Antibiotic resistance is a major risk to human health worldwide due to antibiotic- and multidrug-resistant bacteria, especially in the case of serious infections, which limits the availability of antimicrobial treatment options. Focusing on the bacterial resistance mechanisms against antibiotics and the conventional strategies used to combat antimicrobial resistance, this review highlights the history of antibiotics and their target mechanisms, mentions the strategy limitations, provides the most recent novel alternative therapies to combat resistance, and illustrates their mode of action and applications that may treat several infectious diseases caused by bacterial resistance. Finally, this paper mentions future prospects that we believe would make a considerable difference in the microbial resistance battle. Novel antibiotic alternative therapies, including nanomaterial therapy, antimicrobial photodynamic therapy, hybrid antimicrobial therapy, and phage therapy, are covered in this review.
Bacteria, Antibiotic-resistance, Nanomaterials, Antimicrobial Hybrids, Photodynamic Therapy, Phage Therapy
Antibiotic resistance (AR) is a subset of antimicrobial resistance and refers to a group of bacteria that develop antibiotic resistance. Multidrug-resistant bacteria are resistant to several antimicrobials, making them antimicrobial resistant (AMR). AR typically occurs when bacteria create mechanisms to defend themselves from the effects of antibiotics.1 The World Health Organization (WHO) defines AR as a bacterium with resistance to an antimicrobial medication that allows it to mediate the effects of an antimicrobial that it was previously exposed.2 Bacterial AR has a significant impact on health; social identifiers have been established for worldwide group movements to resolve the problem, including recommendations for global AR agreements.3 AMR is expected to cause over 700,000 deaths globally each year, of which at least 35,000 deaths are in the United States (US), making AR a significant public health problem.4 On April 17, 2018, the Saudi Arabian Ministry of Health established stringent restrictions for antibiotic prescriptions. The availability of over-the-counter (OTC) antibiotics that could be acquired without a prescription was identified as a significant contributor to the spread of AR bacteria in the Kingdom of Saudi Arabia (KSA). Consequentially, according to the executive regulations of health practice law in the Kingdom, pharmacists are prohibited from dispensing any antibiotics without a doctor’s prescription.5 Regardless, the spread and development of AMR bacteria is on the rise because of the large population of expatriates, linked to an increased risk of contracting and transferring infectious illnesses, such as returning Al-Hajj travelers that contracted MDR A. baumannii and E. coli during the Al-Hajj event.6 AMR bacteria and improper use of antibiotics by patients are on the rise, and the situation has worsened during the COVID-19 pandemic. SARS-CoV-2, the new coronavirus, is partially responsible for these widespread, extreme AMR infections.7 Antimicrobial consumption in hospitals and in general for COVID-19 symptom treatment or those that may be confused with the symptoms of a common cold. Self-medication, noncompliance with antibiotic treatment, and using sub-optimal doses are possible reasons for the increase in AR. The global use of antibiotics remained to increase during the COVID-19 pandemic, and there is public concern that resistance to antibiotics will develop. If AR is not managed, we will be confronted with a “post-antibiotic era,” in which simpler bacterial diseases will be difficult to
treat. 8 AR is now threatened by a number of factors, including microbiological (natural) causes and AMR, which is produced by changes in bacteria.9 This can be demonstrated by various methods such as antibiotic misuse, diagnostic and antibiotic prescription errors, patient sensitivities, losses, self-medication, poor healthcare surroundings, and personal hygiene practices.10
This review discusses the history, discovery, and updates of the global antibiotic development pipeline, the mechanism of antibiotics, traditional strategies applied to face AR, and their limitations, highlighting the main aim of the major developments in current novel therapeutic strategies and their mode of action and applications. Some of the innovative antibiotic alternatives discussed in this paper include nanomaterials, antibiotic photodynamic therapy, antimicrobial hybrids, and phage therapy. Therefore, we aim to provide useful information to readers looking for solutions to the AMR problem.
A Brief Antibiotics History
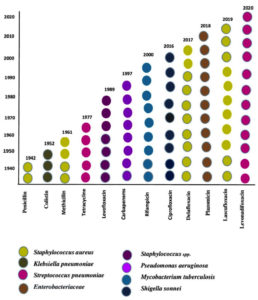
Antibiotics inhibit bacterial growth, by blocking or reducing the bacterial activity, treating infections and are important to deter and prevent illness.11 Furthermore, antibiotics are considered the most important type of medication and one of the most crucial medical advancements of the 20th century, which has saved millions of lives. Figure 1 illustrates the first antibiotics that emerged when Alexander Fleming isolated the first naturally occurring antibiotic, penicillin, from Penicillium notatum in 1928 and its first use in medical practice in 1941.12 Most antibiotics in the current industry were developed and launched before 1987, a period termed the “Golden Decade”.13 Only a few new antimicrobial classes were developed and released onto the market since 1987.14 Currently, we are in the post-antibiotic age, with a slow rate of novel drug discovery and rapid development of AR bacteria.15 As shown in Figure 1, novel antibiotics against carbapenem-resistant bacteria have been examined, including delafloxacin (Baxdela, 2017), and ceftazidime/avibactam that was the first antibiotic authorized by the FDA in 2018, followed by Lascufloxacin (La Svic, 2019), and the most recent antibiotic, imipenem/relebactam and cefiderocol (Paulin, 2020).16
Figure 1. Shows a timeline of antibiotic resistance detection. The x-axis depicts the numerous forms of antibiotics, while the y-axis shows the years of discovery. Distinct circles represent different species of bacteria.
The Global Antibiotics Discovery and Development Pipeline
Antibiotics Approved Between July 1, 2017, and September 1, 2020 (the time frame for this update)
Based on public data, the WHO published the first complete assessment of the developmental antibacterial pipeline. The Observer created a comprehensive visualization that allows users to review each pre-clinical pipeline by type, pathogen category, and pre-clinical stage of development.16 Since the initial assessment of the WHO on the clinical antibacterial pipeline in 2017, 11 new antibiotics have been registered, including one for the treatment of tuberculosis in humans. Vaborbactam (in combination with meropenem) and lefamulin, two authorized agents, are novel chemical classes.17 By the end of 2020, 43 antibiotics had entered clinical trials, including 15 in stage I, 13 in stage II, and 13 in stage III. Pathogens of the “ESKAPE” classification, which includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, have been shown to be effective infection treatments that are caused by the six major common nosocomial diseases in vitro.18 Although several of these novel antibiotic drugs have been licensed for use, clinical trials are currently ongoing. Eight new carbapenem-resistant antibiotics, the bulk of which are used in Complicated intraabdominal infections (cIAI), have been investigated. As previously mentioned, Ceftazidime/avibactam was the first antibiotic approved, followed by ceftolozane/tazobactam, and then imipenem/relebactam, and cefiderocol in (2020).19 In 2021, it is expected to be updated. According to the classification system of the WHO (AWaRe) all new antibiotics drugs licensed until 2019 will be designated under AWaRe drugs, reaching a total of 258 antibiotics.20 AR affects the ability of bacteria to resist drug treatment, and it is expected that this resistance will increase in the future. By understanding the mechanisms of working and how bacteria develop resistance, alternative therapies could be developed to address this resistance.
