ISSN: 0973-7510
E-ISSN: 2581-690X
Escherichia coli is the greatest cause of primary urinary tract infections. The present study aimed Urinary tract infection in female with special reference Escherichia coli and its antibiotic susceptibility pattern. A total of 100 urine samples were collected from different hospitals of Allahabad. The samples were cultured on Cystein lysine electrolytes deficiency media, MacConkey’s agar, Nutrient agar and Hicrome UTI agar media. Based on the results it was found that different media had different properties and Hicrome UTI agar was the best and convenient media for the isolation of Escherichia coli and other uropathogens. Further it was also observed that Escherichia coli was the most common organism for UTI in female.
Urinary tract infection, Female, Escherichia coli, Nutrient agar, MacConkey’s agar, Cystein lysine electrolytes deficiency, Hicrome UTI agar.
Urinary tract infections are among the most common bacterial infections that lead patients to seek medical attention and it has large socio-economic impacts. It is also one of the most common infectious diseases diagnosed in outpatients as well as in hospitalized patients and can lead to significant mortality. (Adedeji and Abdulkadir., 2009). Urinary tract infection (UTI) is an infection of one or more components of the urinary tract. Many infection resolve spontaneously but other can progress to destroy the kidney. Urinary tract is made up of two sections: the lower urinary tract and the upper urinary tract. Lower urinary tract contains the bladder and urethra. Upper urinary tract contains two kidneys and the tube that connects them, called the ureters. Urine does not normally contain microorganisms. Most urinary tract infections are due to bacteria that enter the opening of the urethra. Bacteria stick to the walls of the urethra, multiplying and moving up the urethra to the bladder. Most UTIs remain in the lower urinary tract where they cause annoying symptoms, such as a burning sensation during urination. These infections are easily treated in most cases. Subjects were considered to have a culture-confirmed urinary tract infection if they had dysuria, frequency, or urgency (or all three) and e”102 colony-forming units of an uropathogen per milliliter of midstream urine (Hooton et al., 1996).
Escherichia coli has been documented as the most important pathogen associated with urinary tract infections. The organisms isolated were Escherichia coli, Staphylococcus saprophyticus, Klebsiella aerogenes, Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus faecalis and Proteus mirabilis (Adedeji and Abdulkadir., 2009). UTI’s more commonly progress to bladder infections in females due to the much shorter length of the female urethra. Among the elderly, UTI frequency is roughly equal proportions in women and men.
Risk factors – Women who experience acute UTI are characterized by both a genetic predisposition and behavioral factors. In healthy pre-menopausal women, sexual intercourse, and a history of recurrent UTI have been shown to be strong and independent risk factors for UTIs. Even recent antimicrobial use, by adversely effecting vaginal flora is considered as a potent risk factor (Giamarellou, 2001). Other behavioral factors include: frequency of urination, aspects of personal hygiene. Despite the frequency of acute uncomplicated UTI, there is little long-term morbidity and no evidence for mortality attributable to this problem. These women are not at increased risk of developing hypertension or renal failure. Short-term morbidity due to acute symptoms may however be substancial, especially for women with frequent UTI. Urinary tract infection is categorized as asymptomatic bacteriuria, acute cystitis ,acute pyelonephritis. Manifestations include burning pain on urination after with turbid foul smelling or dark urine, frequency and lower abdominal discomfort (Nahar et al., 2010).
In the community, bacterial infection of the urinary tract is one of the common causes for seeking medical attention. Although most UTIs are painful nuisance. The present study is undertaken with an aim to study the effect of media on isolation and identification of uropathogens from female patients attending hospital and clinics.
100 urine samples, preferably mid stream urine were collected from local hospitals and pathology center in sterile vials from the district Allahabad, Uttar Pradesh. The samples were stored at 4°C for 24 – 48 hrs. The diagnosis of UTI is based on the microscopic findings of more than 10 pus cells/high power field (40X) in urine.
