ISSN: 0973-7510
E-ISSN: 2581-690X
In recent years, the rapid growth in Antimicrobial resistance (AMR) has become a global concern. Essential oils derived from plants that include bioactive components with proven antioxidative and antibacterial activities could be a potential solution to arrest this problem. In this study, antibacterial activities of DoTERRA essential oils such as Onguard, Clove, Teatree, Lavender and Eucalyptus were evaluated with Indian essential oils against clinical pathogenic bacteria. The GC-MS study revealed that cineole, terpinene, eucalyptol, and eugenol were the most prevalent bioactive components in these essential oils. The purity of the essential oils was confirmed with zeta potential and white light absorption spectrophotometer and shows that the Zeta potential of all the essential oils ranges from -51.4 to 0 mV. Using agar well diffusion and Micro broth dilution procedures, the antimicrobial activity of essential oils of clove, lavender, tea tree, eucalyptus, and On-Guard (combined) was assessed against several multi-drug-resistant bacteria. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of essential oils in aqueous and micellar solutions were determined by Micro broth dilution techniques. The most effective antibacterial essential oils were clove, tea tree, and On guard (a blended essential oil with a predominance of Limonene and Eugenol). The current research could result in development of formulas that contain micelle or colloid suspensions of whole essential oils such as clove, On-Guard, or Tea-Tree oil to aid in antimicrobial treatment.
Agar Well Diffusion, Antibiotic Resistance, Essential Oils, Micro Broth Diffusion, Pathogens
In recent decades, antibiotics have been the cornerstone of contemporary medicine, making it possible to successfully opt for complicated therapeutic options such as complex surgeries and chemotherapy. Antimicrobial resistance, however, is on the rise globally as microorganisms have become resistant to multiple antibiotics due to excessive use of antibiotics and epidemic transmission of specific clones.1 The unchecked spread of antimicrobial resistance has significantly curtailed the efficiency of antibiotics.2 According to historical evidence, the emergence of resistant organisms has occurred after the introduction of each new class of antibiotics.3 The pipeline for new antibiotics remains dry despite an aging society and increased immunocompromised patients who require antibacterial therapy.1,4 As an alternative to traditional antibiotics, it is necessary to investigate more recent choices, such as essential oils.
Essential oils, also known as volatile oils or ethereal oils, are naturally occurring complex molecular metabolic secretions of plants, whose functions have not yet been fully comprehended.5 Plants have been the principal source of medicine for thousands of years in rural areas of emerging nations as well as the rest of the world.6 Essential oils are relatively common in the plant kingdom, with some families having extremely high levels of these compounds both in terms of quantity and number.5
Volatile oils are made up of a complex mixture of organic compounds that give them their distinct note, which is determined by the species, the time of year they are harvested, the climate, and the precise plant component from which they are collected. Extraction of essential oils, by distilling or pressing the plant’s specific parts, is an expensive process because of the large amount of raw material required to produce a few milliliters of oil.5 Hydro distillation is one of the frequent ways to purify volatile oils along with hydro diffusion, and solvent extraction.7 Terpenes (mainly monoterpenes and sesquiterpenes), terpenoids (oxygenated chemicals like phenols, alcohols, aldehydes, ketones, or ethers), and aromatic hydrocarbons make up the majority of the composition. Some of these substances are water-insoluble, whereas others are hydrophobic.8
Each essential oil differs in its constituents thereby endowing each essential oil with unique antibacterial effects.9 Despite the fact that essential oils have antibacterial properties, little is known about their basic elements and mechanisms of action. Essential oils have already been used successfully in the treatment of a variety of conditions such as urinary tract diseases,10 respiratory diseases,11 intestinal disorders,12 and cutaneous wounds.13
Natural essential oils have the potential to be a formidable weapon against bacteria, viruses, and fungi, among other ailments. Given their low toxicity, accessibility, availability, and commercial feasibility, essential oils have attracted global interest. Furthermore, essential oils could be used as disinfectants and air purifiers as these can be dispersed as aerosols that could prove to be an important tool in fighting pandemics such as COVID-19. The goal of this research was to learn more about essential oils’ antibacterial properties so that an effective antibacterial blend could be developed.