Mechanism of Antibiotic
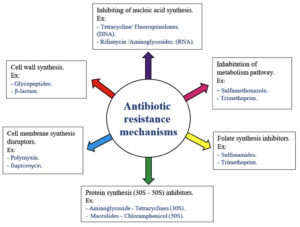
This antibiotic action process can be divided into five steps: termination of microbial protein synthesis, microbial membrane function, metabolic passageway, nucleic acid synthesis, and microbial cell wall synthesis in both Gram-negative (GN) and Gram-positive (GP).21
Cell Wall Inhibition
Bacterial cell walls are composed of cross-linked peptidoglycan, which is a major component of the microbial cell envelope and prevents the cell from bursting due to turgor and maintains the cell shape.22 Antibiotics that block peptidoglycan formation, such as β-lactams, glycopeptides (vancomycin), carbapenems, cephalosporins, and penicillin and its derivatives, make the cell liable to autolysis and osmotic pressure. Therefore, antimicrobial medicines prevent proper cell wall formation.23
β-lactam antibiotics: Both GP and GN bacteria contain peptidoglycan, an ingredient of the bacterial cell wall that provides structural integrity.24 Glycan chain synthesis by pentapeptide chains that cross-link N-acetylmuramic acid and N-acetylglucosamine subunits form peptidoglycan.22 β-lactam antibiotics block the last step of peptidoglycan biosynthesis by inhibiting the enzyme penicillin-binding protein (PBPs), that in turn effect peptidoglycan cross-linking and cell viability and promotes bacterial lysis.25
Antibiotics Protein Synthesis Inhibition
The 30S and 50S subunits make up the 70S ribosome of bacteria (depending on the protein precipitation average, represented as “Svedberg” units). Antibiotics inhibit protein synthesis by binding to the 50S (macrolides and chloramphenicol) subunits or 30S subunit (tetracyclines and aminoglycosides).26
30S Subunit Inhibitors
Aminoglycosides inhibit protein synthesis on 16S ribosomal RNA (rRNA) by binding to the A-site of 30S rRNA with a high affinity. This allows erroneous amino acids to converge inside the polypeptide, resulting in noxa release into the cell membrane.26,27
50S Subunit Inhibitors
Macrolides are linked to the 50S ribosomal subunit in bacteria, where they inhibit bacterial protein synthesis by inhibiting peptide bond formation28 and terminating the transpeptidation phase results in imperfect division of peptide chains.29
Antibiotic Nucleic Acid Synthesis Inhibition
Rifamycin inhibits bacterial transcription (mRNA synthesis) and is effective against intracellular bacteria such as mycobacteria. Bacterial RNA polymerases differ from eukaryotic RNA polymerases regarding their structure, which is responsible for facilitating bacterial cell-specific poisoning.30
Metabolic Pathway Inhibition
Sulfonamides compete with para-aminobenzoic acid (PABA) linked to dihydrofolate biosynthesis, which blocks the enzymatic conversion of PABA and pteridine to dihydropteroic acid by competing with PABA for dihydrofolate synthetase, a stage in the production of tetrahydrofolic acid (THF). THF is required for the production of dTMP and purine, and suppression prevents bacterial development.31 By blocking dihydrofolate reductase with trimethoprim, enzymes divert dihydrofolate (DHF) to (THF). THF is required for the microbial nucleic acid and protein production, and for survival.32
Cell Membrane Function Inhibition
Polymyxins (polymyxin B and E) are a minor family of antibiotics that lyse the bacterial
cell membrane.33 They work as “cleaner” lipophiles that damage the cell membrane by intervening with the GN bacteria lipopolysaccharides (LSPs).34-35
Antibiotic Resistance Mechanisms
The two most common types of AR are acquired AR and natural AR. Normal AR genes can be inherent (frequently expressed in organisms) or mediated (genes that exist in microbes but are activated only after antibiotic exposure).34 Acquired AR occurs when the bacterium obtains genetic material through transposition, translation, conjugation,35 or some change in chromosomal DNA.36 AMR mechanisms can be classified into four groups:
Decreased Drug Uptake
GN bacteria are innately less susceptible to antimicrobials than GP bacteria because of the presence of an additional layer of LPS in their outer membrane, which acts as a permeability shield. The glycopeptide antibiotics, such as the vancomycin, are ineffective against GN bacteria due to the absence of permeation among the outer membrane and is an example of this innate barrier effectiveness. Changes in the bacterial membrane make it more difficult for the antibiotic to succeed. In this case, a lower amount of antibiotics enters the bacteria, such as in Enterobacterales, Acinetobacter spp., and Pseudomonas spp.37 Biofilm formation is another method that aids bacterial colonization.38 Polysaccharides, proteins, and DNA are found in the biofilm extracellular matrix, which makes it difficult for antimicrobial drugs to enter the bacterial cell and consequentially confers protection.39
Drug efflux Some microbes produce a pump located in the cell wall or membrane, called efflux pumps, which are highly prevalent in GN bacteria. These efflux pumps transfer different compounds, such as nutrients, signalling molecules,27 and transport the antibiotics out of the bacteria, decreasing the antibiotic concentration in the bacteria.34 Occasionally, mutations in the DNA can stimulate the bacteria to produce additional pumps, which increase resistance to tetracyclines in P. aeruginosa and Enterobacterales.40
Drug Inactivation
Antibiotics are inactivated by bacteria via destruction by chemically altering drugs.37
Drug Chemical Modification
Bacteria can add various chemical groups to antibiotics by producing specific enzymes that prevent antibiotic from linking to their target in the bacterial cell.41 Aminoglycoside-modifying enzymes (AMEs) change the amino groups of aminoglycoside molecules, making them inefficient. This is an example of medication modification-induced resistance.40.
Drug Destruction
Some bacteria inactivate antibiotics by producing enzymes. The β-lactamase enzyme destroys the β-lactam ring of penicillin, an important antibiotic for treating human infections.25 After several years, bacteria that produce extended-spectrum β-lactamases (ESBL) have become the main problem. These are able to destroy many β-lactam antibiotics, which are typically the last resort of medicine for the treatment of infections with these bacteria.23 The most frequently used antimicrobial drugs are β-lactam antibiotics such as penicillin and cephalosporins.25
Drug Target Modification
Bacterial DNA mutations result in structure changes of the antimicrobial target in bacteria, which prevents antibiotics from reacting with their target.26 Moreover, bacteria can add various chemical groups to the target to protect it from antibiotics. Changes in DNA gyrase or topoisomerase IV, which are enzymes important in the processes of DNA replication, transcription, and recombination, facilitate resistance to drugs that block nucleic acid synthesis, such as fluoroquinolones.42 A frequent process by which bacteria develop resistance to antibiotics is a change in the antibiotic target.34 Other mechanisms of β-lactam resistance to antibiotics include changes in the organization and/or number of PBPs. Modifying the number of PBPs affects the quantity of medication that can bind to a target.23
Traditional Strategies Applied to face AR and their Limitations
Therapeutic strategies for combating bacteria are important for treating, saving lives, and controlling infectious diseases. This review discusses some of the covenantal solutions used to treat ARB and their limitations, and elaborates on novel therapeutic strategies.
New Antibiotics
The early to mid-20th century indicated that the medical advancement and improvement of vaccines and antibiotics is imperative (US Centers for Disease Control and Prevention (CDC)). Antibiotic agents, also known as antibacterial or antimicrobial drugs, are used to control and treat infections associated with bacterial strains by destroying or preventing the growth of these bacteria.43 Antibacterial drugs target cell membranes, cell walls, nucleic acid synthesis, protein synthesis, and biological metabolic compound synthesis according to their mechanism of action (Figure 2).
Limitations
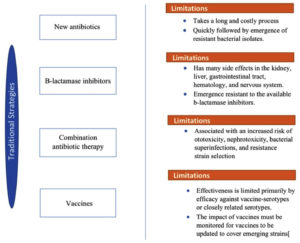
Identifying new antibiotics to combat resistance and developing them into drugs is a long and costly process. It costs approximately $800 million and $1 billion to introduce a new drug, and it takes an average of 10 years to reach medical centers.44 In addition, the introduction of new antibiotics into clinics is quickly followed by the emergence of resistant bacterial isolates.43 Furthermore, the alarming rise in AR rates indicated that the golden era of antibiotics has possibly ended.45
β-lactamase Inhibitors
Combining β-lactamase inhibitors with β-lactamase-sensitive antimicrobials is an effective method for preventing the failure of otherwise effective antibiotics.46 Bactericidal antibiotics that contain β-lactam rings in their molecular structure and β-lactamase antibiotics attach covalently and impair penicillin-binding protein PBPs. PBPs facilitate cross-linking of the bacterial cell wall peptidoglycan layer.47 Since bacteria have shown resistance to these antibiotics, the need of β-lactamase inhibitors is necessary. β-lactamase inhibitors are used in to combat AR by inhibiting serine B-lactamases, which are enzymes that catalyze the hydrolysis of the four beta-lactam ring amide bonds, and then inactive the primary resistant mechanism. All B-lactam antibiotics have the same chemical structure.48 For instance, Enterobacteriaceae, Haemophilus influenzae, Mycobacterium tuberculosis, Neisseria gonorrhoeae, and Pseudomonas aeruginosa are among the most common bacteria that beta-lactamase inhibitors are expected to treat.49
The Limitations
Although this strategy has a major effect on combating β-lactamase-mediated resistance, it has disadvantages that have been linked to adverse effects in the kidney, liver, gastrointestinal tract, hematology and nervous system Figure 3.48 Furthermore, this therapy showed that a number of β-lactamases, that are resistant to usable β-lactamase inhibitors, have increased in recent years.46
Combination Antibiotic Therapy
Another strategy commonly used to cure severe infectious diseases caused by Enterobacter cloacae, Pseudomonas aeruginosa, and Serratia marcescens as well as GP infections caused by Enterococcus and Staphylococcus spp. is combination antibiotic therapy, but it is contentious and debatable.50 Compared to monotherapy, the potential benefits of combinations include a wider antibacterial spectrum, potential interactions, and a lower incidence of resistance development during treatment. Owing to the lack of evidence-based treatment options, combinations are increasingly being used to improve the bactericidal effects of conventional antibiotics against MDR strains. Domestic resistance epidemiology, patient health conditions for resistance, recent colonization or infection with resistant strains, and hospitalization determine the best antibiotics for use. For example, for suspected GN sepsis and severe Pseudomonas spp. infections, broad-spectrum β-lactams, aminoglycosides, or fluoroquinolones are commonly used in combination therapy.51 Colistin combinations, on the other hand, are more commonly used as a last resort treatment for MDR strains.52 Combinations of an aminoglycoside, ampicillin/sulbactam, carbapenem, colistin, and/or rifampin have been found to be effective against MDR acinetobacter spp.53
The Limitations
Recent meta-analyses, however, have reached the conclusion that the present medical evidence is inadequate to support the use of conclusive combination therapy, and that combination therapy is associated with increased ototoxicity, nephrotoxicity, bacterial superinfections, and resistance strain selection. Once the antibiotic sensitivity profile of the causative organism is known, it is strongly advised to post-antibiotic treatment towards the most appropriate single agent.50
Vaccines
Vaccines are a medication that is used to increase the immune response to infection. Vaccines are usually administered via needle injection, but some can also be administered orally or through the nose (CDC, 2014). Similar to antibiotic treatment, vaccines have several advantages regarding AR. For instance, they can avoid infections caused by both AR and antibiotic-sensitive bacteria, provide herd immunity, protect non-vaccinated individuals by minimizing pathogen transmission, decrease the overall numbers of bacteria, and inhibit viral infections that reduce antibiotic administration, and ultimately combat rising AR.44-54 Examples of bacterial vaccines include Bacillus Calmette-Guerin (BCG), adenovirus, and the oral Ty21a Salmonella typhi vaccine.55
The Limitations
There are limitations to what vaccines can offer in this regard. Several obstacles must be overcome for vaccines to be effective as antimicrobial strategies. For example, the pneumococcal vaccine (PCV), introduced in 2001, can defend against seven serotypes identified by capsular polysaccharides. The prevalence of diseases associated with nonpneumococcal serotypes has increased over time. Consequently, a modified PCV was reintroduced, which involves six more serotypes and provides wider protection.44 Vaccine effectiveness is limited primarily by its efficacy against vaccine serotypes or closely related serotypes. As a result, the impact of vaccines must be monitored and updated to cover emerging strains.56
The WHO highlighted the threat posed by MDR GN pathogens in 2017. The exploration, design, and emergence of new and alternative antibiotic therapies are critical for reducing the observable administration of existing antibiotics, thereby lowering the selective advantage for AR strains.45-57 This review aims to illustrate novel alternative strategies that can replace traditional therapies and play a significant role in combating AR in the near future.
