, 246, 212) |
RM(1, 24, 246, 272 ) |
2 |
RM(485, 110, 324, 240) |
RM(154,174, 240) |
RM(110, 279, 324) |
3 |
RM(231,7, 442) |
RM(514,231,7) |
RM(156, 514, 16, 570) |
4 |
RM(307,401,273, 349) |
RM(401, 307, 273) |
RM(124, 470, 261, 142 ) |
5 |
RM(153, 169, 473) |
RM(159, 437, 163) |
RM(13, 413, 122) |
6 |
RM(508, 3) |
RM(204,589) |
RM(510, 204, 435) |
7 |
RM(481, 11,234,248) |
RM(248,481,11, 234) |
RM(10, 234, 70) |
8 |
RM( 407,547,342) |
RM(310,152,223) |
RM(230, 72, 310, 344) |
9 |
RM(296,460,583) |
RM(201,434, 460, 583) |
RM(219, 242, 410) |
10 |
RM(474, 216, 117) |
RM(484, 590, 228) |
RM(244, 271, 228) |
11 |
RM(286,202,473, 254) |
RM(181,167,21,206) |
RM(21, 206, 224) |
12 |
RM(20,19,511) |
RM(17, 270,511) |
RM(519, 463, 313) |
Total |
40 |
39 |
40 |
Obtained 5 gene positive plants were evaluated in multilocation trial for agronomic and yield traits. Seed of each positive plant which has in the background of recipient parent were harvested and were divided in four sets, for multilocation trial as well as glass house trials. The hot spot of BLB, BL and BPH were selected where sufficient pressure of the pest and disease was prevalent. The multilocation trial was conducted at Ambikapur (for blast), Dhamtari for BLB and at Rajahmundry for BPH. The trial was conducted in RBD, the row to row distance was 20 cm and plant to plant distance was 15 cm each selected plant grown in 6 m row length in 12 row plots.
The seeds of five gene positive was planted in Glass house as well as hot spots of Bacterial Leaf Blight, Blast and BPH for phenotypic evaluation.
The following observations were made-
- Days to 50% flowering (days)
- No. of panicles/plant
- Panicle length (average of 10 panicles in cm)
- Yield/ha
- Disease pest reaction in control condition
- Disease and pest reaction in hot spot
Screening Against the Bacterial Blight Pathogen
Pyramided lines of both genotypes were screened using the bacterial blight (BLB) isolates available at Ganga Kaveri Seeds Pvt Ltd, Hyderabad, India, under natural conditions in the field. At maximum tillering stage, the plants’ top leaves were clip-inoculated with bacterial suspension at a density of 109 cells/ml23 to test the reaction of pathogen on plant. The isolate produced average lesion lengths of between 0.1 and 4.8 cm in resistant differentials and between 6.0 and 18 cm in susceptible differentials for evaluating the resistance of introgressed lines developed in this work. Introgressed lines were planted with a distance of 15 to 20 cm (between plants and rows) spacing. Each of the examined lines were undergone clip-inoculations of nine leaves from three distinct plants (three leaves per plant), and 20 days later, both visual score and the measurement of lesion length (LL) were studied to record the phenotypic reactions of the lines. A measurement of 5 cm of LL was used as a differentiating factor between resistant lines and susceptible lines. Plants with LL of 5 cm or less were rated as resistant, and those with LL of 5 cm or more were rated as susceptible, with minor changes.24, 25 For the screening process, three sets of plants from each line containing 18 plants were used. Screening using clip inoculation method in field is shown in Figure 2.
Screening Procedure for Blast Pathogen
A Uniform Blast Nursery (UBN) method was used to screen blast disease for every entry including both pyramided line and parents in a single row of 50 cm and with a 10 cm of spacing between row to row. Local susceptible check and resistant checks were sown in between the nursery (after every 10 entries). HR 12, most commonly used susceptible variety was spread as border in two rows for the entire plot, which in result helps in spreading of disease effectively. Disease scoring was done using 0-9 scale. Susceptible control HR12 and test entries were seen in Figure 3.
