ISSN: 0973-7510
E-ISSN: 2581-690X
The microbiological assessment of the air in operating theatres is critical to control hospital-acquired infections. Regular surveillance is an important tool to evaluate the quality of air and find areas requiring intervention. In this context, the present study is undertaken to assess and compare the microbial contamination levels in operation theatre by active and passive methods. All the environmental surfaces and equipment of OTs and ICU at tertiary care hospital in Vijayapur, included in the study. This study used three sampling procedures: active, passive methods for air sampling, and swabing method for surfaces and equipment. Out of 15 OTs air sampling, the passive method showed more bacterial air contamination than the active method. Statistically, a significant difference was observed with the passive method compared to the active method with p-value of 0.0336 for both bacteria and fungus growth assessment. Out of total 90 swabs collected from all the OTs surfaces and instruments, Pseudomonas species (40%), Bacillus species (40%), Klebsiella species (20%) were the common species isolated. From the 50 swabs collected from in ICUs surfaces and instruments, culture positivity was 16% for pathogenic bacteria; Pseudomonas aeruginosa (62%), Klebsiella pneumonia (25%), and Escherichia coli (13%). The present study showed that the passive method is a better monitoring tool than the active method. So we recommend using passive air sampling method compared to active method, which is easy, cheap, and no instrument is needed for sampling the air.
Microbial contaminations, Active and Passive Methods
Microbial contamination of operating theatre (OT) and other intensive care units (ICU) in hospitals have continued to have major problems leading to nosocomial infections1. Approximately 10% of all infections show serious consequences like increased duration of hospital stay and costs, patient mortality, morbidity among post-operative patients with multidrug-resistant strains2. Environmental contamination plays an important role in the nosocomial transmission of multi-drug-resistant organisms, viruses, mycobacteria, and fungi3. Microorganisms that cause infections in hospitals originate from patients’ own endogenous flora, health care personals and environmental sources4. The contamination of the OT have been reported through multiple reservoirs like air, ventilation systems, wound drainages, surgical team members, and OT traffic, OT dresses like foot wares, gowns, gloves, improperly sterilized OT equipment, contaminated environmental surfaces5. In the past couple of decades, there has been increasing evidence concerning environmental contamination to the acquisition of nosocomial pathogens leading to healthcare-associated infections3. To assess the changing trends and types of microorganisms in the hospital environment monitoring of the air, equipment and environmental surfaces is required. This can be done by microbial testing of air, surfaces and equipment6.
Hospital environmental control procedures effectively reduce nosocomial infections, which can be achieved by strict microbiological control methods. It is possible to assess the high-risk microbial contamination in OTs to prevent nosocomial infections through air sampling. Thus there is the importance of microbial surveillance of environmental matrices. In this context, the present study is undertaken to assess and compare the microbial contamination levels in operation theatre by active and passive methods and assess the microbial contamination of surfaces and equipment in OTs and ICUs. The present study aims to assess and compare the microbial contamination levels in operation theatre by active and passive methods and to assess the microbial contamination of surfaces and equipment in OTs and ICUs by the swab method.
This is an experimental & comparative study conducted from August 2018 to September 2018. at BLDE (Deemed to be University) Shri. B. M. Patil Medical College, Hospital and Research Centre, Vijayapur-Karnataka. A total of 15 Operation Theatres, 10 Intensive care units, Environmental Surfaces like the floor, walls and equipment like OT tables, Anaesthesia machines, Cots, Ventilators, Incubators, Phototherapy units, Cautery machines, Microscopes, Chairs Trolleys were the part of the study. Samples from air, surfaces, equipment from OT’s and ICU’s after fumigation at rest were included and before fumigation were excluded from the study.
Sample collection and processing
In this study, three sampling procedures were used: active, passive air sampling methods and a swabbing method for surfaces and equipment. In all the methods, Nutrient agar, Blood agar, and Sabouraud’s dextrose agar media labeled with sample number, time and date of the collection were used. These culture media plates were taken to OT and ICU in a sealed plastic container. Air samples were taken from all operating theatres by active and passive methods simultaneously from each OT. Swabs from surfaces and equipment’s were taken from all OT’s and ICU’s. The sampling procedure was done in duplicate per each OT and ICU. Each day only one OT or ICUs was sampled.