Novel Alternative Therapeutic Strategies
Antimicrobial Nanomaterial Therapy
Antimicrobial Nanomaterial Therapy Concept and History
Nanomaterials (NM) are materials with diameters ranging from 0.2 to 100 nm, and present substantially larger surface areas, resulting in high surface-to-volume ratios. As a result, electronic energy levels become distinct, yielding notable electrical, magnetic, optical, and dynamic properties.58
NMs are considered a recent science with a history reaching the 9th century. Ancient potters utilize gold and silver nanomaterials (AgNMs) to create a glistening appearance on their creations. Michael Faraday published the first scientific description of NM characteristics (Faraday, 1857). Furthermore, Richard Feynman presented a description of “atomically precise molecular machines” (Feynman, 1960).
In Japan, this is the known formal discussion on nanotechnology. Sugibayashi et al. used albumin nanomaterials to bind 5-fluorouracil and discovered temperature-dependent denaturation variations in drug release and body distribution in mice following intravenous tail vein injection. The NMs were injected intraperitoneally into Ehrlich Ascites Carcinoma-bearing mice, which resulted in an increase in life span.
During the 1950s and 1960s, the global attention was drawn to the use of NMs in medication delivery. Professor Peter Paul Speiser, who invented the first NMs for medical administration and vaccinations, was a pioneer in this field. His Cambridge University research group examined polyacrylic beads for oral delivery before moving on to microcapsules.59.
The first NM for medical administration and vaccination was created in the late 1960s. Graphene was initially proposed in the early 1980s, with K. Eric Drexler of the Massachusetts Institute of Technology presenting the first study on NM in 1981. This was backed by significant progress in creating drug delivery technologies such as transmission electron microscopy (TEM), atomic force microscopy (AFM), and dynamic light scattering (DLS). Current NMs technology has progressed to the point that it would have been seen as the technology of the future.59
NM has also evolved as a creative approach for tackling the bacterial MDR problem caused by antibiotic abuse. Because the microbicidal nature of NM derives from direct contact with the bacterial cell wall without the need to penetrate the cell, the use of its antibacterial agents may bypass resistant bacterial processes. As a result, the development of ABR to NMs is less likely than that to antibiotics. Hence, NM has the potential to be used in medicine for antibacterial therapeutic applications.60
Microbial NM production relies on the capability of microbial cells, enzymes, and biological substances to reduce bacteria, fungi, and plants, and has recently received abundant attention for its ability to biosynthesize metal NMs in an ecologically friendly manner. Many bacteria contribute to the use of green nanotechnology in the synthesis of NMs such as S. aureus, P. aeruginosa,61 E. coli,62 Lactobacillus species63 and K. pneumoniae.64 NMs can also be used as nanocarriers to deliver therapeutic drugs. The functions of NM are driven by its specific physicochemical features, notably its interactions with microbes. They are influenced by chemical parameters such as van der Waals forces, receptor-ligand contacts, hydrophobic interactions, and electrostatic interactions.65
Mode of Action
First, NMs interact with bacteria via electrostatic interactions, which results in membrane damage and cell lysis. Biodegradable cations and amphiphilic polycarbonates are created and self-assembled from methicillin-resistant Staphylococcus aureus killing cationic micellar NM, Methicillin-resistant Staphylococcus aureus (MRSA).66
In the second mode of action, an excessive amount of reactive oxygen species (ROS) is produced. Oxidative stress is caused by the accumulation of ROS, which is deadly. When superoxide and hydroxyl radicals mix with thiols in proteins, ROS may harm cells in several ways, including by deactivating membrane receptors.67 ROS-based antibacterial effects include the production of free copper (Cu+) ions from copper iodide (CuI) NM, which creates ROS and destroys bacterial DNA and intracellular proteins in E. coli and B. subtilis.68
The third mode of action disrupts cellular components; for example, gold-Dual antiplatelet therapy (Au–DAPT) NM inhibits the growth of MDR E. coli and P. aeruginosa strains by binding to ribosomes and chromosomes and destroys the cell membrane. Polymer-coated (AgNMs), which disrupt both the Krebs cycle and amino acid metabolism, also damages E. coli cells.69
Application of Nanomaterials Therapy
Delivery of Therapeutic Agents
The FDA has approved and improved accessibility to the therapeutic use for many treatments that use NMs termed “nano drugs”, which are liposomal nano-formulations for the treatment of a variety of diseases, especially cancer.70 Additionally, antimicrobial agents can be delivered using NMs as carriers71 or medications can be encapsulated or bonded to the surfaces of NM.72
NM protects antimicrobial agents from enzymes and chemicals that would otherwise destroy them. This defense can improve drug treatment effectiveness, leading to a reduced dose to improve therapeutic effects while reducing host toxicity.73
The delivery method is used to improve the solubility, stability, and biocompatibility of antibiotics, which are typically difficult to pharmacologically administer successfully. Through the administration of drugs that induce several mechanisms of action and customized payload release, NM can restrict resistance development by preventing bacteria from being exposed to sub-inhibitory amounts of drugs.74 For example, the antibiotic levofloxacin, which was packed into silver core-embedded MSNs, successfully treated MDR E. coli isolates while exerting minimal toxic effects and causing minimal damage to the hepatic peritoneum. The silver component functioned similarly as a transporter, but also produced silver ions that were antimicrobial. Bacterial burdens were reduced by three orders of magnitude in in vitro-treated mice.75
Jimenez-Jimenez et al. indicated that NM therapies for various tumors might be a distinct and appealing technique for improving cancer treatment. (AgNMs) might be employed to deliver cytotoxic drugs or boost the anti-cancer effectiveness of combinational partners through chemo- or radiation therapy.76
NMs and their nanocomposites can be used in a broad variety of scientific and technological fields, including biomedical and therapeutic applications. Thus, they may be promising antibacterial, anticancer, and antifungal properties. The potential health and environmental consequences of (AgNMs) remain unknown, and additional research regarding toxicity is urgently needed.77 In 2013, toxicology research on mice following exposure to carbon nanotubes (CNT) revealed that the cytotoxicity of gold nanomaterials (AgNMs) depended on the type of toxicity testing employed and the cells used. There are variations in the cytotoxic profile of human lung and hepatic cancer cell lines. Multi-walled carbon nanotube (MWCNT) demonstrated minimal lung inflammatory ability at doses comparable to normal inhalation and exposure to element such as carbon concentrations observed only in CNT facilities in the United States.78 However, NMs effectiveness for viral inhibition has been demonstrated. NM, when paired with enhanced bioavailability, can efficiently carry medications and genes to select cells and tissues while avoiding exterior risks.79 Antiviral drugs with controlled-release drugs may also be administered by NM at all stages of virus-cell replication, whereas vaccines with enhanced immune response only interfere at a certain time.80 The COVID-19 vaccine is an example in which NM has the potential to increase effectiveness. The numerous properties of nanomedicine, such as medication transport, diagnostics, and theragnostic, can function as a catalyst for innovative treatment techniques.81 NMs provide a direction for cancer treatment, despite many technical limitations in clinical application. It is reasonable to assume that therapeutically relevant NMs will be created in the coming years, followed by well-designed clinical studies demonstrating their use in clinical settings.
Wound Therapy
Wound infections usually afflict 300 million people globally, with treatment expenses in the US alone estimated at $25 billion. Damaged tissue increases bacterial adhesion and produces nutrients that enhance bacterial multiplication during these infections. Wound infections are routinely treated using (AgNMs) included in wound dressings.82 Modern medicine has improved the antibacterial capabilities of standard medicines against MDR microorganisms. Copper nanoparticles inhibit the formation of preformed P. aeruginosa and S. aureus biofilms, thereby reducing biofilm-infection wounds, and their potential use in wound dressings is currently being researched. Additional in vitro and in vivo research is being conducted to establish the utility of this method for wound dressings. Gentamicin-loaded PLGA NMs can aid drug administration technique development based on antibacterial smart wound dressings.83 Shalaby et al. thoroughly examined current wound healing dressings, highlighting antibiotic-based nanocomposites, indicating the combination of antibacterial and removable NMs that aid wound healing. NM-based customizable bandages offer considerable potential for healing wounds in a personalized way.84
Oral Therapy
Biofilms are ubiquitous in the oral cavity, and S. mutans is a common oral biofilm pathogen. Dental caries are caused by the degradation of tooth apatite caused by the acidic milieu of dental biofilms (i.e., plaque).85 Yin et al. found that dental metals, dental resins, prostheses, and dental coatings include NMs, which prevent implants from developing biofilms and help maintain lengthy oral hygiene.86 Oral biofilm therapy has also been investigated in NM, which causes the production of ROS and in turn EPS matrix destruction. The application of FDA-approved polymers to iron oxide NMs, such as dextran, increases their aqueous formulation stability and biocompatibility with oral soft tissue structures.87 An oral biofilm containing iron based NMs reduce acid deposition and inhibit tooth cavities, while posing no danger to epithelial and mucosal tissues. Ferumoxytol might bind to the biofilm matrix and cause free radical destruction from H2O2, resulting in bacterial death and EPS decomposition in situ.88 Overall, NMs improve many bactericidal mechanisms that kill bacteria while avoiding AR. The size, surface properties, and shape may all be modified to create a broad design field for novel antibacterial agents. This was evidenced by its anti-MRSA activity.
