ISSN: 0973-7510
E-ISSN: 2581-690X
The current COVID-19 pandemic is caused by the novel SARS-CoV-2 coronavirus strain. Although SARS-CoV-2 infection can affect everyone, the kind and degree of infection and sickness vary widely between individuals and populations. It has been crucial since reported disease loads and case fatality rates vary greatly among countries. However, there are still uncertainties about the severity of the illness in certain people and, in other cases, the aetiology of a more severe illness. Various chronic conditions, such as diabetes, cardiovascular diseases, respiratory ailments, and immunodeficiency disorders, have been identified as significant risk factors for COVID-19. These comorbidities not only increase the susceptibility to contracting the virus but also exacerbate the severity of symptoms and the likelihood of adverse outcomes, including hospitalization, intensive care unit admission, and mortality. The objective of this article is to point out the proliferation of COVID-19 in relation to different diseases affecting the clinical outcome of COVID-19. The study included 1500 patients with various diseases such as HCV, HBV, kidney disease, heart disease, asthma, T.B., arthritis, smokers, and vaccinated or unvaccinated. Results showed that 22% of diabetic patients, 40% of heart patients, 40% of asthma patients, 26% of kidney patients, 25% of T.B patients, and 41% of smokers had high corona positive. Coronavirus positivity was found in 34% of vaccinated patients and 72% of non-vaccinated patients, with an overall calculated p-value of 0.0001 by ANOVA statistical analysis. The overall outcome of the results showed that the severity of Corona disease increased in relation to different diseases.
COVID-19, Proliferation, SARS-CoV-2, Diseases, CT-Value, Vaccination
Particular people may suffer a variety of therapeutic scenarios following a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, extending from an asymptomatic illness to a life-threatening sickness that may be treatable with a combination of drugs. The majority of severe cases of coronavirus disease 2019 (COVID-19) appear to be mediated by an unregulated immune reaction that results in the large multiplication of immune cells and the overproduction of cytokines. The kidney is one of the impacted organs that is being more targeted by COVID-19 problems, and patients with severe illness who have aberrant renal function are at a high risk of dying.1 Additionally, among COVID-19 patients, renal dysfunction was linked to in-hospital mortality.2 Since some investigations have supported indirect pathomechanisms,3-5 others have shown a direct viral infection of the kidney with infection of glomerular and tubular cells.6 Together with the airways and lungs, the cardiac system is frequently implicated in COVID-19 early on. This is demonstrated by the release of cytokines like interleukin-6 and highly sensitive troponin and natriuretic peptides, which are all highly predictive, in specific in those displaying substantial increase. Many COVID-19 infected patients pass away from cardiogenic shock; this is generally a combination of primary cardiac participation and systemic manifestations, including severe hypoxia, multiorgan failure syndrome, and systemic inflammatory response syndrome, among others.7 The upregulation of TMPRSS2 and ACE2 receptors most likely contributed to viral multiplication. After illness, ACE2 expression was also decreased, which most likely contributed to the immune activation.8 In Egypt and Italy, two nations with very distinct COVID-19 histories, disruptions to hepatitis programming across the cascade of care have already been observed. These interruptions are also anticipated in many other nations. The Italian government passed a law requiring graded birth cohort hepatitis testing in February 2020; however, as of May 2020, the program still haven’t been put into effect. The Ministry of Health-affiliated HCV treatment and cirrhosis follow-up facilities’ operational numbers decreased by more than 75% in Egypt, where all active screening program were stopped in March 2020.9 Chymotrypsin-like protease (3CL pro) may break viral polyprotein during the life span of SARS-COV-2 to create the RNA replicase-transcriptase complex, which is necessary for both viral transcription and replication.10,11 Protease inhibitors are thought to have the ability to treat COVID-19 since the proteases of HCV and HIV shown comparable functions to those of SARS-COV-2. According to homology modelling