ISSN: 0973-7510
E-ISSN: 2581-690X
Ear infection or otitis is a generic term used to refer to ear infection or inflammation. The purpose of the present study was to compare culture and Multiplex PCR methods to identify Alloiococcus otitidis, Moraxella catarrhalis, and Haemophilus influenza as three fastidious pathogens causing otitis. 100 patients, who had the physician-confirmed infection of the external ear and otitis media, were randomly sampled. The samples were analyzed with culture and Multiplex PCR. None of the three bacteria were found in samples of otitis externa by culture or PCR. In the case of otitis media three isolates of Alloiococcus otitidis, one isolation of Moraxella catarrhalis, and three strains of Haemophilus influenza were found using culture method. In the case of otitis media three isolates of Alloiococcus otitidis, one isolation of Moraxella catarrhalis, and three strains of Haemophilus influenza were found using culture method, but the rate of these bacteria were 25, 11 and 28 isolates using Multiplex PCR. The Multiplex PCR method was found to be furthermost sensitive, truthful and reproducible screening method for detection of fastidious bacteria.
Otitis media, Otitis externa, Multiplex PCR, Fastidious bacteria
Ear infection or otitis is a generic term used to refer to ear infection or inflammation. Infection can affect the internal or external parts of the ear. Furthermore, the infection can occur suddenly and persist for a short or long time1. Otitis externa, that known as swimmer’s ear, back to infection of the outer ear canal. Very severe types of this infection can spread to the bones and cartilage around the ear2. Moreover, the middle ear infection is one of the most common childhood diseases and a leading cause of general practitioner office visits by children aged under 3. Prevalence of middle ear infection is one of the main reasons leading doctors to be in quest of more affordable ways to manage this disease3. Otitis media (OM) can be considered as a general term to describe different types of infection associated with the middle ear. Otitis media is typically divided into three categories as follows:
First, acute otitis media (AOM), which is an infection with middle ear effusion and inflammation with symptoms such as ear pain, otorrhea, fever, and irritability. Second, otitis media with effusion (OME), which occurs without any symptoms of acute infection; however, it is accompanied by a discharge of fluid from the middle ear and third, Chronic suppurative otitis media (CSOM), which is a chronic inflammation of the middle ear, in which the eardrum is damaged, and pus drainage can be observed from that area. If the ear discharge persists for at least 3-6 weeks, it is called chronic suppurative otitis media4.
By the age of two years, approximately 65-70% of children experience an attack of acute otitis media. Furthermore, half of the children suffer at least 2 or more attacks of acute otitis media by the age of three5. This disease is the main reason for prescribing antibiotics to children (6). Bacteria pathogens that are the leading causes of otitis media are Streptococcus pneumoniae (25-50%), non-typeable Haemophilus influenzae (15-30%), and Moraxella catarrhalis (3-20%) (6-8). Moraxella catarrhalis, Haemophilus influenza, and Alloiococcus otitidis are classified as fastidious Bacteria. Fastidious bacteria are called so as they have complex nutritional requirements. Fastidious bacteria can only grow when its elements are provided within the environment9-11.
Alloiococcus otitidis was first isolated from an ear fluid sample of a child in a clinical study addressing otitis media in Buffalo State, America12. Given the high prevalence of the mentioned bacteria, it can be stated that Alloiococcus otitidis are one of the most frequent pathogens in otitis media among children. Furthermore, the prevalence of infection relapse along with Alloiococcus otitidis following the treatment with antibiotics is more than that of other bacteria. It has been proven that incidence of Alloiococcus otitidis in Nasopharyngeal among otitis-prone children is significantly remarkable13.
Leskinen et al. (2002) examined the prevalence of Alloiococcus otitidis, Moraxella catarrhalis, Streptococcus pneumoniae and Haemophilus influenza with the application of culture and Multiplex PCR methods. The mentioned study addressed 123 children with otitis media with effusion within the age range of 7 months-12 years. Culture results were indicative of 18 isolates of Haemophilus influenza, 14 isolates of Streptococcus pneumoniae, and eight isolates of Moraxella catarrhalis in the samples. The mentioned study was not capable of successfully isolating Alloiococcus otitidis of the samples with the application of culture method. Multiplex PCR results revealed that 25 isolates of Alloiococcus otitidis, 43 isolates of Streptococcus pneumoniae, 40 isolates of Haemophilus influenza and 78 isolates of Moraxella catarrhalis were present in the samples1.