Methods
Active method
In this method, the Air Petri sampling system (Himedia-LA637) was used for air samplings with media like Nutrient agar, blood agar, and Sabouraud’s dextrose agar in a standard petri dish plate. This system sucks air through a perforated plate. The air containing particles were impacted onto the culture media surface on to a standard Petri dish plate. After collecting air samples, the culture media plates were incubated at 37°C for 48 hours for bacterial growth and 7 days for fungal growth. The colonies were counted and expressed as colony-forming units (CFU/m3)7.
Passive method
Passive air sampling (settle plate’s methods) was done by the 1/1/1 scheme. In this, the Petri dish plates of a diameter 9 cm containing culture media were placed for 1 hour, 1 meter above the floor, about 1 meter away from the walls in OT’s. These exposed plates were incubated at 37°C for 48 hours for bacterial growth and 7 days for fungal growth. Results were expressed as CFU/m3.
Samples from surfaces and equipment
Sterile swabs moistened with sterile saline were used to collect samples. Samples from the equipment, operation table surfaces, floor, walls, etc. were collected and placed in a prelabelled sterile tube containing glucose broth and were transported to the laboratory. Swabs were incubated for 48 hours at 37°C and then observed for turbidity. The one which shows turbidity was inoculated on Nutrient agar, Blood agar, and MacConkey agar. These culture plates were incubated at 37°C under the aerobic condition for 48 hours. Colony morphology was noted, and identification of isolate was made according to standard procedures.
Statistical analyses of data were done using Mean ±SD, percentages, and diagrams. Data analysis was performed using SPSS version 17 software. The correlation coefficient was used to assess the correlation between the results of two different methods.
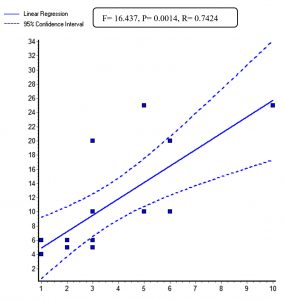
Out of 15 operation theatres air sampling conducted by the active and passive methods after a fumigation at rest, the passive method showed more bacterial air contamination than the active method. The passive method showed more colony-forming units per cubic meter of area. There was a gross difference in CFU/m3 of area between active and passive air sampling methods among operation theatres no. 1,6,10 and 14, in which passive method showed nearly twice the colony-forming units compared to the active method. The permissible limit of bacterial contamination is 10 CFU/m3. The colonies were non-pathogenic, and contaminants like micrococci, Bacillus species, coagulase-negative staphylococci from these OTs. Statistically, a significant difference was observed with passive method compared to active method with p-value of 0.0014 for bacterial assessment of air of operation theatres (Table 1).
Table (1):
Comparison of active and passive air sampling methods to detect Bacterial contamination.
| Operation theatre | No of colony forming units (CFU/m3) | |
|---|---|---|
| Active method | Passive method | |
| O T NO 1 | 10 | 25 |
| O T NO 2 | 3 | 5 |
| O T NO 3 | 1 | 6 |
| O T NO 4 | 1 | 6 |
| O T NO 5 | 3 | 6 |
| O T NO 6 | 6 | 20 |
| O T NO 7 | 5 | 10 |
| O T NO 8 | 3 | 10 |
| O T NO 9 | 3 | 6 |
| O T NO 10 | 3 | 20 |
| O T NO 11 | 2 | 6 |
| O T NO 12 | 2 | 5 |
| O T NO 13 | 1 | 4 |
| O T NO 14 | 5 | 25 |
| O T NO 15 | 6 | 10 |
F= 16.437, P= 0.0014, Correlation coefficient (r) = 0.7424
We also isolated Aspergillus species, Mucor species, Candida species, and Rhizopus species (Table 2). Also, mixed fungal growth was observed in OT no plates 3, 6, 7, 10, 11, 14 by passive method, whereas pure fungus growth was noted in the above-mentioned plates by an active method. The passive method seemed to be a better method of air sampling to assess fungal contamination. A statistically significant difference (P = 0.0336) was observed with the passive method compared to the active method (Table 3).