data, across all licensed medications, HCV protease inhibitors have the maximum binding interactions to SARS-CoV-2 protease.12 Individuals with COVID-19 frequently have liver damage, whether it be in the form of cirrhosis, liver dysfunction, or both,13 and has been demonstrated that this damage is linked to poor outcome measures.14-16 The combination of several COVID-19 therapeutic intervention with HBV and its antiviral treatment is a significant additional problem. Adult patients with COVID-19 who were hospitalized are found to be more prevalent among those who have asthma, according to recent research from the U.S Kingdom.17-19 220 (14%) of the 1526 individuals in Chiba et al. research who had Covid positivity verified by PCR also had concomitant asthma. Comorbid asthma, however, was not shown to raise hospitalization risk in this research cohort.20 A new analysis of COVID-19 positive cases from different hospitals across the United States by the Centers for Disease and Protection revealed that 27.3 percent of COVID-positive hospitalized patients aged 18 to 49 years listed asthma as a comorbid conditions, compared to an occurrence of 8.9 percent in the overall population.21,22 Therefore, asthmatic individuals are hypothesized to be more vulnerable to and more severely affected by COVID-19 due to a weakened immune system to the virus and a higher likelihood of virally caused aggravation.23 Asthma that is just not allergic to things was not linked to this alteration.24 It was also shown that elevated TMPRSS2, a protease that facilitates efficient viral effector function, gene transcription, is related to type II inflammation.25 The small rise in TMPRSS2 gene expression is thought to be overcome by the decline in ACE2 gene regulation, possibly rendering asthma-related type II inflammation a COVID-19 preventive feature.23,25-27
Even though the incidence rate varies between investigations and between countries, accumulating data shows that COVID-19 is frequently found in diabetes patients, antihypertensive, and cardiovascular disease (CVD). Angiotensin converting enzyme 2 (ACE2) is the receptor used by SARS, SARS-CoV2, and MERS, whereas dipeptidyl peptidase-IV (DPP4) is the receptor used by MERS.28,29 The receptor proteins themselves are an improbable explanation for the increased risk because both enzymatic signaling pathways are altered in hyperglycemia, although in distinct ways.30,31 Numerous studies have shown that diabetes individuals are more susceptible to many illnesses, especially those of microbial sources, which is likely due to an immune reaction that is out of balance.32 Patients with diabetes make up a sizable share of COVID-19 hospitalized patients. 7.4 percent, even up to 20 percent, of COVID-19 patients nationwide were found to have diabetes.33-38 As a result, it seems that diabetic patients have a minimally increased risk of contracting SARS-CoV-2 infection. The connection between regular cigarettes and the incidence of acute respiratory infection is once again a hot subject during the coronavirus disease (COVID-19) epidemic. A large portion of the language used to promote e-cigarettes is on the possibility to save countless lives that might otherwise be lost due to these non-infectious effects. These non-infectious consequences are the subject of most of the worldwide effort on tobacco control and reduction. However, in low- and middle-income nations, especially throughout epidemics, the danger of viral consequences is the main emphasis and worry. Furthermore, cigarettes are at a 3- to 5-times increased risk of contracting legionella, meningococcal, or pneumonia bronchitis. Due to the overexpression of the pneumococcal receptor molecule (platelet activating receptor factor), tobacco consumers have enhanced pneumococcal adhesion and colonization. People who smoke are also five times more likely to develop flu than non–smokers.39 In this research article, we will discuss about proliferation of COVID-19 with different commodities like diabetes, cardiac disease, kidney, HCV, HBV, smoking, arthritis, and asthma. Then we provided the statistical analysis to show its effects in patients having different diseases.
Sample collection
Nasopharyngeal swabs and oropharyngeal swabs samples (n = 1500) collected from patients suspected of COVID-19 and received at the CAMB diagnostic lab were included in the study. All the 1500 patients were asked to fill out question-based forms that were further evaluated to find the proliferation of COVID with different diseases.