Kaur et al. (2010) conducted a study in America examining the simultaneous presence of four species in samples obtained from children with otitis media and negative culture results with the application of Multiplex PCR method. In this study, middle ear fluid of 170 children aged 6-36 months with acute otitis media was sampled. After culturing, Streptococcus pneumoniae, Haemophilus influenza, Moraxella catarrhalis, and Staphylococcus aureus were observed in 35, 54, 13, and 2 cases, respectively, and culture results were negative in 49 cases. Furthermore, no instance of Alloiococcus otitidis was observed after the application of culture method. Forty-nine samples that presented negative culture results were examined by Multiplex PCR method. The results of PCR method were positive for 26 samples of the mentioned 49 samples. In detail, Streptococcus pneumoniae, Haemophilus influenza, Moraxella catarrhalis, and Alloiococcus otitidis were observed in 15, 8, 4, and 10 cases, respectively2.
Evaluation of the presented studies indicates the presence of significant discrepancies in the prevalence of these bacteria in the otitis media samples examined by culture and molecular methods. The significance of early detection of disease-causing pathogens, as well as pathogens predisposing children to experience otitis media, motivated the scientists to employ faster and more accurate and sensitive molecular techniques to examine the pathogens before and during the illness.
The aim of the present study was to compare culture and Multiplex PCR methods to detect Alloiococcus otitidis, Moraxella catarrhalis, and Haemophilus influenza as three fastidious pathogens causing otitis. Multiplex PCR method is capable of examining the presence of more than one gene as more than one pair of primers can be used in the test solution. Multiplex PCR method has the potential to significantly reduce testing costs and time without the need for any new devices in the laboratory. Since the introduction of Multiplex PCR method, this method has been successfully tested in various DNA research areas such as gene deletions, mutations, and polymorphism analyses, quantitative evaluations, and RNA detection. Concerning infectious diseases, it has been specified that this method is a valuable technique for the detection of viruses, bacteria, fungi, or parasites3.
Sampling
The study involved patients referring to the ear, nose, and throat clinic of Amir Alam Hospital, Tehran, Iran from March to July 2014. Fifty patients, who had the physician-confirmed infection of the external ear, were randomly sampled. With the implementation of the same method, 50 samples were collected from patients with otitis media. Before the sampling stage, the patients were informed and informed consents were obtained from all of them. The samples were collected during the surgery by a collector. Furthermore, age, gender, and patients’ referring date were recorded at the time of sampling. Otitis samples, which were not maintained for more than two hours from the time of testing, were transferred for cultivation and diagnostic tests to the microbiology center of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Half of the samples were used for culture and diagnostic tests, and the other half were saved at a temperature of -20 degrees Celsius to perform molecular tests (1).
Culture and Identification
The samples were cultured on Chocolate agar and blood agar plates enriched with factors X and D. Alloiococcus otitidis has small alpha-hemolytic colonies on blood agar plate, which are observable after 2-5 days of incubation. Moraxella catarrhalis colonies have about 1 mm diameter, white-gray color, and a hemispherical shape. Colonies are fragile, easily break down, and their surface appears to be covered with a waxy layer. Haemophilus influenza colonies on chocolate agar and blood agar plates enriched with factors X and D are convex, smooth, white-gray, and transparent. Following cultivation and purification of bacteria, those with the morphology of the bacteria colonies were examined by Gram stain technique. Alloiococcus otitidis in the process of Gram stain method are observed under the microscope as gram-positive cocci, large, and often in double or quadruple forms. Moraxella catarrhalis is a kidney-shaped gram-negative diplococcus. Haemophilus influenzae in Gram staining is observed as gram-negative coccobacilli. To identifying the bacteria using culture method, the study made use of biochemical tests of Bacteriology according to CLSI guidelines1.