Media, chemicals, essential oils and bacterial strains
Essential Oils (EO) such as (Tea Tree, Onguard, Eucalyptus, and Lavender) and antibiotics are used in this study. These EOs are purchased from Indian Market (non branded) as well as from US based company DoTERRA. The antibiotic discs of amikacin and Mueller-Hinton (MH) broth were obtained from Himedia, India. Amikacin is a very effective drug against gram negative bacilli particularly multi drug resistant organisms. The human clinical bacterial strains Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii, Staphylococcus aureus, Enterococcus fecalis, and Klebsiella pneumoniae were obtained from SRM Medical College Hospital patients and isolated and confirmed with standard techniques.
GC-MS analysis
The essential oils were subjected to GC- MS (Agilent Technologies GC-780B and MS-5977A) analysis to characterize the functional compounds in the EO. 1µl of essential oils was injected with 1:10 slit ratio with the injector operated at 250°C. The oven was initially programmed at 60°C for 2min then raised to 270°C at rate of 3°C/min for 10 minutes. The total run time was 87 minutes with scan interval of 0.5 sec.14 The mass spectral scan range was set at 30-550 (m/z) and the peaks were characterized by comparing with standard library.
Antimicrobial susceptibility testing (AMST)
The study was conducted on 100 clinical strains obtained from patient samples. Ten strains each of Staphylococcus aureus and Enterococcus fecalis and twenty strains each of Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii, and Klebsiella pneumoniae were included in the study. Of these, 40 strains of bacteria belonged to the multidrug-resistant category that is resistance to at least three classes of drugs. The testing was conducted as per the broad guidelines given by the Clinical & Laboratory Standards Institute (CLSI).
Antimicrobial susceptibility test using essential oils by agar well diffusion method15
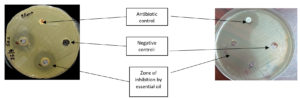
The effect of the essential oils on the bacteria was determined by the agar well diffusion method. The isolated bacteria were inoculated in peptone water and incubated at 37°C for 20 minutes. The turbidity was standardized to 0.5 McFarland turbidity. The Mueller-Hinton agar plate surface was inoculated by swabbing the standardized microbial suspension over the entire agar surface. Essential oils were primed in an organosulfur solvent DMSO (C2H6OS) at final concentrations of 20µg/ml, 30µg/ml, and 40µg/ml. Four wells of 8mm diameter were punched aseptically with a sterile tip. Each well got the designated volume of oils, along with placement of amikacin disc which acted as the positive control & a negative control of Millipore water. The dispersion of oils in the well lasted around 30 minutes at 25°C (room temperature). The agar plates were incubated for 24 hours at 37°C. Plates were observed after incubation to determine the zone of inhibition around the wells which corresponded to antibacterial activity. The diameter of the zone of inhibition was measured in mm. This was carried out in triplicate and the average diameter for each EO-bacteria combination was noted. (Figure 1)
Antimicrobial susceptibility test using essential oils by micro broth dilution method15
The Antimicrobial activity was carried out using the broth microdilution method using essential oils. Direct two-fold dilutions of each essential oil were prepared in the fractions of 1, 0.5, 0.25, 0.125, and 0.06 using an organosulfur solvent DMSO (C2H6OS). The test isolates were standardized to 0.5 McFarland turbidity in peptone broth. 200 µl of the bacterial suspension was added to a 96-well microplate. Each row of wells received an increasing concentration of the prepared essential oil. The positive control was a well with only the bacterial inoculum and the negative control was Millipore water. The microplate was incubated for 24 hours at 37°C. Microplates were observed after incubation to determine the minimum inhibitory concentration (MIC) as the well showing no visible turbidity. Minimum bactericidal concentration (MBC) was also determined.