Artificial Inoculation
The nursery was planted during weather conditions conducive to blast disease development, ensuring favourable conditions for infections and the polycyclic growth of the disease. To intensify the incidence of blast, we introduced additional inoculum by harvesting diseased leaves, cutting them into small fragments, and evenly distributing them across the nursery. This process could be carried out, particularly during prolonged wet weather periods. The assessment of blast severity was conducted using the SES scale, with a minimum of two evaluations performed at 10-day intervals spanning from day 25 to day 30.26, 27 The screening involved three separate sets of plants for each line, with each set comprising 18 plants.
BPH Bioassay for the selected NILs and their Hybrids
BPH introgressed lines were evaluated for resistance in a net house. For this experiment IR71033 and TN1 were used as resistant and susceptible controls, respectively. The BPH samples employed in the test were initially gathered from the field and subsequently maintained through continuous rearing on TN1 in the laboratory. A modified seedling bulk test, in accordance with the SSST protocol, was conducted for the bioassay.28,29 Thirty pre-germinated seeds of each entry, comprising both the improved lines and controls, were uniformly planted in plastic plates measuring 7 × 7 × 8 cm. This process was replicated three times. Once the seedlings had advanced to the second leaf stage, a thinning process was carried out, reducing the number to 20 plants per plate. Upon reaching the third-leaf stage, the seedlings underwent infestation with 2nd to 3rd-instar BPH nymphs, with an application rate of 8 to 10 insects per seedling. Throughout the evaluation period, the water level was consistently maintained at approximately 0.5 cm above the root. Twenty-four hours after infestation, all seedlings received a gentle pat to ensure the even distribution of nymphs. Release of BPH nymphs on seedlings were shown in Figure 4. Subsequently, once all TN1 plants had died, the remaining plants underwent examination, and assessments were assigned according to the following modified scoring criteria, which are based on the Standard Evaluation System for Rice (IRRI 2002):
- No damage observed.
- Very slight damage detected.
- Most plants displayed yellowing in their first and second leaves.
- Yellowing was widespread, with nearly half of the plants wilting or succumbing.
- More than half of the plants had perished, and the remaining ones exhibited severe stunting.
- All plants had died.
In accordance with the criteria established by IRRI (2002), an average resistance score falling within the range of 0.1 to 1.9 was categorized as highly resistant (HR), while scores between 2.0 and 3.9 were classified as resistant (R). Scores ranging from 4.0 to 5.9 were denoted as moderately resistant (MR), whereas scores between 6.0 and 7.9 were considered susceptible (S). Finally, scores in the range of 8.0 to 9.0 were characterized as highly susceptible (HS).
The fifteen introgressed lines having all five genes multiplex were planted at Dhamtari (CG), Ambikapur (CG) and Rajahmundry (AP). The Dhamtari is a hot spot of BLB, Ambikapur is a hot spot for Blast and Rajahmundry is a hot spot for BPH.
Fifteen introgressed lines along with recipient, donor parent and susceptibly check was the experimental material. The experiment was conducted in all the three locations in RBD design, each entry were planted in 20 rows of 6 m in 20 cm row to row and 15 cm plant to plant distance in four replications. The susceptible variety fence 2 meter in all sites of the experimental plots. As per the IRRI standard scale was used for observing the incidence of BLB, Blast and BPH
Evaluation for Agronomic performance and Grain yield
In all the three locations, viz., Dhamtari, Ambikapur and Rajahmundry in middle rows ten individual plants randomly selected for taking observations for agronomic characters, the days to 50% flowering was recorded in plot basis.
In all the three locations the incidence of respective disease/pest was well above the threshold level, the susceptible line showed very high level of susceptibility. Thus it was ideal for evaluating lines for agronomical level. The susceptible check TN1 found to have very high level of incidence of BLB, BL and BPH in all the test locations as well as in control condition.
International Rice Research Institute (IRRI) scale for observing reaction of resistance/susceptibility used for taking observation.