Antimicrobial Photodynamic Therapy
Antimicrobial Photodynamic Therapy Concept and History
Photodynamic therapy (PDT), also called photodynamic inactivation (PDI), is a promising treatment strategy for eradicating pathogenic microbes, such as GP and GN bacteria, yeasts, and fungi. PDT may be superior to traditional antibiotic therapy in the quest for alternative approaches that can kill bacteria without causing undesirable drug-resistant strains. When a light source, oxygen, and a dye or photosensitizer (PS) are present simultaneously, a non-thermal photochemical reaction occurs.89 PTD is also an oxygen-dependent photochemical reaction that occurs when a photosensitizing component is activated by light, producing cytotoxic ROS, most notably singlet oxygen.90 PS, which is known to be activated by light, generates ROS, which can affect cell structures in microorganisms or infected mammalian cells, eventually leading to cell death.91 ROS are produced by type I or type II mechanisms (these types will be clarified later in this review) and can inactivate a variety of microbial cells, including GN bacteria such as Pseudomonas aeruginosa, which have an impenetrable external cell membrane consisting of endotoxins to prevent antibiotics, dyes, and cleansers from passing through.90
Light has long since been used as a therapeutic agent in medicine and surgery. Phototherapy has also been used in Greece, India, and Egypt, but it was forgotten for decades until it was reintroduced via Western culture at the turn of the century. Niels Finsen, a Danish physician, was the first to examine patients with advanced photodynamic therapy. He illustrated PDT for the treatment of Lupus vulgaris, a tuberculous skin condition, using heat-filtered light from a carbon arc lamp (the Finsen lamp).92
The concept of cell death caused by light interactions was proposed by Oscar Raab, a Munich medical resident working with Prof. Herman von Tappeiner. He observed that the use of acridine red in conjunction with light was potent against infusoria, a paramecium species, during his research into the impact of acridine on paramecia cultures.93 Later research, “Photodynamic action” was coined by Von Tappeiner’s laboratory who highlighted the importance of oxygen. PDT has afterward clinically proven by researchers from the Cancer Institute in Buffalo, New York’s Thomas Dougherty, and his coworkers. They reported their findings in 1978, after treating 113 dermal or intradermal malignant tumors and analyzing the partial or full resolution of 111 tumors. A hematoporphyrin derivative was used as an active photosensitizer in this clinical PDT trial. It was renamed PDT by John Toth.94
While PDT has been used to treat cancer for over 25 years,95 PDT as an antimicrobial therapy was revealed for the first time in the early 1990s in the healthcare field, ushering in a “photo-antimicrobial renaissance era.”.96 Antimicrobial PDT (aPDT) is effective against major MDR bacteria, regardless of their drug-resistance characteristics.97 To date, only a few cases of aPDT resistance have been reported, suggesting that the potential of microbes to adjust and avoid this treatment occurs, but is still enclosed. Light-based approaches have shown promising results as existing antimicrobial therapeutic alternatives.98
Mode of Action
The APDT method causes cell death is a non-toxic way, a technique known as lethal photosensitization, in which microbial cells are pre-impregnated with PS and afterward exposed to a particular light source.99 Pursuing sensitization, the dye that accumulates on targeted bacteria converts O2 into ROS, which is highly toxic to microbial cells. When exposed to a specific wavelength of light, the elements of PS obtain an excited state via electron transition to a greater energy level. Through this excited singlet state, PS interacts with O2 to initiate ROS production (process type II), even with other molecules to generate hydroxyl radicals and many sustainably sourced radicals (process type I).100 These reactions can cause a wide range of damages to microbial cell materials or constantly alter their metabolic functions, resulting in death.102 In general, energy is absorbed through intracellular photosensitization and is transferred to O2, resulting in destruction of the oxidative reaction pathways103 inside the cytoplasmic membrane and the genetic materials of the pathogen cells while having no toxic effects on the host cells.101 PS has several important properties, including the ability to absorb light at a certain wavelength (preferable permeability in the 400–800 nm range), photostability, low cytotoxicity in the dark, and most importantly, antimicrobial activity aided quantum yield, which is quite high in the excited triplet state. Due to the high “oxidative power,” the ROS produced by excited PS can be used for localized killing of cells and microorganisms at the site of irradiation.100
Application of Antimicrobial Photodynamic Therapy
Photodynamic antimicrobial chemotherapy is an alternative treatment for drug-resistant microbes that is antibacterial, antifungal, and antiviral.104 The use of PDT in dental care is increasing rapidly. They are also widely used for the treatment of fungal and bacterial infections as well as in the photodynamic diagnosis of oral cancer.93 Photodynamic inactivation of microorganisms (PDI) is an extended form of PDT for the inhibition of microorganisms such as bacteria, yeasts, fungi, and viruses. Nontoxic dyes (photosensitizers) are combined with harmless visible light to render these microbes inactive. PDI may be employed instead of antibiotics and antiviral drugs, which are known to cause resistance, in a variety of fields, including veterinary medicine, humans, agricultural processing, food processing, biosafety, and treatment of wastewater. PDT is increasingly being used in a variety of applications, including the destruction of bacteria, fungi, tumor tissues, and viruses (including COVID-19).105
Viruses
PDT was previously investigated for the treatment of patients with COVID-19. Weber and colleagues (2020) examined whether the PDT process with riboflavin and blue light could be used efficiently as a treatment for patients exposed to the virus with severe symptoms. COVID-19-positive patients who received PDT were included in the study, as were COVID-19-positive patients who received standard care. PDT improved significantly within 5 days. The findings indicate that the PDT procedure has the potential to cure infections caused by coronavirus in its early stages of infection, with clinical symptoms ranging from mild to severe.106 This novel application of PDT has the potential to reduce hospitalization and intensive care treatments.107
Fungi
Oral candidiasis is a renowned fungal disease induced by Candida spp., primarily Candida albicans, which causes surface mucous membrane infections in the mouth, which can escalate to bloodstream infections.108 Innovative research is being conducted to develop less invasive alternative therapies for treating oral illnesses with limited negative side effects. Photodynamic therapy is a treatment option that has shown promising outcomes in the treatment of this disease.109
Bacteria
Dental caries are a common microorganism-related disease in the dental field.110 This disease is primarily caused by lactobacillus, Streptococcus mutants, and Streptococcus sobrinus bacteria which, as they adhere to the teeth, hydrolyze the sugar and generate acidic substances that demineralize dental plaque, leading to the formation of lesions with caries.111 Among the bacteria are Treponema species, Streptococcus species, Fusobacterium species and Enterococcus faecalis which cause endodontic diseases.112 Furthermore, dental diseases are also common and are distinguished by deep-tissue inflammatory responses that destroys the gums.113 Associated bacteria include Fusobacterium periodonticum, Porphyromonas gingivalis, Tannerella forsythia, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Eubacterium saburreum, and Treponema denticola.114 Mechanical extraction (a physical process) combined with antimicrobial administration at the systemic level is the standard treatment for removing or controlling these oral pathogens, which frequently results in the selection of resistant species and the occurrence of health risks.110
Therefore, antimicrobial drug resistance requires the development of alternative therapeutic options. The most common AMR pathogens are MDR strains of microorganisms that are classified as ‘ESKAPE’ pathogens since they are responsible for the vast majority of care facility infections and effectively ‘escape’ the impacts of antibacterial drugs. As a result, alternative, safer, and more efficient antimicrobial strategies, particularly against ‘ESKAPE’ superbugs, are urgently needed. Photodynamic inactivation of antimicrobials is a treatment option for infectious diseases that uses a photosensitizer, light, and oxygen to eliminate highly metabolically active cells.115
Furthermore, PDT can be integrated into medical devices. Light delivery capsules have been studied for the treatment of gastrointestinal tumors with mTHPC-mediated PDT. Because the effective photodynamic reaction when the light power density and the light fluence rate are low, rehabilitation time can be reduced to a few minutes, allowing PDT to be integrated into independent medical devices, including endoscopic capsules with extremely small diameters, to provide advanced therapeutic capabilities.116
Finally, aPDT is regarded as a promising therapy for the treatment of ARB and several oncologic human diseases because PDT is a minimally invasive/non-invasive therapy with rapid microbial cell elimination, short recovery time, low PS concentration level, and toxic selectivity for only microorganism cells in light without seriously affecting the host.109
Phage Therapy
Phage Therapy Concept and History
Bacteriophages (phages) are viruses that infect bacteria and multiply within them. They are the most common organisms on the planet and play an important role in the physiology of microorganisms, population dynamics, and treatments.117 Hankin, Gamaleya, Twort, and d’Herelle were credited with inventing and developing the application of phages.118 Humans were not infected with any of these viruses. Phages are viruses that are harbored by bacteria. The human population is expected to exceed eight billion people by 2050, but the kind of phages found in ecosystems is anticipated to exceed 1031. They can be found anywhere in which bacteria exist. Since the turn of the 20th century, humans have learned to know and identify them. They were discovered in the feces of convalescing dysentery patients in 1917 by Félix d’Hérelle while working at the Pasteur Institute in Paris. They were first reported by Frederick Twort in 1915. Despite the fact that the nature of bacteriophages was a point of contention until the introduction of the electron microscope in the early 1940s, d’Hérelle used his discovery immediately to treat patients with bacterial illnesses and claimed significant success. During these numerous studies, d’Hérelle partnered with George Eliava, who later oversaw the establishment of a facility dedicated to this discipline in Tbilisi, Georgia, which was named.119
The use of phages presents several benefits and setbacks. Setbacks are based on several arguments, including the difficulty of using phages and efficacy problems. Moreover, antibiotics and sulfa drugs were one of the medicines’ greatest triumphs, and as a result of the phage therapy field faded. By the end of the 20th century, there was widespread interest towards the use of phages for therapeutic reasons. While phage treatment grew rapidly in Europe and the U.S., American pharmaceutical companies for producing phages began to decrease in the 1940s, primarily surviving in a covert way. However, it has progressed greatly in former Soviet nations; it was most widespread in Georgia, but it was also common in Poland and Russia.120
In the 2000s, various initiatives to create phage-based biotechnologies, both for human and animal health or biocontrol in the growing food business occurred. Both the FDA and Canadian Environmental Protection Authority have authorized two anti-Listeria phage combinations since 2007. The therapeutic use of phages never completely ceased, such as in France and Belgium, where phage therapy is still employed to treat patients as a last resort treatment. Phage treatment has recently (in the last 15 years) been resurrected in laboratories and hospitals. The first study of phagoburn, which is based on a European collaboration, remain equivocal, and indicated that phages are being used to treat an increasing number of patients in France and Belgium.121
However, there are numerous causes for the demise of phage treatment, and two evaluations of phage treatment published in the renowned Journal of the American Medical Association (JAMA) in 1934 and 1941 revealed challenges in the use of phages, as well as effectiveness issues. However, these assessments clearly indicated that there are still some disagreements concerning the methods of action of these viruses. Phage treatment completely vanished around the same time that sulfonamides were invented, and the widespread use of antibiotics and their use in the USA in the 1940s and beyond. This theory is consistent with the continued use of phages in many Soviet Union.120
This revival of phage treatment owes much to some of its most passionate supporters, but it also answers a rising concern about the rise of “superbugs” since the dawn of the 21st century. This is due to the fact that, whereas phages and antibiotics both have biocidal abilities, their mechanisms of action are principally different. Currently, there are no therapeutic phages licensed for use in the U.S., despite substantial expertise and commercial availability in other countries. Empirical data supporting the efficacy of phage treatment from other nations frequently fall short of the FDA guidelines, which generally involve randomized controlled clinical studies. Thus far, clinical studies have failed to demonstrate sufficient effectiveness. According to previous studies, phages are generally specific strains of bacteria found in geographic locations from where they are obtained. As a result, phage libraries must not only be updated on a regular basis to keep up with evolution, but phages may also need to be procured locally to maintain efficiency against bacterial strains specific to a given location.122
Several clinical studies are currently underway or will commence in the following months, and the number of scholarly papers on this issue has increased substantially. Recently, microbiology conferences have focused on the potential therapeutic uses of phages, particularly ARB.