Table (2):
Comparision of Fungal contamination by active and passive method.
| Operation theatre | Isolated organism | |
|---|---|---|
| Active method | Passive method | |
| O T NO 1 | – | Mucor sp |
| O T NO 2 | – | Candida sp |
| O T NO 3 | Mucor sp | Rhizopus sp & Mucor sp |
| O T NO 4 | – | Mucor sp |
| O T NO 5 | – | Aspergillus sp |
| O T NO 6 | Mucor sp | Rhizopus and M ucor sp |
| O T NO 7 | – | Rhizopus and Mucor sp |
| O T NO 8 | – | Aspergillus sp |
| O T NO 9 | – | Mucor sp |
| O T NO 10 | Aspergillus sp | Aspergillus sp&Mucor sp |
| O T NO 11 | Mucor sp | Mucor sp & Candida sp |
| O T NO 12 | – | Mucor sp |
| O T NO 13 | – | Candida sp |
| O T NO 14 | Mucor sp | Aspergillus sp & Candida sp |
| OT NO 15 | – | Candida sp |
Table (3):
Comparision of fungal contamination level by active and passive methods.
Name of organism |
Active Method (%) |
Passive method (%) |
|---|---|---|
Mucor sp |
04 (58) |
09 (39%) |
Aspergillus sp |
01 (14) |
06 (26%) |
Candida sp |
1 (14) |
05 (22%) |
Rhizopus |
1 (14) |
03 (13%) |
Total |
07 (100%) |
23 (100%) |
P value= 0.0336
A total 140 swabs were collected, of which 90 were from all the Operation theatres surfaces, and 50 swabs were from intensive care units surfaces. Instruments like OT table, anaesthesia machine, trolley, floor, and shadow-less lamp from all the Operation theatres showed 10 (11%) swabs culture positivity for bacteria, and these were identified as Pseudomonas species 4(40%), Bacillus species 4 (40%), Klebsiella species 2 (20%). Cots, floors, ventilators, phototherapy units, suction machines, lamps, incubators, and drug trollies from various intensive care units showed 8 (16%) culture positivity for pathogenic bacteria. These bacteria were identified as Pseudomonas aeruginosa 5 (62%), Klebsiella pneumonia 2 (25%), and Escherichia coli 1 (13%).
Microbial contamination of OT and ICU in hospitals is becoming a major problem leading to nosocomial infections1. Approximately 10% of all nosocomial infections show serious consequences like increased duration of hospital stay and cost, mortality, morbidity among admitted patients with multidrug-resistant strains2. The most important goal for any OTs and ICUs should be reducing microbial contamination of air, surfaces, and equipment. This can be monitored by environmental samplings like air and surface sampling. The active air sampling procedure method is applicable when the microbial load is less like in an operating theatre. The passive air sampling method provides us a valid risk in assessing the microorganisms as it analyses the harmful airborne microorganisms falling onto a surgical site and on the instruments9. For these reasons, it is important to know the method used to assess the microbiological quality of air, surfaces, and equipment in the hospital environment. Thus, we have compared two air sampling methods, active and passive, to assess the microbial quality of air, surfaces, and equipment in the hospital environment.
In the present study, 15 operation theatres air samplings were taken by the active and passive method after a fumigation at rest and observed that the passive method showed more bacterial contamination (CFU/m3) compared to the active method. There was a gross difference in the CFU/m3of area between active and passive air sampling methods among operation theatres no 1,6,10 and 14. Passive method showed nearly twice the colony-forming units than the active method. In the active method of assessing bacterial contamination of air, the maximum value of detection reached a level of 10 CFU/m3. In contrast, in the passive method of assessing bacterial contamination of air, the maximum value of detection reached a level of 25 CFU/m3. Statistically significant finding with the passive method (p-value 0.0014) was found for bacterial growth assessment. This clearly indicates that the passive method was better than the active method for assessing bacterial and fungal contamination of air. Kaur et al. reported that the settle plate method was a crude method to analyze airborne contamination. However, it provides a simple and cost-effective means of assessing microbial contamination10.