RNA extraction
The QIAamp DSP Viral RNA Mini Kit, which includes QIAamp Mini spin columns, was used to isolate high-purity viral RNA from nasal swab samples. It enables the rapid and simple isolation of very pure viral RNA from virus-containing cell-free body fluids. High efficiency and purity were reached by the end of the isolation phase. It is possible to isolate viral RNA from several samples at the same time using very basic laboratory equipment. The total sample volume input was 140 µl. The QIAamp DSP Viral RNA mini-Kit purifies viral RNA that is further used for real-time PCR.
Real time PCR
The genesig® Real-Time PCR Coronavirus COVID-19 (CE IVD) Kit was used to detect SARS-CoV-2 viral RNA isolated from nasopharyngeal swabs and oropharyngeal swabs from patients using a CE IVD extraction system and the specified PCR platforms. The Rotor-Gene Q Real-Time PCR was utilized in conjunction with the Rotor-Disc 100, which is comparable to a 96-well plate with an additional four reference wells. Rotor-Gene Q software was used to analyze the data.
Data analysis
All data results were compiled in Excel, and the data was analyzed with GraphPad prism. GraphPad Prism combines scientific graphing, extensive curve fitting (nonlinear regression), comprehensible statistics, and data organization. Prism can readily perform simple statistical tests needed by laboratory and clinical researchers.
Inclusion criteria
The age range for patients from 20 to 60 was included in the study.
- These conditions include hyperlipidemia, HCV, HBV, ischemic heart disease, hypertension, smoking, diabetes, kidney disease, heart failure, chronic obstructive pulmonary disease, rheumatoid arthritis, and asthma. We then analysed the use of risk factors that may lead to the proliferation of COVID in the eligibility criteria.
- Patients with or without symptoms of COVID were also included in the study.
Exclusion criteria
- Patients aged older than 60 were excluded from the study.
- Patients with diseases like cancer or Alzheimer’s disease and related disorders or senile dementia, depression, and osteoporosis were excluded from the study.
- Patients with already identified COVID and hospitalised patients were excluded from the study.
RNA extraction
The recovered viral RNA was used directly in downstream applications for Real Time-PCR, for viral detection and viral load determination.
Real time PCR
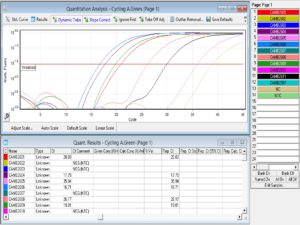
The results of each cycle were analyzed at the end through Rotor-Gene Q software. and compared CT value results with internal control. CT value ranged between 14 and 25, considered high corona positive. CT value ranged between 25 and 30, considered medium corona positive, and CT value ranged between 30-35, considered low corona positive. Some graphs with high CT values of corona-tested patients are given below.