Mode of Action
Phage treatments, such as antibody therapy, have a long history that predates the invention of antibiotics. Viruses that infect bacteria only are known as phages. The bactericidal properties of these viruses provide a precise strategy for treatment. Although certain phages can infect a wide spectrum of bacteria, the vast majority of phages are unique to a single species or strain. The type of bacterial receptor determines the host range. Phages insert genetic material after recognizing a receptor in the host cell and attaching it to it.118 Lytic phages hijack the bacterial replication machinery and use it to create their own phage offspring in the bacterial cytoplasm. When a certain number of offspring are achieved, lytic enzymes encoded by the phage are activated, hydrolyzing the peptidoglycan cell wall. Freshly synthesized phages can then escape and restart the lytic cycle. Lytic phages can operate as self-amplifying therapeutic agents with highly targeted bactericidal action in this way.45
Phage Engineering to Create a More Secure Phage Product
Particular phages have the ability to resist antimicrobials, toxins, virulence, and genes in their genomes,123 the horizontal gene transfer of viral infection works to participate in causing morphological changes in the bacterial host, which can enhance their hosts’ virulence in some situations. This is a big handicap in the marketing of phages as drugs, making the use of phages harder. Furthermore, there are several genes in the phage genome that encode proteins that have not yet been identified or functionally described; in many phage genomes published now, fewer than half of the phage genes can be assigned to known proteins.122 Owing to the difficulties in obtaining uniformity and the risk of unexpected reverse effects, together these characteristics restrict the safety of phage treatment. Genetic engineering techniques, on the other hand, have enabled the creation of far safer phage products. Unique Bacteriophage production and that do not contain undesirable genes is possible.117 This rapid development in scientific, technological, and biological discoveries is creating desirable modified phages that will have critical uses in present medicine and in the future.123
Phage Engineering to Increase Host Range and Reduce Phage-resistant Bacteria Emergence
Previously, it was believed that bacterial resistance to phages does not occur.124 However, recent experiments showed the rapid development of resistant bacteria during treatment and that could not be avoided. Generally, scientists support the idea of targeting multiple microbial receptors with a phage cocktail or using both phages and antibiotics simultaneously to stop the growth and development of resistance to phages. The host range can also be increased by engineering single phages or using multiple phages.125 However, work on phages, including isolation and characterization, requires a long time and stringent regulatory approval before therapeutic application. These problems can be solved by protein modification of the binding between the phage and tail, as well as critical determinants of phage-to-host communication, that may assist homologous reconnection to similar phages or restarting the generated genome. Chimeric bacteriophages can be produced from families such as T2, T4, and T7, which target multiple bacterial receptors that are used for synergistic therapy, which in turn leads to synthetic biotechnique delay producing sterility-resistant clones. Using similar techniques, viable L. monocytogenes phages were established by increasing the host range.126
Application of Phage Therapy
The therapeutic potential of phage treatment can be increased by genetically engineering of phages and/or products derived from phages. Research by the Stevens laboratory revealed that phage editing can improve the therapeutic potential of bacteriophages.117 Phage Ef11 was initially identified in an oral biofilm isolates of Enterococcus faecalis by prophage induction and succeeded in eradicating E. faecalis. Recently, for the first time, a trial of modified phage therapy was published in a patient with cystic fibrosis and Mycobacterium abscess, and the case improved considerably.118
Phages can be used alone or in cocktail mixtures to increase their efficacy against ARB. Fixed phage cocktails contain a predefined composition of lytic phages that target certain bacterial species, whereas personalized phage cocktails have been developed by evaluating a specific bacterial isolate against a large variety of phages.127 For example, Intestiphage, a fixed phage cocktail popular in Eastern Europe, was designed to attack approximately 23 distinct intestinal infections. Antibiotics and phages have been shown to function together. Sublethal dosages of antibiotics that restrict cell division without causing cell death may boost the metabolic capacity of bacteria, resulting in an increase in the production of lytic phages. β-lactams, quinolones, and tetracyclines have all been shown to have synergistic effects.126
Studies indicate that Phagoburn, Phase 1 / 2 clinical experiments on the use of phages in the treatment of infected burn wounds have recently been completed and have been shown to be effective in some patients.127 Furthermore, recent studies have shown the effectiveness of phage treatment against Acinetobacter baumannii secondary infection in COVID-19 patients. This finding demonstrates that phage treatment can be used to treat secondary bacterial infections in COVID-19 patients. As a result, concerted efforts should be undertaken to establish phage therapy as a standard therapeutic option for COVID-19 patients with secondary infection.128
In 2021, a single case report was published for a patient exposed to a MDR strain of Burkholderia cepacia complex (Bcc), and phages were used to treat it, and the case is still under medical follow-up. A patient with cystic fibrosis with a Bacillus dulus strain received a lung transplant. After six months, phages were incorporated into the pharmacological regimen, and the patient showed rapid improvement. Nine months later, owing to antibiotic toxicity, the treatment was discontinued, and the patient died 11 months after transplantation. The simultaneous use of both phages and antibiotics from the start of treatment may lead to treatment success and patient survival of the patient.129
Recently (2019) an individual was infected with a resistant type of Acinetobacter baumannii in Egypt, and the infection developed severely. Upon returning to the U.S., the use of phages was tested, and the case was cured, and follow-up is currently underway to prove the effectiveness of phages.117
Antibiotics Hybrid
Antibiotics Hybrid Concept and History
A hybrid antibiotic is defined as a synthetic form composed of two or more pharmacophores from an existing medication that has been shown to have a desirable antimicrobial action. These include antibiotic conjugates, chimeric antibiotics, multivalent antibiotics, and double-action antibiotic hybrids. In future antibiotic hybrids, the concept of bimodality (dubbed “dual action” in 1994) suggests that covalently attached drugs retain their recognized biological activities. The first hybrid prodrug is a cefamandole derivative connected to omadine (hybrid prodrug 1), which was first described in 1976. Omadine (2-mercaptopyridine-N-oxide or pyrithione) is a metal chelator that inhibits bacterial ATP production. Hybrid prodrug 1 was demonstrated to be active against some GN bacteria.131 The effect of a hybrid of cecropin-A (1-13) and melittin (1-13) was highly bactericidal and not non-toxic to the cell host, and it was also found to have both antibacterial and antitumor activities.137 A mutual effort between academia and industry has created a series of peptidomimetic hybrids derived from polymyxin B and murepavadin.133 The acceptance of novel chemical entities has shifted to clinical trials owing to the application of an antibiotic hybrid paradigm to macrocycle trials. For example, multi-targeted TD-1792 exhibited significant antibacterial activity. Against a variety of bacteria resistant to vancomycin, one of its constituents. Furthermore, rifamycin antibiotic conjugation has created hybrid clinical candidates with promising effects, such as effectiveness and safety. The U.S. and Drug Administration (FDA) granted orphan drug status in 2020. TNP-2092, a rifamycin-fluoroquinolone combination, was developed for the treatment of prosthetic infections. Currently, a novel antibacterial substance, DSTA4637S, is being tested in clinical trials. Infections of the joints Antibody–antibiotic conjugates are a unique type of bacterial treatment.132
Antibiotic Hybrid Mode of Action
Many groups of hybrid antibiotics have been discovered, each of which has a dissimilar mode of action to fight pathogens.136
Arginine-isoleucine-rich and proline-rich AMPs
The merging of two different classes of AMPs can result in better antimicrobial activity. They combine the activities of arginine-isoleucine-rich and proline-rich AMPs. The hybrid peptide hyP7B5GK is a hybrid molecule that is organized to disintegrate into the cytosol in bacteria owing to the reduction potential of the media.136
Cephalosporin
The hybrid prodrug 1 is mainly cephalosporin along with a few contributions from its omadine pharmacophore, including its minimum inhibitory concentration and a reduction of 4-32 folds in GP and GN strains that represent lactamases. A study verified the hybrid prodrug concept, but also showed that it has no significant therapeutic relevance, where an undesirable systemic toxicity was observed with omadine. In 1986, a desacetylcephalothin joined for the inhabitation of alanine racemase (hybrid 2) to chloroalanyl dipeptide which was active towards E. coli with MIC of (7.05 to 14.1 g/ml) pharmacophore of the cephalosporin is still applied as an important most hybrid prodrugs’ structural element.130
Sideromycins
Sidromycins, which act as prodrugs, are analogous to dipeptide prodrugs similar to tabtoxins, where the siderophore component makes cell entry easier to access in a manner similar to the dipeptide prodrug. The release of antibiotics from siderophores is mainly determined by the nature and location of the biological target required for target engagement.131
Albomycin