Nine essential oils were assessed for their chemical composition and antibacterial activity. Mass spectrometry revealed that On-guard essential oil is the richest in Limonene (43.86%) and Eugenol (40.84%), with lower concentrations of Eugenol-acetate (4.05%) and Caryophyllene (2.38%). Clove essential oil contains in majority compounds such as Eugenol (58.71%), Eugenol acetate (29.15%) and Caryophyllene (8.04%) and p-Allylphenol (0.56%) in a very minimal amount. Tea tree essential oil majorly consists of (+)-Terpinen-4-ol (38.94%), Crithmene (20.41%) and Terpinene (10.86%) and Eucalyptol (5.31%) in a minute amount which is also a major component of Eucalyptus oil. Eucalyptus essential oil majorly consists of Eucalyptol (74.29%), L-α-Terpineol (11.84%) and β-Pinene (1.73%) and α-Phellandrene (1.1%) in a very minute quantity. Lavender oil was found to be potent in Linalyl alcohol (32.43%), and Linalyl anthranilate (25.77%) and consists of minimal amounts of Caryophyllene (4.22%) and Linalyl acetate (4.42%). (Table 1)
Table (1):
GC-MS of essential oils: Major components
ON guard |
D-Limnene (43%), Eugenol )(40%), Phenol-2-Methoxy-4-(2-propenyl)-acetate (4%), Caryophyllene (2.3), 2-Prpenal 3-phenyl (1.9%), Cinnamaldehyde (E) (1.3%) |
Tea tree |
(+)-Terpinen-4-ol (38.94%), Crithmene (20.41%), Terpinene (10.86%), Eucalyptol (5.31%) |
Lavender |
Linalyl alcohol (32.43%), Linalyl anthranilate (25.77%), Caryophyllene (4.22%), Linalyl acetate (4.42%). |
Eucalyptus |
Eucalyptol (74.29%), L-α-Terpineol (11.84%), β-Pinene (1.73%), β- Phellandrene (1.1%) |
Clove |
Eugenol (58.71%), Eugenol acetate (29.15%), Caryophyllene (8.04%), p-Allylphenol (0.56%) |
Tea tree India |
(+)-Terpinen-4-ol (34.6%), Crithmene (24.14%), Terpinene (12.54%), Eucalyptol (3.36%) |
Lavender India |
Linalyl alcohol (29.87%), Linalyl anthranilate (26.21%), Caryophyllene (4.51%), Linalyl acetate (5.69%). |
Eucalyptus India |
Eucalyptol (78.96), L-α-Terpineol (8.35%), β-Pinene (1.53%), β- Phellandrene (1.21%) |
Clove India |
Eugenol (57.82%), Eugenol acetate (30.17%), Caryophyllene (8.59%), p-Allylphenol (0.31%) |
Most of the tested essential oils have a negative zeta potential value because of their surface charge which interacts with the essential oil. On Guard essential oil displayed a greater zeta potential and demonstrated effective antibacterial activity against pathogens in agar well diffusion method. The lavender essential oil had zero as the zeta potential value which showed lower antibacterial activity against pathogens in agar well diffusion method. The oil samples were between 90 and 95 percent pure, with Tea Tree and lavender being ionically neutral, according to UV spectrophotometry. For the 200-400 nm wavelength, the absorbance varied between 3.0 and 4.0. On guard essential oil had a maximum value of -51.4 mV, while Lavender India had a maximum value of 0 mV.
Antibacterial activities of the essential oil compounds were tested against 100 bacterial strains belonging to Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii, Staphylococcus aureus, Enterococcus fecalis, and Klebsiella pneumoniae by well diffusion and the broth microdilution method. The antibiotic susceptibility of the test isolates to selected antibiotics revealed that high degree of resistance is noted to penicillin, early generation of cephalosporins and fluoroquinolones while injectable drugs such as aminoglycosides and carbapenems are more effective (Figure 2).