Based on the statistical analysis and reaction to BLB, Blast and BPH the introgressed lines showed very high level of resistance compared to the susceptible TN 1. Along with the resistance these introgressed lines also showed similarity for all the agronomical traits of recipient parents.
However, the introgressed lines showed resistance to BLB, Blast and BPH, but the line which were similar to background selection were subjected to trials at hot spots viz Dhamtari (for BLB), Ambikapur (for Blast) and Rajahmundry (for BPH).
The yield per ha were recorded in all the three locations and amongst all the fifteen lines GK 101-12 (6532 Kg) found 12.32% over the recipient parent, GK 101-15 (6510 Kg; 12.28%), GK 101-9 (6351Kg; 11.98%), GK 101-5 (6292; 11.87%) and GK 101-2 (6238; 11.72%) were other introgressed lines found superior (Table 3).
Table (3):
Location Wise Grain Yield (Kg/ha)
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
Mean |
% of Increase over recipient |
|---|---|---|---|---|---|---|
1 |
GK 101-1 |
5812 |
5554 |
5438 |
5601 |
|
2 |
GK 101-2 |
6231 |
5773 |
6712 |
6238 |
11.72 |
3 |
GK 101-3 |
5632 |
5423 |
5493 |
5919 |
|
4 |
GK 101-4 |
5921 |
5529 |
5523 |
5657 |
|
5 |
GK 101-5 |
6521 |
5723 |
6632 |
6292 |
11.87 |
6 |
GK 101-6 |
5722 |
5776 |
5463 |
5653 |
|
7 |
GK 101-7 |
5888 |
5589 |
5667 |
5714 |
|
8 |
GK 101-8 |
5914 |
5593 |
5632 |
5713 |
|
9 |
GK 101-9 |
6448 |
5823 |
6783 |
6351 |
11.98 |
10 |
GK 101-10 |
5763 |
5623 |
5628 |
5671 |
|
11 |
GK 101-11 |
5832 |
5493 |
5702 |
5675 |
|
12 |
GK 101-12 |
6821 |
5962 |
6813 |
6532 |
12.32 |
13 |
GK 101-13 |
5947 |
5531 |
5673 |
5717 |
|
14 |
GK 101-14 |
5878 |
5626 |
5627 |
5710 |
|
15 |
GK 101-15 |
6773 |
5932 |
6827 |
6510 |
12.28 |
16 |
Donor 1 |
5897 |
4978 |
4982 |
5285 |
|
17 |
Donor 2 |
5962 |
5967 |
6432 |
6120 |
|
18 |
TN -1 |
3222 |
2923 |
3218 |
3121 |
|
19 |
Recipient |
5938 |
5021 |
4938 |
5299 |
|
CD at 5% |
1173 |
1350 |
900 |
|||
CV |
14.69 |
11.84 |
12.71 |
|||
SE+ |
410 |
472 |
315 |
Panicle/Sq m was highest in GK 101-9 (6551) followed by GK 101-12 (6510), GK 101-15 (6510), GK 101-9 (6351), GK 101-5 (6292), GK 101-2 (6238), whereas it was 5299 in recipient parent (Table 4).