Fusing two antibiotic scaffolds can serve as an advantage to hybrid antibiotics by using the dilated target bond and leverage entrance to the membrane transport pathway. In the presence of albomycin, a peptide bond adherent to the cytoplasmic peptidase PepN is formed between a thionucleoside Ser-tRNA synthetase inhibitor and a ferrichrome-like trihydroxamate siderophore, which is observed in many GP bacteria. Resistance to albomycin is formed when PepN mutants are deleted, and in antibacterial activity, the siderophore is vital when released from the antibiotic. Another reason for resistance could be related to mutation or deletion of transport proteins, demonstrating that targeted uptake pathways are required for microorganism virulence. The NRPS assembly line forms a Ferrichrome siderophore, and a separate sequence of enzymes encoded in the same BGC produces thionucleoside. Whereas albomycin acts as an example template for the hybrid biosynthesis.131
Antibiotic Hybrid Applications
The molecular mechanisms underlying ARB removal and antibiotic resistance genes, as well as effluent toxicity testing, remain unaddressed and require more attention, where biofilm and intracellular infections utilizing nucleic acid self-assembled hybrid nanocarriers as antimicrobial treatments offer a significant chance. The combination of these two therapeutic drugs could result in an unexpected third effect. Furthermore, several antibiotic hybrids have the capacity to “resurrect” past antibiotic effectiveness. Simultaneous co-delivery systems show intriguing promise for these hybrids. Nucleic acid nanostructures have been found to have anti-inflammatory characteristics, which are important in a variety of infections, including applications in topical wounds.134 Ex. Murepavadin and polymyxin B were combined in a collaborative work between industry and academics to create a chain of peptidomimetic hybrids (murepavadin is a -turn peptidomimetic of protegrin I). Murepavadin peptidomimetics were synthesized before combining a derivative of one of the peptidomimetics with a fragment of polymyxin B to create a hybrid. This antibiotic hybrid helps to develop AMPs as antiviral, antiparasitic and antifungal peptides.133
To gain a better understanding of how the hybrids alter the microbiota and their effectiveness at various locations like ocular or oral systemic wounds. The effectiveness of antibacterial platforms may be created by employing nucleic acid hybrid platforms to address these challenges and acquire better knowledge of in vivo action.134 This combination was the addition of a second interaction including lipid A via the polymyxin piece to infections of the joints DSTA4637S mechanism of action, which interacts with the outer membrane proteins of GN bacteria. A recent experiment performed by Polyphor, (a Swiss company), has moved one of the most powerful chimeras to pre-clinical investigations under the POL7306 designation. A murine model that was infected by peritonitis (K. pneumoniae and E. coli), exhibited all-encompassing antibacterial action and low mammalian cell toxicity (E. coli, P. aeruginosa and A. baumannii). Additionally, the treatment showed action against the extent of MDR strains in a separate research.133 Another application was in an early study of bacterial keratitis (BK), the leading global cause of corneal blindness, and inspired the development of an incoming type of antimicrobial treatment based on human-derived hybrid host defense peptides (HyHDPs), which is a hybridization of functional amino acids for treating BK.135
Future Perspectives
These novel alternative therapeutic strategies continue to develop and garner more attention, and their applications and roles against AR bacteria will make a great difference and will become more expand in the future. NMs have several technical constraints in clinical experiments. We assume that clinically featured NMs will be created in the near future, followed by well-designed clinical studies to demonstrate their practical use in clinical settings. The potential health consequences of NMs not yet known; however, NMs have the ability to reduce systemic toxicity and research on AR mechanisms with proof of toxicity in the human body is urgently needed. It is necessary to understand the complete mechanism of action of NMs as antimicrobials. To date, several clinical studies have been performed on murine models. The toxicity of NMs in humans is currently being investigated. We expect to develop more effective applications by understanding the natural properties and engineering of NMs to provide a large design field for new antibacterial agents. We look forward to using and improving NM, including tracking what is occurring inside the human system. Drug sensitivity might occur due to long-term exposure to NMs. For example, in the treatment of cancer by delivering NMs, it is still necessary to evaluate the many characteristics of cell cancer as well as NMs. It is essential to consider the function of the TME in cancer development and therapy. This will enable the creation of NMs carrying tailored medications capable of penetrating tumors and influencing their biological processes. NMs and drug interactions occur in the blood circulatory system, as well as in cells, tissues, and organs, all factors that must be considered. These factors point us on the right path for alternatives to the traditional treatment of cancer. We predict that targeted NMs will have a huge influence on human health for years if nanotechnologies continue research to be advanced for the benefit of humanity. The use of NMs in combination with other medications may be a promising strategy for combating MDR. Furthermore, in the coming years, we anticipate that there will be a large number of studies to understand the mechanism of PS in order to effectively design new PS with enhanced properties. For instance, the design of a novel water-soluble PS that generates a large amount of singlet oxygen, such as chlorine-based PS, which has been used and has shown great results but has not yet been approved, and PS dyes with strong absorption of light. In addition, we believe that future research will focus on finding light sources with long wavelengths based on electromagnetic radiation, such as near infrared radiation (NIR), 750 nm to 1300 nm, which pass through deep tissues. However, NIR radiation is limited by a lack of suitable PSs. Some of these approved NIR-absorbing PSs are still under investigation. In addition, we believe there is a need to optimize the current light penetration depth using continuous-wave (CW) and pulsed light waveforms that activate PS in deeper regions of the tissue, resulting in ideal Ps active against both GP and GN bacteria, which are known to react differently to treatment. The use of phages has increased in many modern applications because of their ability to chemically modify their genetic engineering. In the coming years, we expect widespread application of phages in hospitals. For example, our increasing understanding of the nature of microbe-host-phage interaction prior to a clinical trial of phage therapy will resolve the debate between using phages as a substitute or combining them with antibiotics at the same time, particularly MDR. In the near future, we anticipate that treatment with a combination of phages, bioengineered phages, and/or antibiotics will be developed, as it will be necessary to treat the increasing issue of antibiotic-resistant infections. In addition, in the next decade, we hope that phage therapy research will focus on the need to use phages in coordination with antibiotics from the beginning of the disease, rather than as a final solution, as we believe that such combination therapy may decrease the long-range reliance on antibiotics and thus mitigate toxicity, which often leads to death. Furthermore, we anticipate that the phage and antibiotic capture mechanism will be another promising strategy against ARB, which causes serious illness in immunocompromised populations, in the coming years, because efflux pumps represent a major function in AMR. We have understood that hybrid techniques have been used to improve and develop AMR over the years, but when it comes to the hybridization of AR, they have shown progress in preventing enzymatic degradation and increasing binding specificity for their targets. Although few cases have been reported, we believe that the hybrid antibiotic concept proposes a sensible way to advance combinations of pharmacophores as multi-targeting agents. This review reflects on how hybrid techniques will help future antimicrobials to be resistant and provide benefits in making new strains that are less resistant and more specific against a large variety of microbes.
This review demonstrates the increasing ABR hazards globally, as well as novel resistance mechanisms that are forming and expanding internationally, posing a threat to our capacity to treat prevalent infectious diseases. This paper discusses the history of antibiotic discovery, the reasons behind resistance, such as overuse and misuse of antibiotics, and newly discovered antibiotic classes. It also discussed the mechanism of action of antibiotics against bacteria and their targets and indicated some covenantal strategies that it used to combat ABR and their limitations. Finally, this review provides novel alternative therapeutic strategies that are recommended recently to stop and reduce bacterial resistance that has increased over time with their mode of action and applications that have treated many infectious diseases, such as antimicrobial NMs therapy, antimicrobial photodynamic therapy, phage therapy, and antibiotic hybrids, and concludes with the authors’ expectations about future potential for the aforementioned therapies.
ACKNOWLEDGMENTS
The authors would like to thank Deanship of Scientific, Vice Presidency for Graduate Studies and Scientific research, King Faisal University, Al-Ahsa, Saudi Arabia, for supporting this work (Project Grant Number 760).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No.760].
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. ASM.org. 2022. https://www.asm.org/Protocols/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Pro. Accessed 21 May 2022.
- Antimicrobial resistance. Who.2021. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 20 May 2022.
- Biswas S, Brunel J, Dubus J, Reynaud-Gaubert M, Rolain J. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther. 2012;10(8):917-934.
Crossref - UK 20-year vision for antimicrobial resistance. GOV.UK. https://www.gov.uk/government/publications/uk-20-year-vision-for-antimicrobial-resistance. 2019. Accessed 20 May 2022.
- MOH Launches a campaign to supervise dispensing of non-prescribed antibiotics. MOH News [Internet]. Published 2018. Accessed September 9, 2022. https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pa es/news-2018-05-06-001.aspx.
- Leangapichart T, Gautret P, Griffiths K, et al. Acquisition of a High Diversity of Bacteria during the Hajj Pilgrimage, Including Acinetobacter baumannii with blaOXA-72 and Escherichia coli with blaNDM-5 Carbapenemase Genes. Antimicrob Agents Chemother. 2016;60(10):5942-5948.
Crossref - Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128-132.
