ISSN: 0973-7510
E-ISSN: 2581-690X
The use of alternative substrates for biomass production has advantages for the production of non-conventional sources of protein and functional products. Qualitative and quantitative differences were determined in two culture media for the production of biomass by Pleurotus djamor (Rumph. : Fr.) Boedijn (P-19) and P. ostreatus (Jacq. : Fr.) Kumm. (P-11). Growth rate and biomass yield increased when non-conventional culture media were used. In liquid culture, the specific growth rate (µ) for the strain P-19 on Liquid Extract of Malt (EML+BF) medium was of 0.196 h-1 and 0.403 h-1 for the strain P-11, while on Buffer of Liquid Phosphates added with cereal (BFL+C) medium it was of 0.233 h-1 for the strain P-19 and 0.395 h-1 for the strain P-11. There were significant differences among strains studied. The production of mycelial biomass was of 0.07 g/l in EML+BF medium and 0.49 g/l in BFL+C medium for the strain P-19, while for the strain P-11 it was of 0.18 g/l and 0.70 g/l in EML+BF and BFL+C media, respectively. There were significant differences among treatments. The use of substrates containing lignocellulosic material was suitable for biomass production, increasing growth rate.
Growth mycelial, non-conventional culture media, speed of growth.
The study of mushrooms day to day generates major importance, due to the nutritious, functional, and medicinal point of view. On one hand, edible species are exploited to the maximum trying to maintain their own characteristics. On the other hand, the species of medicinal interest are manipulated to increase the biomass and yields of the metabolites of interest.1 Commercial and wild different strains of genus Pleurotus, have been subjected to study in order to check characteristic such as: flavor, color, texture, as well as, adaptability to sustrates.15 Parameters that determine the characteristics of the final product, are: species, geographical origin, substratum, condition of cultivation, enzymatic capacity, and rate of mycelium production. Production of biomass and speed of growth, are associated to the production rate and the capacity of absorption of nutrients.14
Mushrooms, besides being a food are natural sources of metabolites for medicinal use. They have impacted in the health sector, to figurate like an alternative of the main illnesses of national and world interest, such as: antitumoral12, antimutagenic8, antiinflamatory13, antiviral17, antioxidant6 and which are capable to reduce the level of cholesterol in blood16, besides, presenting properties as anticonvulsive and neuroprotector.1, 22 The production of biomass and metabolites are related, being important the nitrogen and carbon sources. The use of non-conventional culture media goes in increase, having as objective to increase biomass yield and reduction of production time, besides working with standardized systems that assure a homogeneous production of functional metabolites.9,20
In the present study, it was evaluated the effect of a cereal like as a non-conventional source of nitrogen and carbon over the cellular growth of two strains of Pleurotus, and its production of biomass and speed of growth.
Biological material and culture media
The strains used in the present study are registered in the strain collection of edible and medicinal mushrooms (COBIOCHUAEMor) of the Autonomous University of the State of Morelos, deposited in the Center of Genetic Resources of Edible and medicinal Mushrooms (CREGENHC) of the School of Postgraduates, campus Puebla. The commercial strain of Pleurotus ostreatus CP-50 was donated by the School of Postgraduate campus Puebla, with registration number COBIOCHUAEMor P-11, the strain COBIOCHUAEMor P-19, of Pleurotus djamor was gathered in state of Morelos growing in a wild way on dead trunk of Ipomoea murucoides, strain with number of CREGENHC, CP -468.
The strains were grown in agar from malt extract (EMA+BF) analytical grade (Difco), also was used as a non-conventional culture media, as source of carbon material whit lignin cereal to 2% (All-BranR). The culture media were resuspended in Buffer of Phosphates (BF) at pH 6.0 to maintain the constant conditions during the kinetic and they were sterilized before using.18 The cultivations were incubated 27 ± 3°C until the mycelia covered the Petridishes, registering the specific rate of growth (Kr) daily.23 For liquid cultivation, flasks of 125 ml were used with 50 ml of culture media, (EML+BF and BFL+C), cultivations stayed with constant agitation (150 rpm) at 27 ± 3°C. Samples by triplicate were taken every day by a period of 10 days, carrying out 2 replicas for each strain. It was registered the speed of growth (µ) also.19
Buffer of phosphates 60 mm pH 6.0
The buffer of phosphates (BF) was prepared of the following way; (g/l): 119.99 of NaH2PO4 H2O 141.96 of Na2HPO4, the pH was adjusted to 6.0 value.