Table (4):
Location Wise Panicle /Sq m
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
Mean |
|---|---|---|---|---|---|
1 |
GK 101-1 |
248 |
201 |
254 |
234.33 |
2 |
GK 101-2 |
253 |
245 |
263 |
253.66 |
3 |
GK 101-3 |
245 |
206 |
259 |
236.66 |
4 |
GK 101-4 |
249 |
199 |
251 |
233 |
5 |
GK 101-5 |
262 |
231 |
258 |
250.33 |
6 |
GK 101-6 |
253 |
207 |
251 |
237 |
7 |
GK 101-7 |
251 |
235 |
258 |
248 |
8 |
GK 101-8 |
249 |
239 |
253 |
247 |
9 |
GK 101-9 |
257 |
229 |
261 |
249 |
10 |
GK 101-10 |
252 |
240 |
259 |
250.33 |
11 |
GK 101-11 |
246 |
237 |
252 |
245 |
12 |
GK 101-12 |
268 |
234 |
259 |
253.66 |
13 |
GK 101-13 |
257 |
229 |
253 |
246.33 |
14 |
GK 101-14 |
251 |
232 |
255 |
246 |
15 |
GK 101-15 |
264 |
236 |
262 |
254 |
16 |
Donor 1 |
248 |
204 |
256 |
236 |
17 |
Donor 2 |
259 |
232 |
252 |
247.66 |
18 |
TN -1 |
247 |
201 |
254 |
234 |
19 |
Recipient |
252 |
229 |
258 |
246.33 |
The days to 50% flowering was lowest in GK 101-9 (100.66 days), it was 102.66 days in GK 101-5 and GK 101-12, it was103.66 in GK 101-2 and GK 101-15 and was 105.33 in recipient parent (Table 5). Panicle length was highest in GK 101-15 (24.16 cm), followed by GK 101-12 (23.43cm), GK 101-9 (23.3 cm), GK 101-2 (23.13 cm), GK 101-5 (22.66cm), it was found 23.8 cm in recipient parent (Table 6).
Table (5):
Location wise Days to 50% flowering
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
Mean |
|---|---|---|---|---|---|
1 |
GK 101-1 |
105 |
95 |
109 |
103 |
2 |
GK 101-2 |
112 |
92 |
106 |
103.66 |
3 |
GK 101-3 |
107 |
97 |
101 |
101.66 |
4 |
GK 101-4 |
109 |
93 |
107 |
103 |
5 |
GK 101-5 |
110 |
94 |
104 |
102.66 |
6 |
GK 101-6 |
108 |
99 |
108 |
105 |
7 |
GK 101-7 |
107 |
95 |
109 |
103.66 |
8 |
GK 101-8 |
106 |
92 |
106 |
101.33 |
9 |
GK 101-9 |
107 |
91 |
104 |
100.66 |
10 |
GK 101-10 |
106 |
94 |
102 |
100.66 |
11 |
GK 101-11 |
105 |
93 |
109 |
102.33 |
12 |
GK 101-12 |
109 |
95 |
104 |
102.66 |
13 |
GK 101-13 |
111 |
96 |
103 |
103.33 |
14 |
GK 101-14 |
108 |
97 |
107 |
104 |
15 |
GK 101-15 |
109 |
93 |
109 |
103.66 |
16 |
Donor 1 |
115 |
97 |
110 |
107.33 |
17 |
Donor 2 |
113 |
98 |
113 |
108 |
18 |
TN -1 |
101 |
95 |
105 |
100.33 |
19 |
Recipient |
110 |
101 |
105 |
105.33 |
Table (6):
Location Wise Panicle Length (cm)
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
Mean |
|---|---|---|---|---|---|
1 |
GK 101-1 |
21.2 |
19.2 |
23.6 |
21.33 |
2 |
GK 101-2 |
23.8 |
21.2 |
24.4 |
23.13 |
3 |
GK 101-3 |
21.6 |
20.3 |
23.8 |
21.9 |
4 |
GK 101-4 |
20.9 |
20.2 |
23.8 |
21.63 |
5 |
GK 101-5 |
23.7 |
20.2 |
24.1 |
22.66 |
6 |
GK 101-6 |
21.6 |
20.3 |
23.4 |
21.76 |
7 |
GK 101-7 |
20.9 |
20.4 |
22.8 |
21.36 |
8 |
GK 101-8 |
21.3 |
21.2 |
23.9 |
22.13 |
9 |
GK 101-9 |
24.6 |
20.2 |
25.1 |
23.3 |
10 |
GK 101-10 |
20.8 |
20.3 |
23.1 |
21.4 |
11 |
GK 101-11 |
21.2 |
20.5 |
23.7 |
21.8 |
12 |
GK 101-12 |
24.4 |
20.7 |
25.2 |
23.43 |
13 |
GK 101-13 |
21.4 |
20.8 |
23.4 |
21.86 |
14 |
GK 101-14 |
21.6 |
20.5 |
23.6 |
21.9 |
15 |
GK 101-15 |
24.9 |
21.7 |
25.9 |
24.16 |
16 |
Donor 1 |
22 |
20.6 |
23.3 |
5285 |
17 |
Donor 2 |
21.8 |
20.2 |
22.6 |
21.96 |
18 |
TN -1 |
19.9 |
20.1 |
22.2 |
20.73 |
19 |
Recipient |
24.2 |
21.6 |
25.6 |
23.8 |
Results of disease reaction for BLB, Blast, BPH and all three diseases together can be seen in Table 7, Table 8, Table 9 and Table 10, respectively.