Crossref - Clancy C, Nguyen M. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin Infect Dis. 2020;71(10):2736-2743.
Crossref - Ukuhor HO. The interrelationships between antimicrobial resistance, COVID-19, past, and future pandemics. J Infect Public Health. 2021;14(1):53-60.
Crossref - Antibiotic prescribing and behavior change in healthcare settings. GOV.UK. https://www.gov.uk/government/publications/antibiotic-prescribing-and-behaviour-change-in-healthcare-settings. Published 2022. Accessed 20 May 2022.
- Gaynes R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg Infect Dis. 2017;23(5):849-853.
Crossref - Lawrie R. First clinical use of penicillin. BMJ. 1985;290(6465):397.
Crossref - Hutchings M, Truman A, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72-80.
Crossref - Ventola CL. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm Ther. 2015;40(4):277–283. PMCID: PMC4378521
- Kwon JH, Powderly WG. The post-antibiotic era is here. Science. 2021;373(6554):471-471.
Crossref - 2020 antibacterial agents in clinical and preclinical development: an overview and analysis. WHO. 2022. int. https://www.who.int/publications/i/item/9789240021303. Accessed 20 May 2022.
- Hasan CM, Dutta D, Nguyen ANT. Revisiting Antibiotic Resistance: Mechanistic Foundations to Evolutionary Outlook. Antibiotics. 2021;11(1):40.
Crossref - Al-Tawfiq JA Momattin H, Al-Ali AF, et al. Antibiotics in the pipeline: a literature review (2017–2020). Infection. 2022;50(30):553-564.
Crossref - Tracking the Global Pipeline of Antibiotics in Development, March 2021. Pewtrusts.org. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/03/tracking-the-global-pipeline-of-antibiotics-in-development/. 2022. Accessed 20 May 2022.
- 2021 AWaRe classification. Who. int. https://www.who.int/publications-detail-redirect/2021-aware-classification. 2022. Accessed 20 May 2022.
- Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol Clin Pharmacol. 2017;33(3):300-305.
https://doi.org/10.4103/joacp.JOACP_349_15 - Levin PA, Angert ER. Small but Mighty: Cell Size and Bacteria. Cold Spring Harb Perspect Biol. 2015;7(7):a019216.
https://doi.org/10.4103/joacp.JOACP_349_15 - Bush K, Bradford PA. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb Perspect Med. 2016;6(8):a025247.
Crossref - Mai-Prochnow A, Clauson M, Hong J, Murphy AB. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep. 2016;6(1):38610.
Crossref - Uddin T, Chakraborty A, Khusro A, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750-1766.
Crossref - Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: An Overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029.
Crossref - Grossman TH. Tetracycline Antibiotics and Resistance. Cold Spring Harb Perspect Med. 2016;6(4):a025387.
Crossref - Dinos GP, Athanassopoulos CM, Missiri DA, et al. Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics (Basel). 2016;5(2):20.
Crossref - Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225-245.
Crossref - Nainu F, Masyita A, Bahar MA, et al. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics (Basel). 2021;10(7):822.
Crossref - Akter T, Shahriar A, Alo M, Rahman T, Emran TB. Survival assessment of pathogenic bacteria with antibiotic resistance traits from fresh summer royal grape: in vitro microbial challenge test. J Microbiol Biotechnol Food Sci. 2020;10(3):344-349.
Crossref - Wrobel A, Maliszewski D, Baradyn M, Drozdowska D. Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs. Molecules. 2019;25(1):116.
Crossref - Poirel L, Jayol A, Nordmann P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin Microbiol Rev. 2017;30(2):557-596.
Crossref - Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482-501.
Crossref - Kittredge H, Dougherty K, Evans S. Horizontal gene transfer facilitates the spread of extracellular antibiotic resistance genes in soil. 2021.
Crossref - Culyba MJ, Mo CY, Kohli RM. Targets for Combating the Evolution of Acquired Antibiotic Resistance. Biochemistry. 2015;54(23):3573-3582.
Crossref - Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42-51.
Crossref - Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177-192.
Crossref - Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41(3):276-301.
Crossref - Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4(2).
Crossref - Lin J, Nishino K, Roberts MC, Tolmasky M, Aminov RI, Zhang L. Mechanisms of antibiotic resistance. Front Microbiol. 2015;6(74):34. https://www.frontiersin.org/articles/10.3389/fmicb.2015.00034/full
- Peterson E, Kaur P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front Microbiol. 2018;9:2928.
Crossref - Ternent L, Dyson R, Krachler A, Jabbari S. Bacterial fitness shapes the population dynamics of antibiotic-resistant and -susceptible bacteria in a model of combined antibiotic and anti-virulence treatment. J Theor Biol. 2015;372:1-11.
Crossref - Lobanovska M, Pilla G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J Biol Med. 2017;90(1):135-145. https://pubmed.ncbi.nlm.nih.gov/28356901/
- Kortright KE, Chan BK, Koff JL, Turner PE. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe. 2019;25(2):219-232.
Crossref - Chen J, Shang X, Hu F, et al. β-Lactamase inhibitors: an update. Mini Rev Med Chem. 2013;13(13):1846-1861.
Crossref - Sykes J. Canine And Feline Infectious Diseases. 1st ed. St. Louis, Mo.: Elsevier/Saunders. 2014:66-86.
- Khanna NR, Gerriets V. Beta Lactamase Inhibitors. In: StatPearls. Treasure Island (FL): StatPearls. 2022. https://www.ncbi.nlm.nih.gov/books/NBK557592/
- Curello J, MacDougall C. Beyond Susceptible and Resistant, Part II: Treatment of Infections Due to Gram-Negative Organisms Producing Extended-Spectrum β-Lactamases. J Pediatr Pharmacol Ther. 2014;19(3):156-164.
Crossref - Tangden T. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci. 2014;119(2):149-153.
Crossref - Urban C, Mariano N, Rahal JJ. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob Agents Chemother. 2010;54(6):2732-2734.
Crossref - Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother. 2002;50(6):1045-1049.
Crossref - Manchanda V, Sanchaita S, Singh N. Multidrug resistant acinetobacter. J Glob Infect Dis. 2010;2(3):291-304.
Crossref - Petrosillo N, Taglietti F, Granata G. Treatment Options for Colistin Resistant Klebsiella pneumoniae: Present and Future. J Clin Med. 2019;8(7):934.
Crossref - Lipsitch M, Siber GR. How Can Vaccines Contribute to Solving the Antimicrobial Resistance Problem? mBio. 2016;7(3):e00428-16.
Crossref - Rima M, Rima M, Fajloun Z, Sabatier JM, Bechinger B, Naas T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics (Basel). 2021;10(9):1095.
Crossref - Buchy P, Ascioglu S, Buisson Y, et al. Impact of vaccines on antimicrobial resistance. Int J Infect Dis. 2020;90:188-196.
Crossref - Satalkar P, Elger B, Shaw D. Defining Nano, Nanotechnology and Nanomedicine: Why Should It Matter? Sci Eng Ethics. 2016;22(5):1255-1276.
Crossref - Kreuter J. Nanoparticles—a historical perspective. Int J Pharm. 2007;331(1):1-10.
Crossref - Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine. 2017;12:1227-1249.
Crossref - Peiris M, Gunasekara C, Jayaweera P, Arachchi N, Fernando N. Biosynthesized silver nanoparticles: are they effective antimicrobials? Memorias do Instituto Oswaldo Cruz. 2017;112(8):537-543.
Crossref - Peiris M, Fernando S, Jayaweera P, Arachchi N, Guansekara T. Comparison of Antimicrobial Properties of Silver Nanoparticles Synthesized from Selected Bacteria. Indian J Microbiol. 2018;58(3):301-311.
Crossref - Garmasheva I, Kovalenko N, Voychuk S, Ostapchuk A, Livins’ka O, Oleschenko L. Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. BioImpacts. 2016;6(4):219-223.
Crossref - Kalpana D, Lee Y. Synthesis and characterization of bactericidal silver nanoparticles using cultural filtrate of simulated microgravity grown Klebsiella pneumoniae. Enzyme Microb Technol. 2013;52(3):151-156.
Crossref - Gupta A, Mumtaz S, Li C, Hussain I, Rotello V. Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev. 2019;48(2):415-427.
Crossref - Hurdle J, O’Neill A, Chopra I, Lee R. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 2010;9(1):62-75.
Crossref - Memar M, Ghotaslou R, Samiei M, Adibkia K. Antimicrobial use of reactive oxygen therapy: current insights. Infect Drug Resist. 2018;11:567-576.
Crossref - Dong X, Liang W, Meziani M, Sun Y, Yang L. Carbon Dots as Potent Antimicrobial Agents. Theranostics. 2020;10(2):671-686.
Crossref - Ashmore D, Chaudhari A, Barlow B, et al. Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Revista do Instituto de Medicina Tropical de Sao Paulo. 2018;60(0).
Crossref - Ventola CL. Cancer Immunotherapy, Part 3: Challenges and Future Trends. P T. 2017;42(8):514-521.
- Mu H, Tang J, Liu Q, et al. Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria. Sci Rep. 2016;6:18877.
Crossref - Abdelghany SM, Quinn DJ, Ingram RJ, et al. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int J Nanomedicine. 2012;7:4053-4063.
Crossref - Li CH, Chen X, Landis RF, et al. Phytochemical-Based Nanocomposites for the Treatment of Bacterial Biofilms. ACS Infect Dis. 2019;5(9):1590-1596.
Crossref - Grumezescu A. Drug Targeting And Stimuli Sensitive Drug Delivery Systems. Cambridge, MA: Elsevier Ltd; 2018.
- Wang Y, Ding X, Chen Y, et al. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials. 2016;101:207-216.
Crossref - Jimenez-Jimenez C, Moreno V, Vallet-Regi M. Bacteria-Assisted Transport of Nanomaterials to Improve Drug Delivery in Cancer Therapy. Nanomaterials. 2022;12(2):288.