Culture media solid branflakes and liquid plus buffer of phosphates (BFS+C and BFL+C)
The culture media BFS+BF was prepared of the following way; (g/l): 18 of agar agar (BIOXON, Mexico), 20 of branflakes, all this was added to 1000 ml of buffer of phosphates 60 mm, pH 6. The solution was sterilized in autoclave by 20 min at 129ºC. Later, the culture media was spilled in Petri dishes, approximately 30 ml for each dish and were incubated at 30ºC by 48 hours before using them to verify that the means were free of pollutants. For the liquid cultivation media, the agar was not added to the solution and it was treated under the same conditions.
Culture media extract of malt agar plus buffer of phosphates (EMA+BF and EML+BF)
The culture media extract of malt agar, is composed of the following (g/l): 13 of culture media extract of malt (BIOXON, Mexico), 18 of agar (BIOXON, Mexico), all this was appraised to 1000 ml. The solution was sterilized in autoclave by 20 min at 129ºC. Later, the culture media was incubated during 48 hours before being used to confirm the absence of pollutants. For the liquid cultivation, agar was not added to the solution and it was treated under the same conditions.
Morphological characterization of the mycelium of Pleurotus spp.
The characteristics in general that presented the strains of Pleurotus were: texture of woolly to lightly woolly, the observed density was from regular to scarce. The growth for all the strain was radial and of air appearance. The color type was from white to white hyaline. In spite of the differences between the species, the color and the texture, were not significant, however, the density was from regular to dense when the culture media was added with cereal (BFS+C). (Table 1, figure 1). A growth is generally attributed to favorable conditions and readiness of the nutrients in the culture media. The lignocellulosic substrate used in this study maintains a constant supply of appropriate nutrients for the cultivation of species of mushrooms, favoring the mycelial growth, high rates of colonization, and appropriate morphological characteristics.
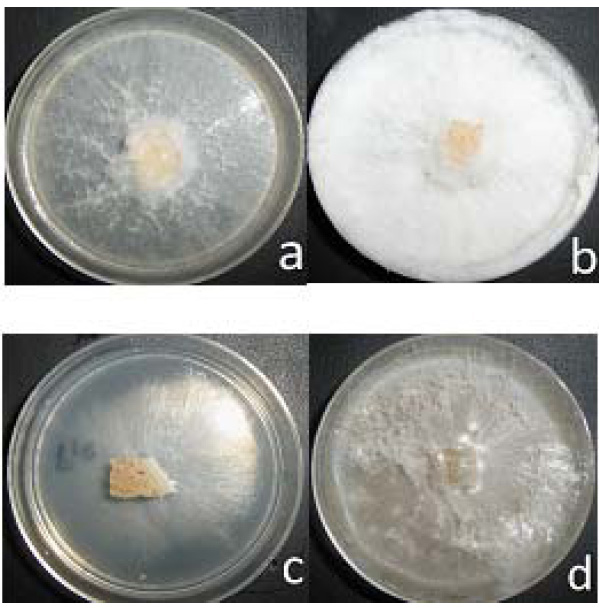 Fig. 1. Characteristic morphological of the mycelium. a) strain P-11 EMA, b) strain P-11 BFS, c) strain P-19 EMA, d) strain P-19 BFS
Fig. 1. Characteristic morphological of the mycelium. a) strain P-11 EMA, b) strain P-11 BFS, c) strain P-19 EMA, d) strain P-19 BFSTable (1):
Characteristics morphological of strain P-19 y P-11, in different culture media.
Strain |
Culture media |
Color |
Texture |
Density |
|---|---|---|---|---|
P-19 |
EMA+BF |
White – hyaline |
Flat |
Very scarce |
P-11 |
EMA+BF |
White |
Cottony |
Scarce |
P-19 |
BFS+BF |
White |
Cottony |
Scarce |
P-11 |
BFS+BF |
White |
Cottony |
Abundant |
EMA = Extract Malta Agar. BFS = Buffer of Phosphates Solid + Bran Flakes. COBIOCHUAEMor = Strain collection of Universidad Autónoma del Estado de Morelos.
The presence of fructifications in Petri dishes for the strain P-19 is possibly due to the competition for the readiness of the nutrients that exert other organisms in free life.
Kinetic Study of growth
Rate specifies of growth
In the analysis of the specific rate of growth (kr) when using EMA+BF, of the wild strain P-19 and the commercial strain P-11 values obtained were of 1.49 ± 0.16 mm / day and 1.74 ± 0.017 mm / day, respectively. While in BFS+C values obtained were of 1.98 ± 0.03 mm / day and 9.40 ± 0.0 mm / day, respectively, observing a bigger specific rate of growth when the strains were incubated in presence of lignocellulosic material.