Table (7):
Location wise reaction to Bacterial Leaf Blight
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
|---|---|---|---|---|
1 |
GK 101-1 |
R |
R |
R |
2 |
GK 101-2 |
R |
R |
R |
3 |
GK 101-3 |
R |
R |
R |
4 |
GK 101-4 |
R |
R |
R |
5 |
GK 101-5 |
R |
R |
R |
6 |
GK 101-6 |
R |
R |
R |
7 |
GK 101-7 |
R |
R |
R |
8 |
GK 101-8 |
R |
R |
R |
9 |
GK 101-9 |
R |
R |
R |
10 |
GK 101-10 |
R |
R |
R |
11 |
GK 101-11 |
R |
R |
R |
12 |
GK 101-12 |
R |
R |
R |
13 |
GK 101-13 |
R |
R |
R |
14 |
GK 101-14 |
R |
R |
R |
15 |
GK 101-15 |
R |
R |
R |
16 |
Donor 1 |
R |
R |
R |
17 |
Donor 2 |
S |
S |
S |
18 |
TN -1 |
S |
S |
S |
19 |
Improved GK4602 |
R |
R |
R |
Table (8):
Location wise reaction to Blast
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
|---|---|---|---|---|
1 |
GK 101-1 |
R |
R |
R |
2 |
GK 101-2 |
R |
R |
R |
3 |
GK 101-3 |
R |
R |
R |
4 |
GK 101-4 |
R |
R |
R |
5 |
GK 101-5 |
R |
R |
R |
6 |
GK 101-6 |
R |
R |
R |
7 |
GK 101-7 |
R |
R |
R |
8 |
GK 101-8 |
R |
R |
R |
9 |
GK 101-9 |
R |
R |
R |
10 |
GK 101-10 |
R |
R |
R |
11 |
GK 101-11 |
R |
R |
R |
12 |
GK 101-12 |
R |
R |
R |
13 |
GK 101-13 |
R |
R |
R |
14 |
GK 101-14 |
R |
R |
R |
15 |
GK 101-15 |
R |
R |
R |
16 |
Donor 1 |
S |
S |
S |
17 |
Donor 2 |
R |
R |
R |
18 |
TN -1 |
S |
S |
S |
19 |
Recipient |
S |
S |
S |
Table (9):
Location wise reaction to Bph
No |
Designation |
Dhamtari |
Ambikapur |
Rajahmundry |
|---|---|---|---|---|
1 |
GK 101-1 |
R |
R |
R |
2 |
GK 101-2 |
R |
R |
R |
3 |
GK 101-3 |
R |
R |
R |
4 |
GK 101-4 |
R |
R |
R |
5 |
GK 101-5 |
R |
R |
R |
6 |
GK 101-6 |
R |
R |
R |
7 |
GK 101-7 |
R |
R |
R |
8 |
GK 101-8 |
R |
R |
R |
9 |
GK 101-9 |
R |
R |
R |
10 |
GK 101-10 |
R |
R |
R |
11 |
GK 101-11 |
R |
R |
R |
12 |
GK 101-12 |
R |
R |
R |
13 |
GK 101-13 |
R |
R |
R |
14 |
GK 101-14 |
R |
R |
R |
15 |
GK 101-15 |
R |
R |
R |
16 |
Donor 1 |
S |
S |
S |
17 |
Donor 2 |
R |
R |
R |
18 |
TN -1 |
S |
S |
S |
19 |
Recipient |
S |
S |
S |
Table (10):
Reaction of Blb, Blast and Bph under Control Conditions.