Molecular Method
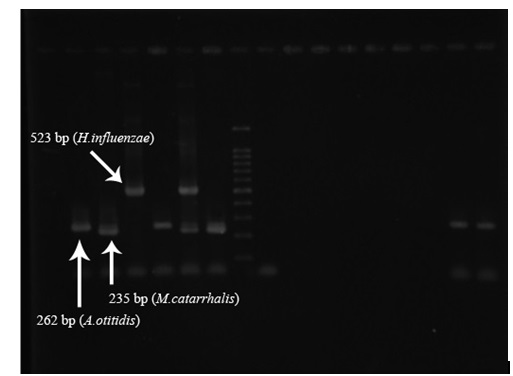
The phenol-chloroform method was used to extract DNA. Then, the three studied bacteria, which were employed in Hendolin’s study (Table 1), were examined by Multiplex PCR method using four 16srRNA gene-specific primers. PCR kits and the required materials were obtained from Sina Qiagene Company. Then, with the consideration of the number of samples and based on Table 2, the reaction mixture was prepared. In the present study, the solutions were collected with a 25-ml volume. Following the preparation of the reaction mixture, the calculated values were taken to each of the 0.5 ml microtubes, and the desired amount of DNA was added to each of the microtubes. After preparation of the reaction mixture and its division to specific microtubes on the number of samples, PCR cycles were regulated for PCR of genes as presented in Table 3. The positive control bacterial strains were isolated from clinical specimens and identified by the microbiological methods. Moreover, distilled water was used as the negative control. To performing electrophoresis of PCR products, agarose gel with a concentration of 2.1% and TBE 0.5X buffer was used. Ten microliters of each PCR product was mixed with 2 ml of loading dye, and the mixture was placed within gel sinks. To perform electrophoresis, the researchers applied 100 mV voltage for 1 hour. Upon completion of electrophoresis, the gel has been put in Ethidium Bromide at a concentration of 10 micrograms per liter for 30 minutes. Then, observation and imaging of the bands were followed using a Gel Doc. Device1.
Table (1):
Used primers
Pathogens |
Primer sequences )51→ 31) |
Fragment length (bp) |
|---|---|---|
A.otitidis (Fw) |
GGG GAA GAA CAC GGA TAG GA |
262 |
M.catarrhalis (Fw) |
CCC ATA AGC CCT GAC GTT AC |
235 |
H.influenzae (Fw) |
CGT ATT ATC GGA AGA TGA AAG TGC |
523 |
Universal (Rw) |
CTA CGC ATT TCA CCG CTA CAC |
——- |
Table (2):
The amount of material in each reaction tube
Material |
Volume (ml) |
Concentration |
|---|---|---|
Distilled water |
5,5 |
– |
A.otitidis (Fw) primer |
1 |
10 pico mol |
M.catarrhalis (Fw) primer |
1 |
10 pico mol |
H.influenzae (Fw) primer |
1 |
10 pico mol |
Universal primer (Rw) |
1 |
10 pico mol |
Master mix |
12,5 |
1.5 unit |
DNA pattern |
3 |
20 ng |
Total volume |
25 |
– |
Table (3):
Response times
| Number of cycles | phase | Temperature (°C) | Time (s) |
|---|---|---|---|
| 1 | initial denaturing | 95 | 480 |
| 30 | denaturing | 95 | 45 |
| Connection | 58 | 45 | |
| Elongation | 72 | 60 | |
| 1 | Final Elongation | 72 | 300 |
| 1 | Maintenance | 4 | Till the required time |
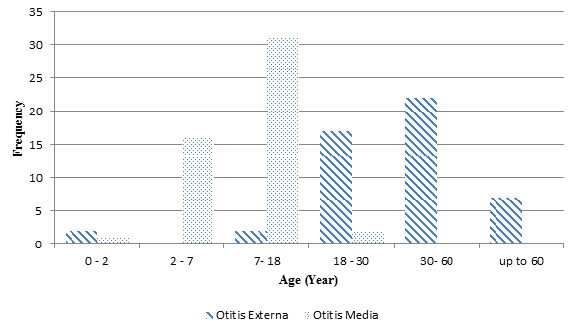
Of total 50 patients with otitis media, 29 (58%) were females, and 21 (42%) were males. The majority of the patients with otitis media were within the age range of 7-18 and then 2-7 years, respectively. Moreover, of 50 patients with otitis externa, 21 (42%) were females, and 29 (58%) were males, and the majority of the patients were in the age range of 30-60 years.