Figure 2. Resistance of the bacterial isolates against selected antibiotics
Abbreviations used: BL+BLI= β-Lactams+ β Lactams Inhibitors; G. CEPH= Generation Cephalosporins; AG= Aminoglycosides, FQ= Fluoroquinolones
In the well diffusion testing, Tea tree (DoTERRA) oil showed the maximum zone of inhibition against Klebsiella pneumoniae (27mm at 20 ug/ml) and Escherichia coli (26mm at 20 ug/ml). Clove oil (I) demonstrated the maximum zone of inhibition against Acinetobacter baumanii (36 mm at 20 ug/ml); Enterococcus fecalis (37mm at 20 ug/ml) and Pseudomonas aeruginosa(24 mm at 20 ug/ml). Staphylococcus aureus was maximally inhibited at Tea tree (I) showing a zone of inhibition of 32 mm at 20 ug/ml. The essential oils were tested in increasing concentrations against the pathogens (20 ug/ml, 30 ug/ml, and 40 ug/ml) which paralleled a corresponding increase in the zone of inhibition. There was no major difference in the effect of essential oils against the relatively sensitive strains as well as against the multidrug resistant bacteria. The details of the average zone of inhibition in mm against different bacteria are detailed in Table 2.
Table (2):
Average zone of inhibition of essential oils against bacteria
Bacterium |
Conc. pg/ul |
E. coli |
Klebsiella pneumoniae |
Acinetobacter baumanii |
Pseudomonas aeruginosa |
Staphylococcus aureus |
Enterococcus spp |
|---|---|---|---|---|---|---|---|
Essential Oils |
|||||||
On-guard |
20 |
15 |
20 |
23 |
20 |
15 |
10 |
30 |
18 |
22 |
24 |
21 |
17 |
13 |
|
40 |
21 |
24 |
25 |
25 |
19 |
15 |
|
Tea tree DT |
20 |
26 |
27 |
25 |
14 |
23 |
8 |
30 |
33 |
30 |
30 |
15 |
27 |
10 |
|
40 |
40 |
33 |
35 |
16 |
35 |
12 |
|
Tea tree I |
20 |
11 |
7 |
21 |
14 |
32 |
9 |
30 |
12 |
9 |
23 |
16 |
36 |
11 |
|
40 |
15 |
10 |
24 |
17 |
38 |
12 |
|
Eucalyptus DT |
20 |
19 |
15 |
19 |
14 |
16 |
7 |
30 |
20 |
16 |
20 |
17 |
17 |
8 |
|
40 |
21 |
17 |
22 |
18 |
18 |
11 |
|
Eucalyptus I |
20 |
11 |
16 |
15 |
7 |
10 |
6 |
30 |
12 |
17 |
19 |
9 |
14 |
7 |
|
40 |
13 |
19 |
19 |
10 |
16 |
9 |
|
Lavender DT |
20 |
9 |
6 |
12 |
20 |
14 |
11 |
30 |
10 |
7 |
17 |
22 |
16 |
12 |
|
40 |
13 |
8 |
18 |
24 |
19 |
15 |
|
Lavender I |
20 |
9 |
6 |
12 |
13 |
9 |
9 |
30 |
10 |
7 |
15 |
15 |
11 |
10 |
|
40 |
12 |
9 |
17 |
16 |
14 |
13 |
|
Clove DT |
20 |
16 |
16 |
35 |
11 |
19 |
35 |
30 |
17 |
17 |
37 |
14 |
20 |
36 |
|
40 |
18 |
18 |
38 |
15 |
23 |
37 |
|
Clove I |
20 |
20 |
17 |
36 |
24 |
21 |
37 |
30 |
23 |
19 |
37 |
26 |
23 |
38 |
|
40 |
24 |
20 |
39 |
29 |
24 |
39 |
In the micro broth dilution test, the MIC of Onguard was 0.06 ug/ml against Klebsiella pneumoniae, Enterococcus fecalis, and Escherichia coli while clove oil DoTERRA showed MIC of 0.06 ug/ml against Acinetobacter baumanii and Pseudomonas aeruginosa. Clove oil (I) demonstrated MIC of 0.06 ug/ml against Staphylococcus aureus, Enterococcus fecalis, and Klebsiella pneumoniae. The highest antibacterial effect was expressed by Clove oil, On-guard, and Tea tree oil. Lavender and eucalyptus oil did not show strong antibacterial activity. (Table 3). Reversal of antibiotic resistance was not noted as combination of Essentia oil and antibiotics was not tested.