No |
Designation |
BLB |
BLAST |
BPH |
|---|---|---|---|---|
1 |
GK 101-1 |
R |
R |
R |
2 |
GK 101-2 |
R |
R |
R |
3 |
GK 101-3 |
R |
R |
R |
4 |
GK 101-4 |
R |
R |
R |
5 |
GK 101-5 |
R |
R |
R |
6 |
GK 101-6 |
R |
R |
R |
7 |
GK 101-7 |
R |
R |
R |
8 |
GK 101-8 |
R |
R |
R |
9 |
GK 101-9 |
R |
R |
R |
10 |
GK 101-10 |
R |
R |
R |
11 |
GK 101-11 |
R |
R |
R |
12 |
GK 101-12 |
R |
R |
R |
13 |
GK 101-13 |
R |
R |
R |
14 |
GK 101-14 |
R |
R |
R |
15 |
GK 101-15 |
R |
R |
R |
16 |
Donor 1 |
R |
S |
S |
17 |
Donor 2 |
S |
R |
R |
18 |
TN -1 |
S |
S |
S |
19 |
Recipient |
R |
S |
S |
Over fifty percent of the global population relies on rice as a staple to fulfill their nutritional needs. In addition to the expanding population, the productivity and quality of rice crops are consistently limited by biotic stresses such as BPH, blast, and BLB. Developing a robust breeding strategy for enhancing the sustainable cultivation and quality of rice crops involves the strategic combination of BPH, blast, and BLB resistance genes through introgression. Conventional methods of breeding aimed at enhancing resistance against diseases and pests have proven to be relatively ineffective due to their time-consuming nature and high reliance on specific environmental conditions. So conventional and molecular methods of breeding together assisted in achieving the target of selecting best lines with improved resistance and productivity. Molecular breeding to be precise marker assisted selection (MAS) is simple, efficient and accurate. This approach aims to pyramid these genes effectively to counteract biotic stressors and promote resilience in rice cultivation. Therefore, it is crucial to incorporate resistance genes into widely adopted varieties through introgression, aiming to enhance both the productivity and quality of these varieties.
In recent years, there have been initiatives to enhance elite rice varieties by employing marker-assisted pyramiding combined with field phenotyping. This approach aims to introgress multiple targeted genes/QTLs, providing resistance against various stresses while also optimizing grain quality and yield in these preferred varieties. For example, Ramalingam implemented a pyramiding strategy by incorporating BLB resistance genes (xa5, xa13, and Xa21), blast resistance genes (Pi54), and sheath blight resistance QTLs (qSBR7-1, qSBR11-1, and qSBR11-2) into the genetic background of the cultivars ASD 16 and ADT 43.30 Ji developed novel restorer lines by combining blast resistance genes (Pita, Pi1, and/or Pi2), BLB resistance genes (Xa23 and/or xa5), and/or a BPH resistance gene (Bph3).31
In this study, an introgression program was implemented using a series of complex crosses followed by a marker-assisted breeding approach. This study shows the simultaneous introgression of multiple biotic stress resistance through marker-assisted selective intercrossing, where both foreground selection and background selection along with rigorous phenotyping at each generation resulted in the selection of positive lines with targeted traits and with high yield potential. Ten promising lines with 5 targeted genes Xa21, xa13, Pi54, Bph20 and BPh21 which have shown resistance against BLB, Blast and BPH were developed using 3 different donors. The lines which showed variations in agronomical characters were discarded and only these ten lines were evaluated for yield and other agronomical traits in hot spots of the occurrence of the stress. The introgressed lines showed significantly higher yield compared to the recipient parent and all showed more than 11% improvement over the recipient parent.
The bioassays were conducted for BLB, Blast and BPH resistance for the population. 5 gene lines have shown high level of combined resistance for biotic stress compared to individual resistance. Among them these selected ten lines has shown high level of resistance along with yield and agronomical characters matching with the recipient parent.