It is important to identify, treat, and manage COVID-19 as soon as possible. All sufferers with even the smallest indication of the illness receiving positive results on an RT-PCR test or chest CT have received treatment since the beginning of COVID-19’s existence. CT values are a useful approximation for semi-quantitative RT-PCR monitoring systems for contagious pathogen detection, and they may assist in guiding infection prevention decision-making. This work expands the body of research on the length of infectiousness following mild-to-moderate COVID-19 by showing that the contagious virus can linger for a week or longer after the beginning of symptoms before gradually waning. We noticed a significant correlation between CT value and viral recovery efficiency.40 According to our findings, there is a significant correlation between infection and the RT-PCR CT value. CT values ranged from 14 to 25 when COVID-19 was positive and from 30 to 35 when it was negative as shown in Figure 1. The CT value was 25–30 for those with mildly contagious diseases. According to a recent statistical study conducted between March and May 2020 at a sizable quaternary accredited health centre in New York City, USA, surprisingly low CT values upon detection (i.e., significantly higher virus in the body) were linked to markedly elevated mortality rates, including both in- and out-patients.41 Cardiovascular diseases, including hypertension, coronary artery disease, and heart failure, have consistently been identified as notable risk factors associated with severe cases of COVID-19. Research has demonstrated that individuals who have pre-existing cardiovascular conditions exhibit a greater propensity for encountering unfavorable consequences, such as increased rates of hospitalization, admission to intensive care units (ICUs), and mortality.42 It is widely believed that the connection between cardiovascular diseases and the severity of COVID-19 can be attributed to the disruption of the renin-angiotensin system, impairment of endothelial function, and heightened systemic inflammation. Diabetes mellitus has been recognized as a significant comorbidity linked to severe cases of COVID-19.43 Individuals diagnosed with diabetes are at heightened susceptibility to the development of severe respiratory infections, and this vulnerability extends to the COVID-19 virus as well. The population in question is believed to experience increased vulnerability and unfavorable outcomes due to factors such as inadequate glycemic control, persistent inflammation, and compromised immune responses. Chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma, have been widely acknowledged as significant risk factors for severe respiratory infections.44 The COVID-19 virus presents a specific risk to individuals who have pre-existing lung conditions, as it has the potential to cause worsening symptoms and ultimately respiratory failure. Numerous studies have consistently demonstrated that individuals diagnosed with chronic obstructive pulmonary disease (COPD) and asthma exhibit an elevated susceptibility to hospitalization, intensive care unit (ICU) admission, and mortality upon contracting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).45
Figure 1. The figure shows the graph of the CT value of different samples analyzed by Rotor Gene Q software. The sample number CAMB 2606 has shown a low CT value of 16.71 in the graph that showed high corona positivity
Individuals with SARS (caused by SARS-CoV, the “relative” of SARS-CoV-2), who’d never taken glucocorticoids, had vastly greater rising plasma glucose levels than those with non-SARS asthma, according to Yang et al. findings. As ACE2 is expressed on the pancreatic islets, it was hypothesized that SARS-CoV caused harm to the pancreatic B-cells.46 Diabetes has been identified in the first few published case series as a potential cause for COVID-19 initially, as well as a more obvious disease history and death,47-50 along with other often associated disorders such as arterial hypertension, obesity, and cardiovascular disease. The cause of illness is yet unknown, but the risk group pattern resembles earlier deadly coronavirus epidemics of zoonotic origin, SARS and MERS,51 in a startlingly comparable pattern. 180 of the 1500 COVID-19 patients, according to our findings, had diabetes mellitus. 130 of the 180 individuals had COVID-19 infections of varying severity. A patient has an extremely high chance of developing a serious illness, developing acute respiratory distress syndrome, and eventually passing away due to the complicated interplay between COVID-19 and diabetes mellitus. Furthermore, the concurrent COVID-19 is likely to make it difficult for people with insulin resistance to control their blood sugar levels. People who are elderly, fragile, or who have one or more comorbid conditions seem to be more adversely impacted by the progression of the disease. Particularly in males and those with cardiovascular illness, the condition progresses more rapidly and can be fatal.52-54 In Chinese research, it was shown that 58 percent of patients were male and that high blood pressure (26 percent), type-2 diabetes (10 percent), and heart disease were the most often seen comorbidities. According to research conducted in the USA, hypertension, diabetes mellitus, severe respiratory illnesses, and cardiomyopathy are the most common morbidities among patients, accounting for 59.6 percent of patients (13.1 percent).55