Crossref - Cho K, Wang X, Nie S, Chen Z, Shin D. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin Cancer Res. 2008;14(5):1310-1316.
Crossref - Erdely A, Dahm M, Chen B, et al. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10(1):53.
Crossref - Kashkooli FM, Soltani M, Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J Control Release. 2020;327:316-349.
Crossref - Rashidzadeh H, Danafar H, Rahimi H, et al. Nanotechnology against the novel coronavirus (severe acute respiratory syndrome coronavirus 2): diagnosis, treatment, therapy and future perspectives. Nanomedicine. 2021;16(6):497-516.
Crossref - Tabish T, Hamblin M. Multivalent nanomedicines to treat COVID-19: A slow train coming. Nano Today. 2020;35:100962.
Crossref - Dai X, Liu J, Zheng H, et al. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017;9(3):e368.
Crossref - Sun Y, Bhattacharjee A, Reynolds M, Li Y. Synthesis and characterizations of gentamicin-loaded poly-lactic-co-glycolic (PLGA) nanoparticles. Journal of Nanoparticle Research. 2021;23(8):155.
Crossref - Shalaby M, Anwar M, Saeed H. Nanomaterials for application in wound Healing: current state-of-the-art and future perspectives. J Polym Res. 2022;29(3):91.
Crossref - Allaker RP, Yuan Z. in Nanobiomaterials in Clinical Dentistry 2nd edn (eds Subramani K & Ahmed W.). Elsevier. 2019:243–275.
- Yin I, Zhang J, Zhao I, Mei M, Li Q, Chu C. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int J Nanomedicine. 2020;15:2555-2562.
Crossref - Naha P, Liu Y, Hwang G, et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano. 2019;13(5):4960-4971.
Crossref - Gao L, Liu Y, Kim D, et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272-284.
Crossref - Sperandio FF, Huang YY, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov. 2013;8(2):108-120.
Crossref - Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39(1):1-18.
Crossref - Grumezescu A. Nanobiomaterials In Antimicrobial Therapy. Kidlington, Oxford, UK: William Andrew is an imprint of Elsevier. 2016:1-27.
- Daniell MD, Hill JS. A history of photodynamic therapy. Aust N Z J Surg. 1991;61(5):340-348.
Crossref - Rajesh S, Koshi E, Philip K, Mohan A. Antimicrobial photodynamic therapy: An overview. J Indian Soc Periodontol. 2011;15(4):323-327.
Crossref - Dougherty TJ, Marcus SL. Photodynamic therapy. Eur J Cancer. 1992;28A(10):1734-1742.
Crossref - Shi X, Zhang CY, Gao J, Wang Z. Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(5):e1560.
Crossref - Wainwright M, Maisch T, Nonell S, et al. Photoantimicrobials-are we afraid of the light? Lancet Infect Dis. 2017;17(2):e49-e55.
Crossref - Sabino CP, Wainwright M, Ribeiro MS, et al. Global priority multidrug-resistant pathogens do not resist photodynamic therapy. J Photochem Photobiol B. 2020;208:111893.
Crossref - Youf R, Muller M, Balasini A, et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics. 2021;13(12):1995.
Crossref - Yildirim C, Karaarslan ES, Ozsevik S, Zer Y, Sari T, Usumez A. Antimicrobial efficiency of photodynamic therapy with different irradiation durations. Eur J Dent. 2013;7(4):469-473.
Crossref - Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57(4):680-684.
Crossref - Zanin IC, Goncalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother. 2005;56(2):324-330.
Crossref - Hamblin MR, O’Donnell DA, Murthy N, et al. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49(6):941-951.
Crossref - Munin E, Giroldo LM, Alves LP, Costa MS. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J Photochem Photobiol B. 2007;88(1):16-20.
Crossref - Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother. 1998;42(1):13-28.
Crossref - Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics. 2021;13(9):1332.
Crossref - Weber H, Mehran Y, Orthaber A, Saadat H, Weber R, Wojcik M. Successful Reduction of SARS-CoV-2 Viral Load by Photodynamic erapy (PDT) Veried by QPCR – A Novel Approach in Treating Patients in Early Infection Stage. Medical & Clinical Research. 2020;5(11):311-325.
- Miyashiro CA, Bernegossi J, Bonifacio BV, et al. Development and characterization of a novel liquid crystalline system containing sodium alginate for incorporation of trans-resveratrol intended for treatment of buccal candidiasis. Pharmazie. 2020;75(5):179-185.
Crossref - Silvestre ALP, Di Filippo LD, Besegato JF, et al. Current applications of drug delivery nanosystems associated with antimicrobial photodynamic therapy for oral infections. Int J Pharm. 2021;592:120078.
Crossref - de Oliveira AB, Ferrisse TM, Marques RS, de Annunzio SR, Brighenti FL, Fontana CR. Effect of Photodynamic Therapy on Microorganisms Responsible for Dental Caries: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2019;20(14):3585.
Crossref - Aida KL, Kreling PF, Caiaffa KS, et al. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int J Nanomedicine. 2018;13:3081-3091.
Crossref - Reis ACM, Regis WFM, Rodrigues LKA. Scientific evidence in antimicrobial photodynamic therapy: An alternative approach for reducing cariogenic bacteria. Photodiagnosis Photodyn Ther. 2019;26:179-189.
Crossref - Dos Santos DM, Chagas PAM, Leite IS, et al. Core-sheath nanostructured chitosan-based nonwovens as a potential drug delivery system for periodontitis treatment. Int J Biol Macromol. 2020;142:521-534.
Crossref - Akram Z, Hyder T, Al-Hamoudi N, Binshabaib MS, Alharthi SS, Hanif A. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2017;19:86-92.
Crossref - Bapat RA, Joshi CP, Bapat P, et al. The use of nanoparticles as biomaterials in dentistry. Drug Discov Today. 2019;24(1):85-98.
Crossref - Nakonieczna J, Wozniak A, Pieranski M, Rapacka-Zdonczyk A, Ogonowska P, Grinholc M. Photoinactivation of ESKAPE pathogens: overview of novel therapeutic strategy. Future Med Chem. 2019;11(5):443-461.
Crossref - Klausen M, Ucuncu M, Bradley M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules. 2020;25(22):5239.
Crossref - Dedrick R, Guerrero-Bustamante C, Garlena R, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25(5):730-733.
Crossref - Chanishvili N. Phage Therapy—History from Twort and d’Herelle Through Soviet Experience to Current Approaches. Adv Virus Res. 2012:3-40.
Crossref - Herelle F, Dublanchet A, Schwartz M, n.d. Autobiographie de Felix d’Herelle. 1873-1949.
- Myelnikov D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922–1955. J Hist Med Allied Sci. 2018;73(4):385-411.
Crossref - Djebara S, Maussen C, De Vos D, et al. Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses. 2019;11(3):265.
Crossref - MacDonald PDM. The Perfect Predator: A Scientist’s Race to Save Her Husband, by Drs. Steffanie Strathdee and Thomas Patterson. Ann Glob Health. 2020;86(1):97.
Crossref - Pirnay J, De Vos D, Verbeken G. Clinical application of bacteriophages in Europe. Microbiology Australia. 2019;40(1):8-15.
Crossref - Azam A, Tanji Y. Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl Microbiol Biotechnol. 2019;103(5):2121-2131.
Crossref - Gordillo Altamirano F, Barr J. Phage Therapy in the Postantibiotic Era. Clin Microbiol Rev. 2019;32(2):e00066-18.
Crossref - Dunne M, Rupf B, Tala M, et al. Reprogramming Bacteriophage Host Range through Structure-Guided Design of Chimeric Receptor Binding Proteins. Cell Rep. 2019;29(5):1336-1350.e4.
Crossref - Drayton M, Kizhakkedathu JN, Straus SK. Towards Robust Delivery of Antimicrobial Peptides to Combat Bacterial Resistance. Molecules. 2020;25(13):3048.
Crossref - Wojewodzic M. Bacteriophages Could Be a Potential Game Changer in the Trajectory of Coronavirus Disease (COVID-19). PHAGE. 2020;1(2):60-65.
Crossref - Aslam S, Courtwright A, Koval C, et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am J Transplant. 2019;19(9):2631-2639.
Crossref - Domalaon R, Idowu T, Zhanel G, Schweizer F. Antibiotic Hybrids: the Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin Microbiol Rev. 2018;31(2):e00077-17.
Crossref - Wencewicz T. Crossroads of Antibiotic Resistance and Biosynthesis. J Mol Biol. 2019;431(18):3370-3399.
Crossref - Surur A, Sun D. Macrocycle-Antibiotic Hybrids: A Path to Clinical Candidates. Front Chem. 2021;9:659845.
Crossref - Gan B, Gaynord J, Rowe S, Deingruber T, Spring D. The multifaceted nature of antimicrobial peptides: current synthetic chemistry approaches and future directions. Chemical Society Reviews. 2021;50(13):7820-7880.
Crossref - Obuobi S, Skalko-Basnet N. Nucleic Acid Hybrids as Advanced Antibacterial Nanocarriers. Pharmaceutics. 2020;12(7):643.
Crossref - Ting D, Goh E, Mayandi V, et al. Hybrid derivative of cathelicidin and human beta defensin-2 against Gram-positive bacteria: A novel approach for the treatment of bacterial keratitis. Sci Rep. 2021;11(1):18304.
Crossref - Hilpert K, Gani J, Rumancev C, et al. Rational Designed Hybrid Peptides Show up to a 6-Fold Increase in Antimicrobial Activity and Demonstrate Different Ultrastructural Changes as the Parental Peptides Measured by BioSAXS. Front Pharmacol. 2021;12.
Crossref - Ting D, Beuerman R, Dua H, Lakshminarayanan R, Mohammed I. Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front Immunol. 2020;11:983.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.