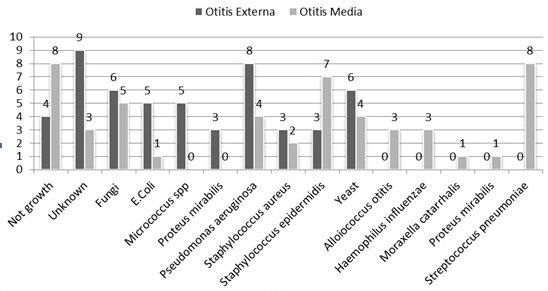
In 50 otitis media, no cases of Alloiococcus otitidis, Moraxella catarrhalis, and Haemophilus influenza were detected using culture method. Most of the bacteria isolated from otitis externa samples were related to Pseudomonas aeruginosa with 8 (38/15%) isolates. In otitis media samples, three isolates of Alloiococcus otitidis, one strain of Moraxella catarrhalis, and three strains of Haemophilus influenza were isolated by culture method. Furthermore, Streptococcus pneumoniae with 8 (16%) isolates presented the highest prevalence in the mentioned samples. Figure 1 indicates the isolated bacteria and their frequency in the otitis externa and media samples.
In molecular analysis using Multiplex PCR method, no cases of the three studied bacteria were found in 50 samples of otitis externa; however, 25 (50%) samples with respect to Alloiococcus otitidis, 28 (56%) samples with respect to Haemophilus influenza, and 11 (22%) samples with respect to Moraxella catarrhalis were positive within 50 samples of otitis media (Fig. 2).
Fig. 1. Frequency of patients with otitis externa and media with respect to age distribution in seven categories
In the present study, otitis media samples were taken from 42% males and 58% females. Gender did not significantly affect the prevalence of otitis media. Moreover, the majority of the patients were in the age range of 7-18 (62%) and then 2-7 years (32%), respectively. The mentioned statistics are in line with those of other studies reporting the highest prevalence of otitis media to be among children. With respect to otitis externa, the majority of the patients were in the age range of 30-60 and then 18-30 years, respectively, who were categorized as young and adult patients. Probably the logic behind the mentioned finding is the increase in a set of behaviors that increase the risk of developing external otitis such as being in places with high humidity and heat, swimming, having injuries in the ears, using hearing aid devices such as hearing aids or ear protective devices such as earmuffs1.
In this study, from among the 50 samples of otitis media, 3 isolates (6%) of Alloiococcus otitidis, 3 (6%) isolates of Haemophilus influenza, and 1 (2%) isolate of Moraxella catarrhalis were isolated by culture method; however, from among the 50 samples of otitis media, 25 (50%) samples with respect to Alloiococcus otitidis, 28 (56%) samples with respect to Haemophilus influenza, and 11 (22%) samples with respect to Moraxella catarrhalis were observed with the application of molecular method.