When comparing the results using variance analysis with multiple differences of means, highly significant differences were observed, with a value of Pr >0.0001. The rate specifies of growth in Extract of Malt Agar (EMA+BF), were of 13.2 mm / day for strain P-19 and for strain P-11 of 14.9 mm / day, while in Buffer of Solid Phosphates + cereal (BFS+C), was of 17.4 mm / day and of 14.9 mm / day, respectively. In the analysis of means for the three groups, the biggest specific rate of growth the strain P-11 and P-19 were obtained when they were grown in presence of lignocellulosic material (BFS+C), while, when strains were grown in Agar Extract of Malt, the specific rate of growth in both strains was not significant, observing significant differences in the use of the culture media (Table 2, figure 2). The use of material liginocelulolitic probably is similar to the substrate in what originally the strains grow favoring the cellular growth when using in a more efficient way the enzymes of degradation of the substrate. The diameter of the extension of the colony was significantly influenced by the interaction of the substrate Lignocellulosic. Similar results were observed when using wheat, corn, and millet for the “spawn” production.5
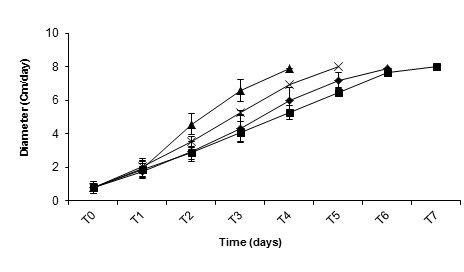 Fig. 2. Kinetics of growth in culture media solid using different substrates. . ▲ P-11 (BFS+C). ♦ P-11 (EMA+BF, XP -19 (BFS+C) AND. ■ P-19 (EMA+BF). N =5
Fig. 2. Kinetics of growth in culture media solid using different substrates. . ▲ P-11 (BFS+C). ♦ P-11 (EMA+BF, XP -19 (BFS+C) AND. ■ P-19 (EMA+BF). N =5Table (2):
Specific rate of radial growth in culture media solid.
Strain |
Culture media |
Rate specific of growth (mm/día) |
|---|---|---|
P-11 |
EMA+BF |
14.9 ± O.16 C |
P-11 |
BFS+C |
19.8 ± 0.03 A |
P-19 |
EMA+BF |
13.2 ± 0.19 C |
P-19 |
BFS+C |
17.4 ± 0.17 B |
*Means with the same letter are not statistically significant for each treatment.
EMA+BF = Extract of malt agar. BFS+C = Buffer of solid phosphates plus cereal.
These results were similar to those reported by20, for the Pleurotus ostreatus strain in which the grown curve was linear too. Authors elsewhere reported that the mycelial growth of other fungi such as Lentinula edodes3, Pycnoporus sanguineus.2
The addition of lignocellulosic material (commercial cereals without supplement obtained by means of a standard process of elaboration) in a culture media as the only source of carbon is adapted for the growth of cellular fungi. The use of alternative culture media in the industry, which offer cellular high proliferation, high yields, decrease in the times of fermentation and of low cost, is a factor that impacts in the production costs, besides obtaining products of high quality.
Production of biomass and speed of growth in culture media liquid
The production of biomass (X) was registered in cultivation liquid for the two strains in two culture media, obtaining the biggest production of biomass from both strains when are grown in presence of lignocellulosic material (BFL+C) presenting values of 14,135 g/l for the strain P-11 and 8,502 for the strain P-19, with regard to the culture media (EML+BF) values of 3,693 were obtained for the strain P-11 and 1,128 for the strain P-19, showing highly significant differences between the use of cultures medias and the strain used. (Figure 3, Table 3).
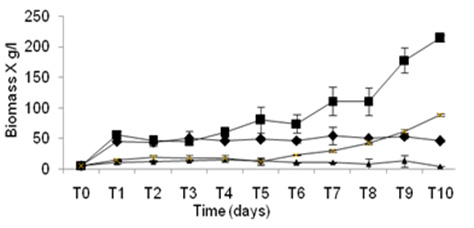 Fig. 3. Kinetics of growth in culture media liquid using different substrates. . ▲= P-19 (BFS+C). ♦= P-19 (EMA+BF), X =P -11 (BFS+C) AND. ■= P-11 (EMA+BF). N =6
Fig. 3. Kinetics of growth in culture media liquid using different substrates. . ▲= P-19 (BFS+C). ♦= P-19 (EMA+BF), X =P -11 (BFS+C) AND. ■= P-11 (EMA+BF). N =6Table (3):
Speed of growth and production of biomass in culture media liquid.