Table (3):
Average zone of inhibition of essential oils against bacteria
| Minimum Inhibitory Concentration (ug/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Essential Oils | Clove I | Clove DT | Lavender I | Lavender DT | Tea tree I | Tea tree DT | Eucalyptus I | Eucalyptus DT | On-guard |
| Bacterium | |||||||||
| E. coli | 0.11 | 0.11 | 0.17 | 0.29 | 0.29 | 0.09 | 0.3 | 0.3 | 0.06 |
| Klebsiella pneumoniae | 0.06 | 0.11 | 0.17 | 0.29 | 0.29 | 0.09 | 0.18 | 0.3 | 0.06 |
| Acinetobacter baumanii | 0.11 | 0.06 | 0.29 | 0.29 | 0.09 | 0.09 | 0.3 | 0.3 | 0.12 |
| Pseudomonas aeruginosa | 0.11 | 0.06 | 0.17 | 0.17 | 0.09 | 0.09 | 0.3 | 0.18 | 0.12 |
| Enterococcus spp | 0.6 | 0.06 | 0.17 | 0.29 | 0.09 | 0.29 | 0.18 | 0.3 | 0.06 |
| Staphylococcus aureus | 0.6 | 0.11 | 0.29 | 0.29 | 0.29 | 0.09 | 0.3 | 0.18 | 0.12 |
Due to excessive use of antibiotics, drug resistance genes have been transmitted across the globe leading to proliferation of multi drug resistant microorganisms. These bacteria even become a part of the individual’s microbiome and can subsequently lead to infections that are challenging to treat with conventional antibiotics.1 To combat the developing resistance of microorganisms to antibiotics, the spotlight has focused on natural compounds, such as essential oils, as a source of powerful antibacterial compounds. Multiple papers have highlighted the use of essential oils as antibacterial agents.10-13
Plant extracts consist of complex mixtures of major compounds and their secondary metabolites which may have possible synergistic effects on the inhibition of bacteria. The use of natural materials as antimicrobial agents has a number of additional benefits, including higher patient tolerance, lower risk of side effects, cost-effectiveness, and widespread acceptance due to their long history of use, renewability, and improved biodegradability.16
Numerous factors, including genotype, environment, seasonal fluctuations, soil composition, plant organ, and harvesting time, can affect the diversity of the chemical composition of essential oils. The geographical origin of the plant, the organ, and the method of extraction are three factors that have a significant impact on the chemical makeup of essential oils.17 Therefore, it is crucial to determine the precise makeup of essential oils and detect any impurities. In our study, the exact composition of similar oil obtained from two manufacturers showed minor changes in the compositions while the proportions of the constituents differed from each manufacturer. This may explain the difference in the results of the AMST where different brands show slight variations in zone sizes and MICs.
This study evidenced that Clove as well as Tea tree EO, show favorable bacteriocidal properties as they contain compounds such as eugenol and terpinen-4-ol having high antiseptic and antifungal properties. These EOs are therefore widely used in traditional medicine for treating various skin infections caused by bacteria and fungi. On guard oil is a blended oil with a predominance of Limonene and Eugenol which contributes to its antibacterial properties. The phytochemical compounds â-linalool, linalyl acetate, and -alpha terpinyl acetate are abundant in lavender essential oil, enhancing its role in treating epilepsy and insomnia.
Zeta potential was strongly influenced by the impurities. From the experimental results that most of the essential oils have negative zeta potential value indicates the purity of the oils and their surface charge is adequate to interact with bacteria.
In this study, Onguard oil, Tea Tree oil, and Clove oil all demonstrated strong overall antibacterial effects. This was reflected by other researchers with Puvaca et al. concluding that tea tree oil demonstrated the strongest antimicrobial effect18 and Santa et al. demonstrating the antibacterial effect of clove EO against E. coli.19 Microbicide activity of clove essential oil has also been described by Anwer et al.,20 Cui et al.,21 and Moon et al.22 The antimicrobial properties of Tea tree oil have been detailed by Li et al.,23 Raman et al.,24 Gustafson et al.,25 and Park et al.26 On guard is a new blended essential oil which is being described first in this paper in reference to its antibacterial activity.