The high detection of these bacteria by molecular methods in comparison with culture method can be found in the other conducted studies. In a survey carried out by Gharibpour et al. (2013), the rate of Alloiococcus otitidis isolation by culture and PCR methods was reported to be 23/7% and 40%, respectively2. In another study, Leskinen et al. reported the isolation of Moraxella catarrhalis and Haemophilus influenza to be 7% and 15%, respectively. The researchers of the mentioned study stated that they did not succeed to isolate Alloiococcus otitidis with the application of culture method; however, the detection rate of Moraxella catarrhalis, Haemophilus influenza and Alloiococcus otitidis with the use of Multiplex PCR method was 63%, 33%, and 20%, respectively3. Kaur et al. (2010) conducted a study on children with otitis media and negative culture results. The obtained samples were examined by Multiplex PCR method, and it was found that from among 49 samples, there were 10 (4/20%) cases of Alloiococcus otitidis, 4 (16/8%) cases of Moraxella catarrhalis, and 8 cases of Haemophilus influenza4. Also, Farajzadah-Sheikh report that detection rate of Alloiococcus otitidis, Moraxella catarrhalis and Haemophilus influenza with culture method (1.4%, 2.9% and 0%) much less than multiplex PCR method (25.7%, 12% and 20%)5. To considering the above-presented results as well as the results of the present study, it can be concluded that Molecular methods in comparison with culture methods and biochemical tests are faster, more accurate, and sensitive; moreover, it is possible to detect these invading pathogens using these methods in a short time span.
As the evidence provided by studies conducted in Iran and other countries, which report the prevalence of Alloiococcus otitidis, Haemophilus influenza, and Moraxella catarrhalis detected by Multiplex PCR method as a more sensitive and accurate technique, it is observed that prevalence of Alloiococcus otitidis reported in the present study is higher than that of identical studies conducted in Sweden (2/19%) and Finland (32/20%)1, 2; however, the obtained results of the present study are in line with those of other studies conducted in Iran (40%) and Australia (40%) (3, 4). It seems that the prevalence of the mentioned bacteria is directly associated with the level of development observed in countries’ public health.
The reported prevalence of Haemophilus influenza in this study was higher than that of Sweden (32/9%), America (21/4%), Finland (16/4%), Australia (4%), and Israel (37/9%)1-5; however, it was less than the prevalence (95/2%) reported by Shishegar, Faramarzi, Kazemi, Bayat, and Motamedifar’ s (2011) study conducted in Shiraz, Iran. Remarkable prevalence of Haemophilus influenza in Iran may be due to lack of vaccination against this disease6.
Prevalence of Moraxella catarrhalis reported in this study was higher than that reported by researchers in America (7/1%), Israel (2/1%), Finland (6/5%), and Iran (16/6%)2, 3, 5, 6; however, it was less than that reported by studies carried out in Sweden (53/4%) and Finland (27/1%)7,8. It seems that the observed prevalence of Moraxella catarrhalis in different studies is related to regional differences in the mentioned countries. The role of the cited factor in the identification and determination of the prevalence of this bacterium requires further examinations.
Examination of the results of the present study and its comparison with other studies reveal that three species of Alloiococcus otitidis, Moraxella catarrhalis, and Haemophilus influenza play a crucial role in the development of otitis media though they cannot be detected and isolated in conventional cultures performed on samples due to their complex nutrition requirements and slow growth. Hence, they are neglected as important factors in the pathogenesis of patients. It seems that administration of health control programs at early ages, such as vaccination against Haemophilus influenza can lead to the reduction of otitis media development in children. Finally, it is recommended to employ Multiplex PCR molecular method as a fast, accurate, low-cost, and capable of identifying multiple pathogens to detect fastidious bacteria in patients, to apply appropriate treatment strategies.
- Goldman, L., Schafer, A.I. Goldman’s Cecil Medicine. (24 ed). Philadelphia, PA: Saunders Elsevier, 2011.
- Sood, S., Strachan, D., Tsikoudas, A., Stables, G.I. Allergic otitis externa. Clin Otolaryngol Allied Sci., 2002; 27: 233-6.
- Berman, S. Otitis media in children. The New England Journal of Medicine., 1995; 332(23): 1560-5.
- Bluestone, C.D., Gates, G.A., Klein, J.O., et al. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Ann Otol Rhinol Aryngol Suppl., 2002; 188: 8-18.
- Behrman, R.E., Kliegman, R., Jenson, H.B. (17th ed): Nelson textbook of pediatrics, New York: Saunders; 2004.
- Kliegman, R., Behrman, R.E., Nelson, W.E., Jenson, H.B., Stanton, B.F. (18th ed): Nelson textbook of pediatrics. Philadelphia: Saunders; 2007.