Isolation and Identification of E. coli
Freshly voided midstream urine was inoculated with calibrated loop on to different agar plates, and the plates were incubated at 37°C for 24 to 48hrs. Isolates were further identified by growth characteristics on selective and specific media, microscopic and biochemical tests. (Holt et al.,1994)
Isolation and Identification
Morphological characteristics
100 urine samples were taken. Mid stream urine were cultured on Nutrient Agar, MacConkey’s Agar, Cystine-Lactose-Electrolyte-Deficient, HiCrome UTI Agar media. One loop full of the culture was streaked on different media and colonies on the media were obtained. (table. No. 1)
Table (1):
Characteristics of E. coli on different agar media.
Name of the media |
Characteristics |
|---|---|
Nutrient agar |
Opaque, smooth, flat, moist, white color colonies. |
CLED |
Opaque, yellow color colonies |
MacConkey’s agar |
Pink color colonies |
Hicrome UTI agar |
Magenta color colonies |
From the study it was observed that the growth of organism over the media was according to the characteristics of the media. Mixed cultures were obtained on Nutrient agar, MacConkey’s agar, and CLED agar. On MacConkey’s agar lactose fermenting organism grows which gives pink to red color colonies. But the organism obtained from the Hicrome UTI agar media was of different colors. E. coli gives magenta color colony, Klebsella spp blue to purple, mucoid etc. Chromogenic media is better media than CLED, MacConkey,s agar and Nutrient agar.
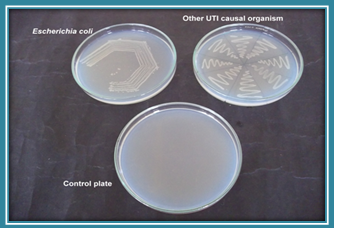 Plate 1. Growth on nutrient agar plate
Plate 1. Growth on nutrient agar plate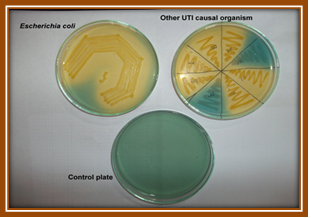 Plate 2. Growth on CLED agar plate.
Plate 2. Growth on CLED agar plate.The present findings were in concordance with the findings of (Aspevall et al., 2002) observed that the chromogenic media tested in this study was slightly better than CLED agar and MacConkey agar. Similar observation was also reported by (Fallon et al., 2003) that use of BBL CHROMagar, UTI medium, or CPS ID2 chromogenic agar as a replacement for cystine lactose electrolyte deficient agar would improve the detection rate of contaminated urine samples. “A cost comparison of the agars suggests that as the use of chromogenic agar in laboratories increases, the purchase cost is decreasing” (Fallon et al., 2003).
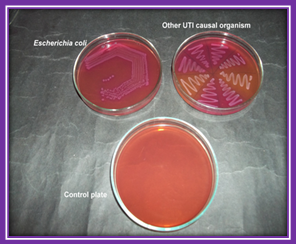
Plate 3. Growth on MacConkey’s agar plate
The overall impression of the color changes produced on chromogenic media by E. coli (purple – magenta), Klebsiella spp., (blue) and the Pseudomonas spp. (colourless) was that they were distinct and easy to perceive. In the study gram positive bacteria were also isolated as one chromogenic substrate is cleaved by ß-glucosidase possessed by Enterococci resulting in formation of bluish green colonies and S. aureus gives golden yellow color colonies. The result was in comparable with the result of (Aspevall et al., 2002). that chromogenic agars effectively supported the growth of gram-negative uropathogens but did not consistently support the growth of gram-positive and fungal uropathogens.
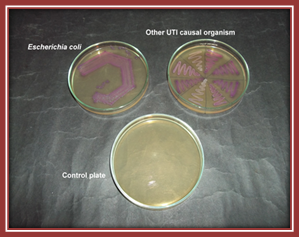
Plate 4. Growth on Hicrome UTI agar plate.

Plate 5. Indole +ve, MR +ve, VP -ve, Citrate utilization –ve for Escherichia coli
Cultural Characteristics
Cultural characteristics were observed by gram staining method. The cells observed were Gram-negative, rod shaped bacteria. Similar result was reported by (Gaafar et al., 2006) E. coli are rapidly growing, Gram-negative, rod-shaped cells measuring approximately 0.5×2 ìm length. Further organisms other than glucose-fermentative and nonfermentative gram-negative rods were identified provisionally by their appearance on Gram stains and colonial morphology on 5% sheep blood agar; identification was confirmed by standard methods (Kodaka et al., 1995).