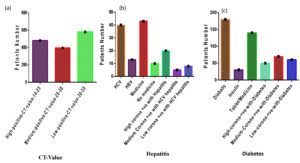
Figure 2. (a) Of the 1500 COVID-infected patients, 480 had a high level of COVID positivity (CT value 14–25), 395 had a medium level (CT value 25–30), and 580 had a low level (CT value 30-35). The data of 1500 patients were further analyzed against different diseases, as presented in the figure. (b) Out of 1500 patients, 53 had hepatitis disease; 20 were high COVID-positive, 5 were medium COVID positive, and 8 were low COVID-positive with hepatitis. (c) Out of 1500 patients, 180 were diabetic, 50 were high COVID-positive, 70 were medium COVID-positive, and 60 were low COVID-positive with diabetes
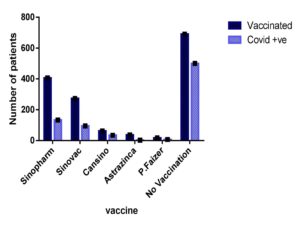
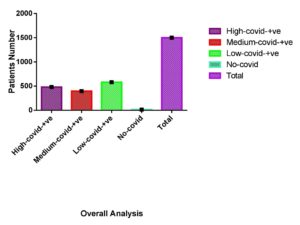
Our investigation revealed that individuals with cardiovascular disease had a significant risk of COVID-19 infection. As shown in Figure 2, out of 50 cardiac patients, 20 had extremely high infection rates, 15 had moderate infections, and 15 had mild infections. Diffuse microangiopathy with thrombosis can be caused by activation of the circulatory system. Acute coronary syndrome, myocarditis, heart failure, cardiac arrhythmias, fast progression, and eventual death can all be caused by myocardial infection. Patients with COVID-19 infections exhibit signs of heart damage with increased troponin levels in between 8 and 28% of cases.55 It was stated that we provided evidence for a lower risk of a SARS-CoV-2 positive test in people with early-onset asthma compared to people without asthma using UK Biobank data from 107 412 people who had been tested for the virus. Only men, nonsmokers, overweight or obese individuals, and people of non-Black ethnicity were associated with this relationship. Contrary to expectations, we also discovered that people with initial asthma who had lung function in the upper quartile had a higher risk than those who had lung function in the bottom quartile.56 Our study showed that patients with asthma had a high infection rate. As shown in Figure 2, 75 patients were suffering from asthma, and all 75 asthmatic patients tested positive for COVID-19. For the predictors, tobacco was not a potential risk on its own. Our findings are somewhat at odds with previous studies that indicate smoking is linked to the development of COVID-19 illness.57 Basic science studies have shown that smoking increases the expression of the coronavirus entrance receptors for the severe acute respiratory syndrome in the respiratory epithelium.58 Even without accessible pack-year data from the patient’s file, it was actually impossible to evaluate the dosage relationship between the cigarettes smoked and the intensity of the radiograph. To properly quantify the danger of smoking, current experiments should collect data on response length. Our results showed that smokers were at high risk for COVID-19. Cigarettes suppress the immune system, which makes it more susceptible to disease (both latent and active). Smoking especially affects macrophage and cytokine activation, which makes it more difficult to fight infection.39 A gradual rise in both vulnerability to SARS-CoV-2/COVID-19 and the probability of unfavourable outcomes were significantly related to kidney function deterioration in a cohort of the overall population across the country.59 Specifically, as shown in Figure 3, of 38 kidney patients, 10 showed a high positivity rate for COVID-19. A potential risk for community-acquired bacteremia, infections, and accompanying adverse consequences, such as influenza-associated fatality, has been identified as renal impairment, which is characterized by either albuminuria or a lower GFR.60 As shown in Figure 4 T.B and arthritis like diseases also increase the risk of corona. A decent level of vaccine coverage has already been attained in the Veneto area, and presumably it will keep increasing. Because the elderly was given priority at the start of the vaccine program, it is not uniform across all age groups. Individuals who were unvaccinated had a higher positive and higher metabolic rate chance of disease than those who were partially or completely immunized. The completely immunized were likewise much more resistant to infection than the partly immunized.61 Our research revealed that vaccination reduced a person’s vulnerability to COVID-19 as shown in Table 1. Additionally, our research shows that those who received the Astrazinca vaccine had the lowest vulnerability to COVID-19 infection as shown in Figure 5. Other research points to a notable decline in the durability of the positive in those who have received a synthetic immunization. As shown in Figure 6, the overall analysis of our study showed that of the 1500 data points, only 15 were negative, and the other 1485 showed positivity in different ranges, like from high positivity to low positivity. As shown in Table 2 overall calculated P-value is P<0.0001 show that results of our study are significant.