The finding of present investigation suggest that while improvement of line need to be done proper care must be taken for background selection as the basic objective of the introgression of resistance to biotic stresses will not be fulfilled until and unless the recovery of host genome in the background of recipient parent, therefore the present investigation the proper selection of plants advanced to next cycle of selection proper due care was taken to recover the host genome.
Considering the outcomes of the study, we believe that MAS (Marker-Assisted Selection) represents an exceptionally potent breeding strategy for transferring disease resistance and pest resistance genes from donor sources into adaptable genetic backgrounds over successive generations.
The fifteen introgressed lines having similar agronomical characters as of recipient parent, they are promising for their direct induction in a breeding program.
Our results indicated that out of fifteen lines GK 101-12, GK101-15, GK 101-9, GK 101-5 and GK 101-2 out-performed in all the locations, they had not only have very high level of resistance to BLB, Blast and BPH but also showed significant higher yield compared to susceptible check as well as recipient parent.
These lines showed along with the genotyping, the phenotyping plays a very important role for recovering the host genome, the introgressed lines showed the very high level of similarity to the recipient parent in major agronomical traits and also have on and average basis more than 10% yield superiority over the host recipient parent.
These lines may be used for improvement of GK 4602 (hybrid) and for developing of new hybrids with other CMS lines. These lines also be useful for developing new set of restorer lines through (R x R) and also useful for introgression of wide spectrum of resistance to the varieties/hybrid.
ACKNOWLEDGMENTS
The authors are thankful to the Management of Ganga Kaveri Seeds Pvt. Ltd., Basheerbagh, Hyderabad, India, for providing assistance for this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Khush GS. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol. 2005;59(1):1-6.
Crossref - Skamnioti P, Gurr SJ. Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol. 2009;27(3):141-150.
Crossref - Jamaloddin M, Durga Rani CV, Swathi G, et al. Marker Assisted Gene Pyramiding (Magp) For Bacterial Blight and Blast Resistance Into Mega Rice Variety “Tellahamsa”. Plos One. 2020;15(6):E0234088.
Crossref - Nino-Liu DO, Ronald PC, Bogdanove AJ. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol. 2006;7(5):303-324.
Crossref - Fiyaz RA, Shivani D, Chaithanya K, et al. Genetic improvement of rice for bacterial blight resistance: present status and future prospects. Rice Science. 2022;29(2):118-132.
Crossref - Zarco-Tejada PJ, Camino C, Beck PSA, et al. Pre-visual symptoms of Xylella fastidiosa infection revealed in spectral planttrait alterations. Nat Plants. 2018;4(7):432-439.
Crossref - Zhang L, Ma B, Bian Z, et al. Grain Size Selection Using Novel Functional Markers Targeting 14 Genes in Rice. Rice. 2020;13(1):-63.
Crossref - Pranamika S, Bora LC, Puzari KC, et al. Review on bacterial blight of rice caused by Xanthomonas oryzae pv. oryzae: Different management approaches and role of Pseudomonas fluorescents as a potential biocontrol agent. Int J Curr Microbiol Appl Sci. 2017;6(3):982-1005.
Crossref - Du XX, Park JR, Wang XH, et al. Genotype and phenotype interaction between OsWKRYq6 and BLB after Xanthomonas oryzae pv. Inoculation in the Field. Plants. 2022;11(3):287.
Crossref - Miah G, Rafii MY, Ismail MR, et al. Blast resistance in rice: A review of conventional breeding to molecular approaches. Mol Biol Rep. 2013;40(3):2369-2388.
Crossref - Srivastava D, Shamim M, Kumar M, et al. Current status of conventional and molecular interventions for blast resistance in rice. Rice Science. 2017;24(6):299-321.
Crossref - Wang X, Lee S, Wang J, Ma J, Bianco T, Jia Y. Current advances on genetic resistance to rice blast disease (Publisher: In Tech, Editor Wengui Yan, 2014)
Crossref - Acharya B, Shrestha SM, Manandhar HK, Chaudhary B. Screening of local, improved and hybrid rice genotypes against leaf blast disease (Pyricularia oryzae) at banke district, Nepal. J Agric Natural Resour. 2019:2(1):36-52.