Though the results broadly corroborated in both the methods, the discrepancy in well diffusion results versus the broth micro dilution can be explained by the low water solubility coefficient of the essential oil in the agar that makes the inhibition zone difficult to compare with the results obtained by the dilution method.17
Previous research has indicated that the mechanism of action of EOs are mainly the damage of the membrane integrity and the resulting increased permeability which leads to cell lysis.27 The targets of EOs differ from those of traditional antibiotics. EOs are also known to have a greater effect on Gram-positive bacteria as compared to Gram-negative bacteria.27 However, in this study, the tested EOs showed a significant inhibitory effect on gram negative bacteria, including on multiple carbapenem resistant bacteria. This is also reflected in recent research where tea tree oils has shown reduced colonization with Carbapenem resistant Serratia.28 Clove oil and cinnamon oils have also shown effect against pathogens.29-34 With limited options for safe antibiotic therapy, the utility of essential oils in treating MDR pathogens is evidenced in this study.
Limitation of this study
No firm guidelines for the testing methodology or interpretation of the zone sizes/ MICs have been published, so only the increasing zone sizes can be noted.
Although the study indicated promising results for the researched essential oils’ effectiveness against pathogenic bacteria in vitro, these results may not yet be replicated in vivo.
Due to their high volatility, administering EOs to produce the appropriate effects might be difficult. Therefore, efforts based on nanotechnology for creating nanoscaled carriers for their effective delivery could present a potential answer.29,35
This research evidenced higher antimicrobial activity of clove essential oil, tea tree essential oil and On Guard essential oil towards Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii, Staphylococcus aureus, Enterococcus fecalis, and Klebsiella pneumoniae as compared to Lavender and eucalyptus essential oils. Based on these in vitro results, additional in vivo study is required to completely assess the examined essential oils’ antimicrobial efficacy. Newer delivery methods are necessary to fully explore the benefits of essential oils.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Friedrich AW. Control of hospital acquired infections and antimicrobial resistance in Europe: the way to go. Wiener Medizinische Wochenschrift. 2019;169(1):25-30.
Crossref - Andersson DI, Balaban NQ, Baquero F, et al. Antibiotic resistance: turning evolutionary principles into clinical reality. FEMS Microbiol Rev. 2020;44(2):171-188.
Crossref - Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277-283. https://pubmed.ncbi.nlm.nih.gov/25859123
- Singer AC, Kirchhelle C, Roberts AP. Reinventing the antimicrobial pipeline in response to the global crisis of antimicrobial-resistant infections. F1000Research. 2019;8:238.
Crossref - Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1(4):35.
Crossref - Munda S, Dutta S, Pandey SK, Sarma N, Lal M. Antimicrobial activity of essential oils of medicinal and aromatic plants of the North east India: A biodiversity hot spot. J Essent Oil-Bear Plants. 2019;22(1):105-119.
Crossref - Khan MF, Dwivedi AK. A review on techniques available for the extraction of essential oils from various plants. Int Res J Engin Technol. 2018;5(5):5-8.
- Masyita A, Sari RM, Astuti AD, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem: X. 2022;13:100217
Crossref - Daynac M, Cortes-Cabrera A, Prieto JM. Application of artificial intelligence to the prediction of the antimicrobial activity of essential oils. Evid Based Complement Alternat Med. 2015;561024.
Crossref - Ebani VV, Nardoni S, Bertelloni F, Pistelli L, Mancianti F. Antimicrobial activity of five essential oils against bacteria and fungi responsible for urinary tract infections. Molecules. 2018;23(7):1668.
Crossref - Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. Essential oils used in aromatherapy: A systemic review. Asian Pac J Trop Biomed. 2015;5(8):601-11.
Crossref - Tanveer M, Wagner C, Ribeiro NC, et al. Spicing up gastrointestinal health with dietary essential oils. Phytochem Rev. 2020;19(2):243-263.
Crossref - Abu-Al-Basal MA. Healing potential of Rosmarinusofficinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J Ethnopharmacol. 2010;131(2):443-450.
Crossref - Hendel N, Napoli E, Sarri M, et al. Essential oil from aerial parts of wild Algerian rosemary: Screening of chemical composition, antimicrobial and antioxidant activities. J Essent Oil-Bear Plants. 2019;22(1):1-7.