Plate 6. Glucose AG+, Dextrose AG+, Lactose AG+
Biochemical Characteristics
Biochemical characteristics were observed by various biochemical tests which were routinely done for identification of culture.
Similar work was done by (Jha et al., 2009) and (Islam et al., 2010). Biochemical tests were carried out on both reference E. coli and isolated strain to compare the result and to confirm the identity of the isolated organism. The tests were urease, oxidase, indole, catalase, TSI agar.
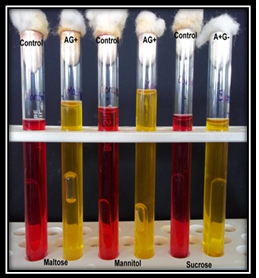
Plate 7. Maltose AG+, Mannitol AG+, Sucrose A+G-
Incidence of uropathogens
Out of 100 samples 88 were found positive. The organism isolated were Escherichia coli (61%), Klebsella spp. (19%), S. aureus (9%), Proteus species (6%), Pseudomonas species (4%). (Fig. 1). Similar finding were reported by (Bashar et al., 2009) which showed that 67% were Escherichia coli, 11% Streptococcus faecalis, 8% Pseudomonas aeruginosa, 2% Enterococcus faecalis, 4% Staphylococcus aureus, 2% Hafnia alvei, 2% Citrobacter freundii, 2% Klebsiella pneumonia and 2% Yersinia enterocolitica. Gram-negative isolates maximum were Escherichia coli 1937 (41.3%) followed by Klebsiella species 622 (15.8%) Pseudomonas species 534 (11.4%) Enterobacter species 376 (8%) and Proteus species 245 (6.2%). Amongst gram-positive isolates maximum were Staphylococcus aureus 361 (7.8%) followed by Enterococcus species 278 (5.9%) (Sonavane et al., 2008).
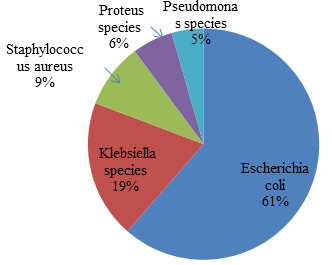 Fig. 1. Percentage of different organism isolated from urine samples
Fig. 1. Percentage of different organism isolated from urine samplesIn the present study, E. coli (61%) was the predominant bacterial pathogen followed by Klebsiella species, non-lactose fermenters, Pseudomonas species and others. Certain strains of E. coli have the ability to persist and predominate in the human intestine, making them more likely than other strains to cause UTI. In contrast to this finding, one study from Tamil Nadu showed Klebsiella and E. coli as the commonest isolate followed by P. aeruginosa and S. aureus. This may be due to the fact that the Klebsiella is one of the most important organisms causing nosocomial infection (Ramesh et al., 2008).

Plate 8. Motility test
Comparison between different types of media used for the isolation of uropathogens
The analysis of the data obtained from Nutrient agar with CLED, MacConkey’s agar, HiCrome UTI agar for the detection of dependence of tables 4.at 5% level of significance were tested. It was observed that N.A, CLED, MacConkey’s agar, Hicrom UTI agar gave significant result when tested for dependence between the media using t-test for paired samples. Result indicated that the growth pattern of the uropathogens were different. It could be due to the different constituents and properties of the media.

Fig. 1. Percentage of different organism isolated from urine samples
The result was similar to the result of Scarparo et al. (2002) that urine specimens were considered positive and clinically significant. In all cultures of the group, the isolates developed a significant colony count. But no significant difference was observed in colony count while comparing both the media.
Number of specimens showing mixed growth were same for both media but was easily appreciated on CUM because of variation in color was reported (Qaiser et al., 2011).
Table (4):
Mean values of number of colonies on different media.
Nutrient Agar media |
CLED |
MacConkey’s Agar media |
Hicrome UTI Agar media |
|---|---|---|---|
25.11 |
12.53 |
11.53 |
10.81 |
- Adedeji, B. A. M. And Abdulkadir, O. A., Etiology and Antimicrobial Resistance Pattern of Bacterial Agents of Urinary Tract Infections in Students of Tertiary Institutions in Yola Metropolis. Advances in Biological Research., 2009; 3(3-4): 67-70.