In the present study, Bacillus species were the commonest contaminant among OT air samples. Similar findings were also reported by Javed I et al.11 and Sharma D et al.12 for microbiological surveillance of air in operation theatres and ICUs. Rajni Sabharwal et al. revealed mean aerobic colony counts in all the OTs within acceptable limits and isolated Bacillus species, Micrococcus species, Staphylococcus aureus, and CoNS from the operating theatre areas, which was similar to our study13.
Among the fungal contamination: Mucor, Rhizopus, Candida, and Aspergillus were common fungi observed in the present study. The passive method successfully detected the fungal growth, namely Mucor, Rhizopus, Candida, and a statistically significant p-value of 0.0336. In another study, air monitoring by active and passive methods, a high correlation coefficient was found between these two methods for A. niger. For S. aureus, a larger CFU/plate was shown in passive air sampling than in the active air sampling method. This study concluded using active and passive air sampling methods to monitor the quality of air in OTs. For the detection of fungal spore, the active method is better, contrary to our study in which the passive method showed more efficiency than the active method14. A study by Napoli et al. also document that the active method is reliable, even though many others do not recommend an active method for assessing airborne contamination in the hospital9. A study conducted in Kashmir showed that air samples monitored by the passive method could detect high bacterial contamination in which 80% of contamination was caused by Bacillus spp.
These two methods have both advantages and disadvantages. The active method needs a device for air sampling, thereby makes it costly than the passive method but allows the analyses of larger volumes of air in a lesser time. The disadvantages are that it produces noise during sampling and thus disturbs the operating team. The main advantage of the active method (slit air sampler) is that all the suspended particles in the air were collected. The passive sampling method is the most commonly used microbiological air sampling technique in the hospital. Advantages are simple and cheap but do not interrupt the microorganisms’ movement in the air during the air sampling procedure. The passive method reproduces contamination by dust particles settling onto the wound site better than the active method. However, its disadvantages are particles that are large enough to be pulled by gravity and collected onto the collecting surface media15. The discrepancies in the values of CFU obtained by these two methods can be clarified by the fact that sampling of air by passive method helps in collecting larger particles which settled by gravity. The slit air sampler in the active method draws a fixed volume of air containing particles of variable sizes16. However, many published research work has been carried out, but the guidelines are still not established to be better and better used. International guidelines offer different methods (active or passive sampling) and different types of sampling instruments, leaving the method of choice to us.
The study conducted by Rumpa et al., at Delhi tertiary care hospital demonstrated that both methods correlate when the procedure is stringently followed. Therefore, any one of the two methods can monitor the microbiological quality of air15. A study by Napoli et al. from Italy also documents a similar finding9. Several studies have evaluated microbial contamination values obtained by active and passive air sampling methods, but with inconsistent and significant correlation results. Our study has shown a significant P-value & significant correlation for the passive method of air sampling. Thus indicating that the passive method of air sampling was better than the active method.
Also, in the present study, the OTs surfaces and instruments showed 10 (11%) swabs culture positive for bacteria and were identified as Pseudomonas species 4(40%), Bacillus species 4 (40%), Klebsiella species 2 (20%). 8 (16%) showed culture positive for pathogenic organisms among ICU’s surfaces and instruments. The organisms isolated were Pseudomonas aeruginosa 5 (62%), Klebsiella pneumonia 2(25%), Escherichia coli 1(13%).