Table (1):
Two-way ANOVA analysis of vaccinated and non-vaccinated patients and the ratio of coronavirus positive among them the overall P-Value calculated for the row factor was P = 0.0038 and the column factor was P = 0.0446
| Two-way ANOVA | Ordinary | ||||
|---|---|---|---|---|---|
| Alpha | 0.05 | ||||
| Source of Variation | % Of total variation | P value | P value summary | Significant? | |
| Row Factor | 87.45 | 0.0038 | ** | Yes | |
| Column Factor | 7.367 | 0.0446 | * | Yes | |
| ANOVA table | SS | DF | MS | F (DFn, DFd) | P value |
| Row Factor | 502888 | 5 | 100578 | F (5, 5) = 16.87 | P = 0.0038 |
| Column Factor | 42364 | 1 | 42364 | F (1, 5) = 7.105 | P = 0.0446 |
| Residual | 29812 | 5 | 5962 | ||
| Number of missing values | 0 | ||||
Table (2):
ANOVA Statistical Analysis of Overall Data by the Use of Software GraphPad Prism The overall calculated p-value of the data was P < 0.0001
| ANOVA summary | |||||
|---|---|---|---|---|---|
| F | 18287 | ||||
| P value | < 0.0001 | ||||
| P value summary | **** | ||||
| Are differences among means statistically significant? (P < 0.05) | Yes | ||||
| R square | 0.9999 | ||||
| Brown-Forsythe test | |||||
| F (DFn, DFd) | +infinity (4, 5) | ||||
| P value | < 0.0001 | ||||
| P value summary | **** | ||||
| Significantly different standard deviations? (P < 0.05) | Yes | ||||
| Bartlett’s test | |||||
| Bartlett’s statistic (corrected) | |||||
| P value | |||||
| P value summary | |||||
| Significantly different standard deviations? (P < 0.05) | |||||
| ANOVA table | SS | DF | MS | F (DFn, DFd) | P value |
| Treatment (between columns) | 2407000 | 4 | 601628 | F (4, 5) = 18287 | P < 0.0001 |
| Residual (within columns) | 164.5 | 5 | 32.9 | ||
| Total | 2407000 | 9 | |||
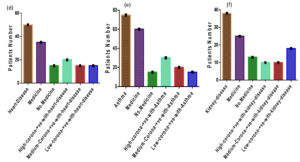
Figure 3. (d) Out of 1500 COVID-suspected patients, 50 suffered from heart disease, 20 were high COVID-positive, 15 were medium COVID-positive, and 15 were low COVID positive. (e) Of the 1500 COVID suspects, 75 had asthma, 30 were high COVID positive, 20 were medium COVID positive, and 15 were low COVID positive. (f) Of the 1500 suspected COVID patients, 38 had suffered from kidney disease10 were high COVID positive, 10 were medium COVID-positive, and 18 were low COVID positive
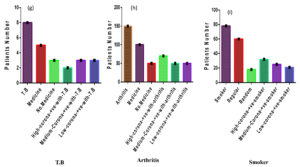
Figure 4. (g) Out of the 1500 COVID-suspected patients, 8 had suffered from T.B disease; 2 were high COVID-positive, 3 were medium COVID-positive, and 3 were low COVID-positive. (h) Of the 1500 COVID suspected patients, 150 had arthritis disease. 70 were high COVID-positive, 30 were medium COVID-positive, and 50 were low COVID-positive. (I) Out of the 1500 suspected COVID patients, 78 were smokers. Of those, 32 were highly COVID positive, 25 were mediumly COVID positive, and 21 were lowly COVID positive
Figure 5. The relationship between the suspected COVID status of 1,500 patients vaccinated with different vaccines and the ratio of COVID positives revealed that 406 patients were vaccinated by Sinopharm, with 133 being COVID positives. 273 were vaccinated by Sinovac, of which 95 were COVID-positive. 62 patients were vaccinated by Cansino, of whom 34 were COVID-positive. Of the 36 patients vaccinated by AstraZeneca, 2 were COVID-positive, and 17 were vaccinated by P. Faizer, of whom 7 were COVID-positive. Of those, 690 received no vaccine, of which 500 were COVID-positive