Crossref - Kumar H, Maurya RP, Tiwari SN. Study on antibiosis mechanism of resistance in rice against brown plant hopper, Nilaparvata lugens (Stal.). Ann. Plant Protec. Sci. 2012: 20, 98-101.
- Wei S, Zhang H, Li B, Ji J, Shao X. Insecticidal effect of aconitine on the rice brown planthoppers. PLoS ONE. 2019;14(8):e0221090.
Crossref - Cabautan PQ, Cabunagan RC, Choi IR. Rice Viruses Transmitted by the Brown Planthopper Nilaparvata lugens Stal. In KL Heong & B. Hardy (Eds.), Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia 2009:357-368.
- Ronald PC, Albano B, Tabien R, et al. Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21 . Molec Gen Genet. 1992;236(1):113-120.
Crossref - Sundaram RM, Laha GS, Viraktamath BC, et al. Marker Assisted Breeding For Development Of Bacterial Blight Resistant Rice, Institute of Biotechnology, Acharya NG Ranga Agricultural University, 2011;500(30):154-182
- Ma BJ, Wang WM, Zhao B, Zhou YL, Zhu LH, Zhai WX. Studies of PCR marker for the rice bacterial blight resistance gene Xa-4. Hereditas. 1999: 21:9-12
- Fjellstrom RG, Conaway-Bormans CA, McClung AM, Marchetti MA, Shank AR, Park WD. Development of DNA markers suitable for marker assisted selection of three Pi genes conferring resistance to multiple Pyricularia grisea pathotypes. Crop Sci. 2004;44(5):1790-1798.
Crossref - Ramkumar G, Srinivasarao K, Mohan KM, et al. Development and validation of functional marker targeting an InDel in the major rice blast disease resistance gene Pi54 (Pik h). Mol Breeding. 2011:27(1):129-135.
Crossref - Rahman ML, Jiang W, Chu SH, et al. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor Appl Genet. 2009;119(7):1237-1246.
Crossref - Kauffman HE, Reddy APK, Hsieh SPY, et al. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis Rep. 1973;57(6): 537-541.
- Dokku P, Das KM, Rao GJN. Genetic enhancement of host plant-resistance of the Lalat cultivar of rice against bacterial blight employing marker-assisted selection. Biotechnol Lett. 2013:35(8):1339-1348.
Crossref - Dokku P, Das KM, Rao GJN. Pyramiding of four resistance genes of bacterial blight in Tapaswini, an elite rice cultivar, through marker-assisted selection. Euphytica. 2013:192(1):87-96.
Crossref - Roumen E, Levy M, Notteghem JL. Characterization of the European pathogen population of Magnaporthe grisea by DNA finger printing and pathotype analysis. Eur J Plant Pathol. 1997:103:363-371.
Crossref - IRRI. Standard evaluation system for rice. International Rice Research Institute, Los Banos, Manila, Philippines. 2002.
- Velusamy R, Heinrichs EA. Field resistance to the whitebacked planthopper Sogatella furcifera (Horvath) in IR rice varieties. Journal of Plant Protection Studies in Tropics. 1986;2(2):81-86.
- Horgan FG. Mechanisms of resistance: a major gap in understanding planthopper-rice interactions. Planthoppers: new threats to the sustainability of intensive rice production systems in Asia 2009: (ed. Heong KL, Hardy B), pp. 281-302. International Rice Research Institute (IRRI), Los Banos, Philippines.
- Ramalingam J, Raveendra C, Savitha P, et al. Gene Pyramiding for Achieving Enhanced Resistance to Bacterial Blight, Blast, and Sheath Blight Diseases in Rice. Front Plant Sci. 2020;11:591457.
Crossref - Ji ZJ, Yang SD, Zeng YX, Liang Y, Yang CD, Qian Q. Pyramiding blast, bacterial blight and brown planthopper resistance genes in rice restorer lines. J Integr Agric. 2016;15(7):1432-1440.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.