In another study from north India, 4.4% of OT surfaces and equipment samples showed bacterial culture positive. The commonest bacterial species were isolated Bacillus species (87.6%), followed by coagulase-negative Staphylococci (8.1%), Staphylococcus aureus (2.9%), and Enterococcus species (1.4%). All ICUs (100%) samples showed growth of contaminants and pathogens16. A similar result had been reported by Javed I et al.11 and Sharma D et al.13. Surface samples by the swab method showed Bacillus spp as the commonest organism17. In contrast, the present study showed pseudomonas to be the predominant environmental contaminant. Our study has also isolated pathogenic organisms like Klebsiella pneumonia, Escherichia coli, similar to a study conducted by Kiranmai et al.18
Regular microbial monitoring tools to evaluate the environmental air, surfaces, and equipment are useful for identifying situations that need intervention. However, there are no specific indications & guidelines to be used in air sampling methods. We recommend the use of passive sampling method compared to the active method, which is easy, cheap, and no instrument is needed. If the infrastructure and facilities are good enough, we can use both methods hand in hand. Swabbing techniques for surfaces are useful, easy, and cost-effective methods in resource-limited settings for surveillance of OTs surfaces and equipment to monitor the nosocomial infections. We conclude that the passive method is a better way for air sampling and to monitor the hospital-acquired infection.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors designed the experiments. RK, SW performed the experiments. RK, SW, SB and LK analyzed the data. RK and H wrote the manuscript. All authors read and approved the manuscript.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript and in the Supplementary Files.
- Bhalla A, Drin, D, Donskey CJ, Staphylococcus aureus intestinal colonization in associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. 2007;7(105)
Crossref - Haque M, Sartelli M, McKimm J, Abu Bakar M. Healthcare-associated infections – an overview. Infect Drug Resist. 2018;11:2321-2333.
- Suleyman G, Alangaden G, Bardossy AC. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr Infect Dis Rep. 2018;20:12.
Crossref - Boyce JM, Potter-Byno, G, Chenevert C, King T. Environmental contamination due to methicillin-resistant S. aureus; possible infection control implication. Infect Control Hosp Epidemiol. 1997;18:622-627.
Crossref - Reddy BR. Management of culture-negative surgical site infections. J Med Allied Sciz. 2012;2:2-6.
- De Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387-397.
Crossref - Desai SN, Kikani KM, Mehta SJ. Microbilogical Surveillance of Operation Theaters and Intensive Care Units of Teaching Hospital in Surendranagar, Gujarat. Gujarat Med J. 2012;67(2):95.
- Biddle C. Semmelweiss revisited; hand hygiene and nosocomial disease transmission in anesthesia workstation. AANA J. 2009;77(3):229-3719.
- Napoli C, Marcotrigiano V, Montagna MT. Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres. BMC Public Health. 2012;12:594.
Crossref - Kaur N, Hans C. Air bacterial isolations from operation theatres in a tertiary care hospital in India. J Clin Diagn Res. 2007; 2:87-89
- Javed I, Hafeez R, Zubair M, Anwar M, Tayyib M, Husnain. Microbiological surveillance of OT’S and ICU’S of a tertiary care hospital, Lahore. Biomedica. 2008;24:99-102.
- Sharma D, Nagarajan S. A study of cleaning/ disinfecting procedures in a primary tertiary care hospital, Delhi. Health and Population Perspectives And Issues. 2001;24(4):189-97
- Rajni SE, Rajni S. Estimation of microbial air contamination by settle plate method: are we within acceptable limit. Sch Acad J Biosci. 2015;3(8):703-707.
- Haas D, Galler H, Fritz C, Hasler C, Habib J, Reinthaler FF. Comparative study of impaction and sedimentation in an aerosol chamber using defined fungal spore and bacterial concentrations. PloS one. 2017;12(12).
Crossref - Saha R, Agarawal S, Khan AM. Air sampling procedures to evaluate microbial contamination: A comparison between active and passive methods at high-risk areas in a Tertiary Care Hospital of Delhi. J Patient Saf Infect Control. 2017;5:18-23.
- Najotra DK, Malhotra AS, Slathia P, Raina S, Dhar A. Microbiological surveillance of operation theatres: Five year retrospective analysis from a Tertiary Care Hospital in North India. Int J App Basic Med Res. 2017;7:165-168.
Crossref - Singh K, Dar A, Kishor K. Bacterial contamination in operating theatres of district Hospital Budgam in Kashmir division. Innova Journ of Medical and Health Science. 2013;3:62-63.
- Kiranmai S, Madhavi K. Microbiological surveillance of operation theatres, intensive care units and labor room of a teaching hospital in Telangana, India. Int J Res Med Sci. 2016;4:5256-5260.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.