Crossref - Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-79.
Crossref - Yap PS, Yiap BC, Ping HC, Lim SH. Essential oils, a new horizon in combating bacterial antibiotic resistance. open Microbiol J. 2014;8:6-14.
Crossref - Ribeiro SO, Fraselle S, Baudoux D, Zhiri A, Stevigny C, Souard F. Proposals for Antimicrobial Testing Guidelines Applied on Ajowan and Spanish Lavender Essential Oils. Planta medica. 2021;87(10-11):754-763.
Crossref - Puvaea N, Milenkovic J, Coghill TG, et al. Antimicrobial activity of selected essential oils against selected pathogenic bacteria: In vitro study. Antibiotics. 2021;10(5):546.
Crossref - Santa Packyanathan J, Prakasam G. Antibacterial effect of clove oil against clinical strains of Escherichia coli. J Pharm Sci Res. 2017;9(7):1203-1204.
- Anwer MK, Jamil S, Ibnouf EO, Shakeel F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J Oleo Sci. 2014;63(4):347-354.
Crossref - Cui H, Zhang C, Li C, Lin L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control. 2018;94:140-116.
Crossref - Moon SE, Kim HY, Cha JD. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch Oral Biol. 2011;56(9):907-916.
Crossref - Li WR, Li HL, Shi QS, et al. The dynamics and mechanism of the antimicrobial activity of tea tree oil against bacteria and fungi. Appl Microbiol Biotechnol. 2016;100(20):8865-8875.
Crossref - Raman A, Weir U, Bloomfield SF. Antimicrobial effects of tea tree oil and its major components on Staphylococcus aureus, Staph. epidermidis and Propionibacterium acnes. Lett Appl Microbiol. 1995;21(4):242-245.
Crossref - Gustafson JE, Liew YC, Chew S, et al. Effects of tea tree oil on Escherichia coli. Lett Appl Microbiol. 1998;26(3):194-198.
Crossref - Park H, Jang CH, Cho YB, Choi CH. Antibacterial effect of tea-tree oil on methicillin-resistant Staphylococcus aureus biofilm formation of the tympanostomy tube: an in vitro study. In Vivo. 2007;21(6):1027-1030.
- Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines. 2017;4(3):58.
Crossref - Das S, Vishakha K, Banerjee S, Nag D, Ganguli A. Exploring the antibacterial, antibiofilm, and antivirulence activities of tea tree oil-containing nanoemulsion against carbapenem-resistant Serratia marcescens associated infections. Biofouling. 2022;38(1):100-117.
Crossref - Vase H, Nemattalab M, Rohani M, Hesari Z. Comparison of Chitosan and SLN nano-delivery systems for antibacterial effect of Tea Tree (Melaleuca alternifolia) oil against P. aeruginosa and S. aureus. Lett Appl Microbiol. 2023;176(11):ovad130.
Crossref - Anandhi P, Tharani M, Rajeshkumar S, Lakshmi T. Antibacterial activity of cinnamon and clove oil against wound pathogens. J Popul Ther Clin Pharmacol. 2022;28(2):e41-6.
Crossref - Mishra N, Gupta E, Singh P. Antibacterial and anti-biofilm activity of cinnamon and clove oils against uropathogens. Ukrainian Journal of Food Science. 2022;10(1):74.
Crossref - Neagu R, Popovici V, Ionescu LE, et al. Antibacterial and antibiofilm effects of different samples of five commercially available essential oils. Antibiotics. 2023;12(7):1191.
Crossref - Hou T, Sana SS, Li H, et al. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Bioscience. 2022;47:101716.
Crossref - Gheorghita D, Robu A, Antoniac A, et al. In vitro antibacterial activity of some plant essential oils against four different microbial strains. Appl Sci. 2022;12(19):9482.
Crossref - Mihai AD, Chircov C, Grumezescu AM, Holban AM. Magnetite nanoparticles and essential oils systems for advanced antibacterial therapies. Int J Mol Sci. 2020;21(19):7355.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.