Cepa |
Medio de cultivo |
Production of biomass weight fresh (X) g/l |
Velocity of growth weight fresh (µ) horas -1 |
|---|---|---|---|
P-11 |
EML+BF |
61,237 ± 2,631 B |
0,403 ± 0,016 A |
P-11 |
BFL+C |
110,795 ± 23,737 A |
0,395 ± 0,003 A |
P-19 |
EML+BF |
13,633 ± 0,940 C |
0,196 ± 0,016 B |
P-19 |
BFL+C |
61,071 ± 4,314 B |
0,233 ± 0,054 B |
Means with the same letter are not statistically significant for each treatment.
EMA+BF = Extract of malt agar. BFS+C = Buffer of solid phosphates plus cereal.
The specific speed of growth µ for the strain P-19 in Liquid Extract of Malt (EML+BF) was of 0.196 hour-1 and 0.403 hour-1 for the strain P-11, while, when Buffer of Liquid Phosphates was added with cereal (BFL+C), a µ presented of 0.233 hour-1 for the strain P-19 and of 0.395 hour-1 for the strain P-11, showing significant differences, among strains and do not between culture media. Table 3. This parameter of bath cultivation assumes significance because it also favors the agitation, which proved to be a very important parameter in bioreactor fermentation.11
Both strains manifested the formation of mycelium (pellets) which are consequence of the orbital agitation in the flasks (Figure 4). Under these conditions, the cultivations presented similar patterns of coloration when mycelium was conglomerated, however, for strain P-11 a better development of the mycelium was observed, there were bigger pellets distributed in the mean and in a regular way, manifesting a better adaptability to the substrate. For strain P-19, pellets of more size were observed in a regular way. Similar results were observer.2 It is possible that these differences are due to the cultivation conditions, since the hydrodynamics inside the system is influenced by the agitation and the aerations of the cultivation broth.4,10
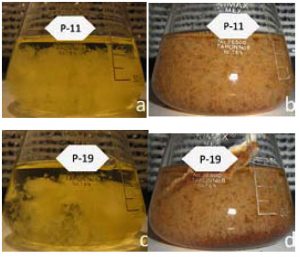 Fig. 4. Growth mycelial in flask, after 5 days of agitation. a) Strain P-11 EML+BF, b) Strain P-11 BFL+BF, c) Strain P-19 EML+BF, d) Strain P-19 BFL+BF
Fig. 4. Growth mycelial in flask, after 5 days of agitation. a) Strain P-11 EML+BF, b) Strain P-11 BFL+BF, c) Strain P-19 EML+BF, d) Strain P-19 BFL+BFHigh concentration of biomass and a fast speed of growth in any production process, assure a fast colonization of the substrate, as well as rapid production. In the process of obtention of inoculum, “spawn” for the production of edible mushrooms is an important factor, since when diminishing the times of colonization, diminishes the probability of contamination for mushrooms like Aspergillus spp., and Tricoderma spp., as well as, the presence of bacteria mainly Pseudomonas spp. Also, when diminishing the times of colonization, the production costs are diminished. Due to developments in the technology of this area, the cost can be significantly reduced. Current research is being direct toward finding new strain which can produce a valuable mycelium by submerged cultivation to manufacture functional products.6,21,24
ACKNOWLEDGMENTS
CONFLICT OF INTEREST
AUTHORS’ CONTRIBUTION
FUNDING
ETHICS STATEMENT
AVAILABILITY OF DATA
- Aguirre A, Villeda-Hernández J, Campos-Peña V, Herrera M, Montiel E, Tello I, Del Río-Portilla F, Estrada-Soto S, Navarrete-Vázquez G, Yolanda Rios M, Aguilar B, Castillo-España P, León-Rivera. I. Immune and neuroprotective effects of oligosaccharides from Ganoderma lucidum mycelium. International Journal of Medicinal Mushrooms. 2013; 15(5):555-568.
- Baumer J, Mas Diego J, Pacheco S, Morgado A, Furigo A. Comparative study of mycelial growth and production of cinnabarin by different strains of Picnoporus sanguineus. Biofar. 2008; 2:1-5.
- Castro M, Souza P, Eira A. Digital monitoring of mycelium growth kinetics and vigor of Shiitake (Lentinula edodes Berk. Pegler) on agar medium. Braz J Microbiol. 2006; 37:90-95.