- Klein, J. Otitis media. Clin Infect Dis., 1994; 19: 823-33.
- Marcdante, K.J., Kliegman, R.M., Jenson, H.B., Behrman, R.E. (6th ed): Nelson essentials of pediatrics. Philadelphia: Saunders/Elsevier; 2010.
- Jordens, J.Z., Slack, M.P.E. Haemophilus influenzae: Then and Now. Eur J Clin Microbiol Infect Dis., 1995; 14: 935-48.
- Leskinen, K., Hendolin, P., Virolainen Julkunen, A., Ylikoski, J., Jero, J. The clinical role of Alloiococcus otitidis in otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2002; 66(1): 41-8.
- Murphy, T.F., Parameswaran, G.I. Moraxella catarrhalis, a Human Respiratory Tract Pathogen. Clinical Practice. 2009; 49: 124-31.
- Bosley, G.S., Whitney, A.M., Prucjler, j.M., Moss, C.Y., Daneshvar, M., Sih, T., Talkington, D.F.,. Characterization of Ear Fluid Isolates of Alloiococcus otitidis from Patients with Recurrent Otitis Media. Journal of Clinical Microbilogy. 1995; 33(11):2876-80.
- Harimaya A, Takada, R., Himi, T., Yokota, Sh., Fujii, N. Evidence of local antibody response against Alloiococcus otitidis in the middle ear cavity of children with otitis media. FEMS immunology and medical microbiology. 2007; 49: 41-5.
- Kaur, R., Adlowitz, D.G., Casey, J.R., Zeng, M., Pichichero, M.E. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. The Pediatric infectious disease journal. 2010; 29(8): 741-5.
- Elnifro, E.M., Ashshi, A.M., Cooper, R.J., Klapper, P.E. Multiplex PCR: optimization and application in diagnostic virology. Clinical microbiology reviews. 2000; 13(4): 559-70.
- Gharibpour, F., Khoramrooz, S.S., Mirsalehian, A., Emaneini, M., Jabalameli, F., Darban-Sarokhalil, D., et al. Isolation and Detection of Alloiococcus Otitidis in Children with Otitis Media with Effusion Using Culture and PCR Methods. J Mazand Univ Med Sci. 2013; 23(100): 52-60.
- Leskinen, K., Hendolin, P., Virolainen-Julkunen, A., Ylikoski, J., Jero, J. Alloiococcus otitidis in acute otitis media. Int J Pediatr Otorhinolaryngol. 2004; 68(1): 51-6.
- Farajzadah Sheikh, A., Saki, N., Roointan, M., Ranjbar, R., Yadyad, M.J., Kaydani, A., et al. Identification of Alloiococcus otitidis, Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae in Children With Otitis Media With Effusion. Jundishapur Journal of Microbiology. 2015; 8(3): e17985.
- Hendolin, P.H., Paulin, L., Ylikoski, J. Clinically Applicable Multiplex PCR for Four Middle Ear Pathogens. Journal of Clinical microbiology. 2000; 28(1): 125-32.
- Ashhurst-Smith, C., Hall, S.T., Walker, P., Stuart, J., Hansbro, P.M., Blackwell, C.C. Isolation of Alloiococcus otitidis from Indigenous and non-Indigenous Australian children with chronic otitis media with effusion. FEMS immunology and medical microbiology. 2007; 51(1): 163-70.
- Broides, A., Dagan, R., Greenberg, D., Givon-Lavi, N., Leibovitz, E. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin Infect Dis. 2009; 49(11): 1641-7.
- Brook, I., Yocum, P., Shah, K. Aerobic and Anaerobic Bacteriology of Concurrent Chronic Otitis Media With Effusion and Chronic Sinusitis in Children. Arch Otolaryngol Head Neck Surg. 2000; 126: 174-6.
- Shishegar, M., Faramarzi, A., Kazemi, T., Bayat, A., Motamedifar, M. Polymerase chain reaction, bacteriologic detection and antibiogram of bacteria isolated from otitis media with effusion in children, shiraz, Iran. Iranian journal of medical sciences. 2011; 36(4): 273-80.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.