The influence of COVID-19 on the global health architecture and development aid programs may have far-reaching impacts on efforts made worldwide to manage HBV in relation to prevention, detection, and medication. We provide the most comprehensive group of kidney specimens from COVID-19 victims to date, demonstrating an elevated incidence of COVAN, PGMID, and myoglobin-cast nephropathy. To evaluate the long-term diagnostic evidence of patients with renal illness in the context of COVID-19, and especially in the context of COVAN, more research is necessary. We draw the conclusion that either asthma is not a premorbid disease that led to the emergence of COVID-19 or physicians and scientists are inaccurately reporting the premorbidities in COVID-19 individuals given the 4.4 percent global incidence of asthma. When working with patients who have certain illnesses, doctors must take note of such results. In order to avoid these possible consequences, people with COPD should take extra care to reduce their risk of COVID-19 consumption.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Zafar Saleem for his assistance throughout the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Ethical Review Board of the Centre for Applied Molecular Biology, University of the Punjab, Pakistan, with certificate reference number CAMB-9871.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Abbate M, Rottoli D, Gianatti A. COVID-19 attacks the kidney: ultrastructural evidence for the presence of virus in the glomerular epithelium. Nephron. 2020;144(7):341-342.
Crossref - Chen Y-T, Shao S-C, Hsu C-K, et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):346.
Crossref - Kudose S, Batal I, Santoriello D, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959-1968.
Crossref - Golmai P, Larsen CP, DeVita MV, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944-1947.
Crossref - Santoriello D, Khairallah P, Bomback AS, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158-2167.
Crossref - Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592.
Crossref - Oudit G, Kassiri Z, Jiang C, et al. SARS coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618-625.
Crossref - Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68-78.
Crossref - Blach S, Kondili LA, Aghemo A, et al. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74(1):31-36.
Crossref - Wan Y, Shang J, Graham R, et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-00120.
Crossref - Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS CoV 2. J Med Virol. 2020;92(6):595-601.
Crossref - Nguyen DD, Gao K, Chen J, Wang R, Wei GW. Potentially highly potent drugs for 2019-nCoV. BioRxiv. 2020.
Crossref - Anjum MR, Chalmers J, Hamid R, Ragoriya N. COVID-19: Effect on gastroenterology and hepatology service provision and training: Lessons learnt and planning for the future. World J Gastroenterol. 2021;27(44):7625-7648.
Crossref - Yip TC-F, Lui GC-Y, Wong VW-S, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70(4):733-742.
Crossref - Weber S, Hellmuth JC, Scherer C, et al. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70(10):1925-1932.
Crossref - Ding Z-y, Li G-x, Chen L, et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74(6):1295-1302.
Crossref - Abrams EM, W’t Jong G, Yang CL. Asthma and COVID-19. CMAJ. 2020;192(20):E551-E551.
Crossref - Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region-case series. N Engl J Med. 2020;382(21):2012-2022.
Crossref - Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. MedRxiv. 2020.
Crossref - Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307-314. e304.
Crossref - Covid C, Team R, COVID C, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019-United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382-386.
Crossref - Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019-COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
Crossref - Liu S, Zhi Y, Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78-88.
Crossref - Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203-206. e203.