- Cui Y, Ouwehand J, Van Der Lans R, Giuseppin M, Luyben K. Aspects of the use of complex media for submerged fermentation of Aspergillus awamori. Enzyme and Microbial Technology. 1998; 23:168-177.
- Elhami B, Naser A. Effect of substrates of spaw production on mycelium growth of oyster mushroom species. Journal of Biological Sciences. 2002; 8(2):474-477.
- Gregori A, Svagelj M, Pohleven J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol Biotechnol. 2007; 45:238-249.
- Guillén-Navarro G, Márquez-Rocha F, Sánchez-Vázquez J. Producción de biomasa y enzimas ligninolíticas por Pleurotus ostreatus en cultivo sumergido. Rev. Iberoam. Mic. 1998;15 :302-308.
- Jose N, Ajith T, Jananrdhanan K. Antioxidant, antiinflammatory, and antitumor activities of culinary medicinal mushroom Pleurotus pumonarius (Fr.) Quel. (Agaricomycetidae). Int J Med Much. 2002; 4:329-334.
- Hadar, Cohen-Arazi E. Chemical composition of the edible mushroom Pleurotus ostreatus produced by fermentation. Appl. Environ Microbiol. 1986; 51:1352-1354.
- Justen P, Paul G, Nienow A, Thomas C. Dependence of mycelial morphology on impeller type and agitation intensity. Biotechnology and Bioengineering. 1996; 52:672-684.
- Lee W, Park J, Ahn K, Park S. Factors influencing the production of endopolysaccharide and exopolysaccharides from Ganoderma applanatum. Enzyme and Microbial Technology. 2007; 40(2):249-254.
- Lindequist U, Timo H, Niedermeyer S, Wolf D. The pharmacological potential of mushrooms. eCAM. 2005; 2 (3):285-299.
- Lull C, Wichers H, Savelkoul H. Antiinflammatory and inmmunomodulating properties of fungal metabolites. Mediators Inflamm. 2005; 2:63-80.
- Mohammadi Goltapeh E, Purjam E. Principles of Mushroom Cultivation. Tarbiat Modarres University Press, UK. 2003.
- Morales P, Sobal M, Bonilla M, Martínez W. Ramírez-Carrasco P, Tello I, Spezzia T, Lira N, de Lima R, Villa S, Montiel E y Martínez -Carrera D. Los hongos comestibles y medicinales en México: recursos genéticos, biotecnología, y desarrollo del sistema de producción-consumo. En. Hacia un desarrollo sostenible del sistema de producción-consumo de los hongos comestibles y medicinales en Latinoamérica: Avances y perspectivas en el siglo XXI. Ed. Red Latinoamericana de Hongos Comestibles y Medicinales Puebla, México. 2012, p. 563-580.
- Nuhu A, Ki N, Jae S, Hae J, Mi J, Tae S. Dietary effect of Pleurotus eryngii on biomedical function and histology in hypercholesterolemic rats. Saudi J Biol. 2001;18:403-409.
- Ng T, Wang H. A novel ribonuclease from fruiting bodies of the common edible mushroom Pleurotus eryngii. Peptides. 2004; 25: 1365-1368.
- Pickard M, Vandertol H, Roman R, Vazquez-Duhalt R. High production of ligninolytic enzimes from white rot fungi in cereal bran liquid medium. Canadian Journal of Microbiology. 1999; 45: 627-631.
- Quintero R. Ingeniería Bioquímica: Teoría y Aplicaciones. Alhambra Mexicana Ed. México DF. 1990.
- Sánchez J, Royse D. La biología y el cultivo de Pleurotus spp. El colegio de la frontera Sur; Editorial Limusa. Grupo Noriega Editores. 2001. 290 pp.
- Tang Y, Zhu L, Li D, and Li H. Significance of inoculation density and carbon sourse on the mycelial growth and Tuber polysaccharides production by submerged fermentation of Chinese truffle Tuber sinece. Process Biochemistry. 2008; 43(5): 576-586.
- Tello I, Montiel E, León I, Martínez Carrera, D. Extracto con actividad anticonvulsiva y neuroprotectora. 2014. IMPI. MX/a/2014/014291.
- Trinci A. A study of the kinetics of hyphal extension and branching initiation of fungal mycelia. J. Gen. Microbiol. 1974; 81: 225-236.
- Wu C, Liang Z, Lu C, Wu S. Effect of carbon and nitrogen source on the production and carbohydrate composition of exopolysaccharide by submerged culture of Pleurotus citrinopileatus. Journal of Food and Drug Analysis. 2008; 16(2): 61-67.
© The Author(s) 2016. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