- Aspevall, O., Osterman, B., Dittmer, R., Ste´N, L., Lindbäck, E. And Forsum, U., Performance of Four Chromogenic Urine Culture Media after One or Two Days of Incubation Compared with Reference Media. Journal of Clinical Microbiology, 2002; 40(4) : 1500-1503.
- Bashar, M. A., Ahmed, M. F., Rahman, S. R. And Gomes, D. J., Distribution and resistance trends of Escherichia coli from urinary tract infections isolated in Dhaka city. Bangladesh Journal of Medical Sciences, 2009; 15(2): 93-98.
- Fallon, D., Ackland, G., Andrews, N., Frodsham, D., Howe, S., Howells, K., Nye, K. J. And Warren, R. E., A comparison of the performance of commercially available chromogenic agars for the isolation and presumptive identification of organisms from urine. Journal of Clinical Pathology, 2003; 56 : 608–612.
- Gaafar, E. S. A., Hanafy, M. S., Tohamy, E. Y. And Ibrahim, M. H., Stimulation and control of E. coli by using an extremely low frequency magnetic field. Romanian Journal of Biophysics., 2006; 16(4):283-286.
- Giamarellou. H., Uncomplicated urinary tract infection European Renal Association–European Dialysis and Transplant Association, 2001; 16 (6): 129-131.
- Holt, J.g., Krieg, N.r., Sneathm, P.h.a., Staley, J.t. And Williams,S.t., 1994 Bergey’s Manual of Determinative Bacteriology, 9th edition.
- Hooton, T. M., Scholes, D., Hughes J. P., Winter, C., Roberts. P. L., Stapleton, A. E., Stergachis, A. And Stamm, W. E., A prospective study of risk factors for symptomatic urinary tract infection in young women. The New England Journal of Medicine. 1996; 335(7) : 469-474.
- Islam, M.s., Khondkar, P. And Islam, M. A., Isolation aand In vitro antibiotic resistance and susceptibility pattern of a strain of E.coli. Bangladesh Pharmaceutical Journal. 2010; 13(1):34-37.
- Jha, B.k., Singh, Y.i., Khanal, L.k., Yadab,V.c. And Sanjana, R.K., Prevalence of asymptomatic bacteriuria among elderly diabetic patients residing in Chitwan. Kathmandu University Medical Journal. 2009; 7(2):157-161.
- Kodaka, H., Ishikawa, M., Iwata, M., Kashitani, F., Mizuochi, S. And Yamaguchi ,K., Evaluation of New Medium with Chromogenic Substrates for Members of the Family Enterobacteriaceae in Urine Samples. Journal of Clinical Microbiology, 1995; 33(1) : 199–201.
- Nahar, S.j., Khanum, H. And Shimasaki, K., Occurrence of Escherichia coli infection among the women of Dhaka city. Asian Research Publishing Network (ARPN) Journal of Agricultural and Biological Science, 2010; 5(6) : 68-73.
- Qaiser, S., Zeeshan, M., Jabeen, K., Ahsan, T. And Zafar, A., Comparison of chromogenic urinary tract infection medium with cysteine lactose electrolyte deficient media in a resource limited setting. Journal of Pakistan Medical Association 2011; 61(7):632-635.
- Ramesh,N., Sumathi, C. S., Balasubramaniam, V., Palaniappan, K. R. And Kannan, V. R. Urinary tract infection and antimicrobial susceptibility pattern of extended spectrum of Beta lactamase producing clinical isolates. Advances in Biological Research. 2008; (5-6) 78-82
- Scarparo, C., Piccoli, P., Ricordi, P. And Scagnelli, M., Comparative Evaluation of Two Commercial Chromogenic Media for Detection and Presumptive Identification of Urinary Tract Pathogens. European Journal of Clinical Microbiology and Infectious Diseases, 2002; 21:283–289.
- Sonavane, A., Mathur, M., Turbadkar, D. And Baradkar, V., Antimicrobial Susceptibility Pattern in Urinary Bacterial Isolates. Bombay Hospital Journal., 2008; 50(2) : 240-244.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