Crossref - Sajuthi SP, DeFord P, Jackson ND, et al. Type 2 and interferon inflammation strongly regulate SARS-CoV-2 related gene expression in the airway epithelium. Biorxiv. 2020.
Crossref - Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146(2):327-329. e324.
Crossref - Morais-Almeida M, Aguiar R, Martin B, et al. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13(5):100126.
Crossref - Vickers NJ. Animal communication: when i’m calling you, will you answer too? Curr Biol. 2017;27(14):R713-R715.
Crossref - Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251-254.
Crossref - McKillop AM, Stevenson CL, Moran BM, Abdel-Wahab YHA, Flatt PR. Tissue expression of DPP-IV in obesity-diabetes and modulatory effects on peptide regulation of insulin secretion. Peptides. 2018;100:165-172.
Crossref - Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132-2139.
Crossref - Guo W, Li M, Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7):e3319.
- Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720.
Crossref - Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
Crossref - Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID 19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797-806.
Crossref - Bai Y, Yao L, Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
Crossref - Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513.
Crossref - Liu K, Fang Y-Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025-1031.
Crossref - van Zyl-Smit RN, Richards G, Leone FT. Tobacco smoking and COVID-19 infection. Lancet Respir Med. 2020;8(7):664-665.
Crossref - Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483.
Crossref - Rabaan AA, Tirupathi R, Sule AA, et al. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics. 2021;11(6):1091.
Crossref - Jo Y, Hong A, Sung H. Density or connectivity: what are the main causes of the spatial proliferation of covid-19 in Korea? Int J Environ Res Public Health. 2021;18(10):5084.
Crossref - Adamo S, Chevrier S, Cervia C, et al. Lymphopenia-induced T cell proliferation is a hallmark of severe COVID-19. BioRxiv. 2020:2008.2004.236521.
Crossref - Chen L, Zhao J, Peng J, et al. Detection of SARS CoV 2 in saliva and characterization of oral symptoms in COVID 19 patients. Cell Proliferation. 2020;53(12):e12923.
Crossref - Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128.
Crossref - Pal R, Bhadada SK. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14(4):513-517.
Crossref - Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372-1379.
Crossref - Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481.
Crossref - Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531-538.
Crossref - Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129-133.
Crossref - Jaly I, Iyengar K, Bahl S, Hughes T, Vaishya R. Redefining diabetic foot disease management service during COVID-19 pandemic. Diabetes Metab Syndr. 2020;14(5):833-838.
Crossref - Suh HJ, Kim DH, Heo EY, et al. Clinical characteristics of COVID-19: clinical dynamics of mild severe acute respiratory syndrome coronavirus 2 infection detected by early active surveillance. Jo Korean MedSci. 2020;35(32):e297.
Crossref - Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
Crossref - Barrera FJ, Shekhar S, Wurth R, et al. Prevalence of diabetes and hypertension and their associated risks for poor outcomes in Covid-19 patients. Journal of the Endocrine Society. 2020;4(9):bvaa102.
- Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. bmj. 2020;369.
- Lodge CJ, Doherty A, Bui DS, et al. Is asthma associated with COVID-19 infection? A UK Biobank analysis. ERJ Open Research. 2021;7(4).
Crossref - Vardavas CI, Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tobacco Induc Dis. 2020;18.
Crossref - Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev cell. 2020;53(5):514-529. e513.
Crossref - Carlson N, Kristensen NKE, Ballegaard FE, et al. Increased vulnerability to COVID 19 in chronic kidney disease. J Intern Med. 2021;290(1):166-178.
Crossref - Wang HE, Gamboa C, Warnock DG, et al. Chronic kidney disease and risk of death from infection. Am J Nephrol. 2011;34(4):330-336.
Crossref - Cocchio S, Zabeo F, Facchin G, et al. The Effectiveness of a Diverse COVID-19 Vaccine Portfolio and Its Impact on the Persistence of Positivity and Length of Hospital Stays: The Veneto Region’s Experience. Vaccines. 2022;10(1):107.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.