ISSN: 0973-7510
E-ISSN: 2581-690X
Acinetobacter baumannii (A. baumannii) is a remarkable opportunistic pathogen responsible for a great proportion of hospital-associated infections and the high prevalence of resistance towards many classes of antibiotics makes the treatment challenging. The present cross-sectional study was conducted in the Department of Microbiology, IMCHRC, Indore. The study was approved by IEC and conducted from October 2019 to September 2021. A total number of 168 Acinetobacter species including 143 A. baumannii were isolated from the various clinical specimens, the majority of the isolates were obtained from the respiratory system (66%), followed by urine, pus/wound swab, blood, fluids and other samples. The majority of the patients who had underlying/diagnosed with a disease such as aspiration pneumonia/pneumonia (35%), cerebrovascular accident/haemorrhagic shock (30.7%), respiratory failure (24%), accelerated HTN/HTN(18%), and less common were septicemia (8.4), acute kidney injury/chronic kidney diseases (7.7%) and trauma/burns (5.5%). The antibiotic susceptibility testing showed higher antibiotic resistance to cefotaxime (94%), ceftazidime (93%), cefepime (92%), imipenem (92%), meropenem (90%) and the resistance was low to doxycycline (39%) Polymyxin B (8%). The association between antibiotic resistance and the clinical profile of patients was found significant (p-value < 0.05). In our study, a remarkably high antibiotic resistance pattern was observed in the classes of antibiotics in A. baumannii isolates, mostly MDR and XDR. To address infection caused by antibiotic-resistant A. baumannii, appropriate antibiotic administration in a clinical setting is essential. Moreover, local and national surveillance data, stringent infection control, and antimicrobial stewardship are required.
Acinetobacter baumannii, Antibiogram, Clinical Profile and MDR
Acinetobacter baumannii (A. baumannii) is a remarkable opportunistic pathogen responsible for a great proportion of hospital-associated infections such as ventilator-associated pneumonia, urinary tract infections, septicemia, endocarditis, meningitis and wound infections.1 Patients in ICUs who are kept on life support for an extended period usually get A. baumannii infections, and treatment failures are common.2 Endotracheal intubation, intravenous (I.V.) catheters, prostheses, prior antibiotic therapy, and underlying illnesses like aspiration pneumonia/pneumonia, respiratory failure, cerebrovascular accident, hypertension, diabetes mellitus, acute kidney injury, chronic kidney disease, and cancer are risk factors for A. baumannii infections. Due to widespread antibiotic resistance and the persistence of microorganisms in hospital settings, such infections are extremely difficult to treat.3
According to the World Health Organization (WHO) published report A. baumannii has been identified as a lead pathogen for the development of newer antibiotics and anti-infectives.4 A. baumannii isolates have developed resistance to the majority of antibiotic classes during the past three decades as a result of both acquired and innate resistance mechanisms.5 The antimicrobial agent A. baumannii has developed resistance to a variety of antibiotics from several classes, including aminoglycosides, cephalosporins, fluoroquinolones, carbapenems, tetracyclines and lipopeptides.6 The main cause of antibiotic resistance is the transmission of resistance genes through the mutation of target genes and plasmids. Given its incredible ability to acquire antibiotic resistance determinants, A. baumannii may leave us with few useful therapeutic alternatives.7
The initiation of effective empiric treatment is challenging due to the high prevalence of resistance towards many classes of antibiotics. As a result of its present antibiotic resistance, A. baumannii has become a “superbug” in hospitals, particularly in intensive care units (ICUs).8 The last-resort medications polymyxins, such as polymyxin B or colistin and tigecycline, or a combination of one of these classes with a second agent, are available as alternative treatments. There are additional reports of Acinetobacter spp. isolates that are resistant to colistin or polymyxin B worldwide. In some situations of infections with pandrug-resistant bacteria, there are no therapeutic choices available because of the rising levels of antibiotic resistance in isolates. Acinetobacter spp. are hard to eliminate because they have adapted to persist in hospital settings.9 In India, studies have reported the antimicrobial resistance pattern and associated with pre-disposing factors in A. baumannii infections.3,6 However limited data are available from India related to antimicrobial resistance pattern of A. baumannii and clinical profile of patients infected with A. baumannii infections.
The present study was based hypothesis that there is association between the antimicrobial resistance pattern and clinical profile (predisposing factors, underlined diseases and diagnosed diseases). The study was undertaken with the objectives to isolate A. baumannii from various clinical samples and evaluate the antibiotic resistance pattern of A. baumannii and clinical profile of patients.
The present cross-sectional study was conducted in the Department of Microbiology, Index Medical College, Hospital & Research Centre (IMCHRC), Indore, from October 2019 to September 2021. A total of 168 non-duplicate, consecutive isolates of Acinetobacter were obtained from different clinical samples such as blood, urine, pus, wound swab, aspirated fluids, sputum, endotracheal- tube/aspirate/ and BAL, etc., from patients admitted in the hospital and who given written informed consent for the study. All the isolates of Acinetobacter were identified by using standard microbiological procedures, including Gram staining, characteristics of the colony on culture media, catalase test, oxidase test and motility.10 Speciation of Acinetobacter (Table 1) was performed based on biochemical tests; urease, citrate, OF glucose, nitrate reduction test, hemolysis, gelatine hydrolysis, growth at 44°C, chloramphenicol sensitivity test and arginine hydrolysis test.3,11,12
Table (1):
Speciation of Acinetobacter species.
| Acinetobacter species | Phenotypic Tests | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalase | Oxidase | Motility | Urease | Citrate | OF glucose | Arginine decarboxylase | Nitrate reduction test | Gelatine hydrolysis | Haemolysis | Growth at 42°C | Chloramphenicol sensitivity | |
| A. baumannii | + | – | – | V | + | + | + | – | – | – | + | R |
| A. calcoaceticus | + | – | – | V | + | + | + | – | – | – | – | R |
| A. lwoffii | + | – | – | V | – | – | – | – | – | – | – | S |
| A. haemolyticus | + | – | – | – | + | V | + | – | + | + | – | R |
| A. junii | + | – | – | – | + | – | + | – | – | – | – | R |
| A. radioresistens | + | – | – | – | – | – | + | – | – | – | – | R |
A. baumannii: Acinetobacter baumannii, A. calcoaceticus: Acinetobacter calcoaceticus, A. lwoffii: Acinetobacter lwoffii, A. haemolyticus: Acinetobacter hemolyticus, A. junii: Acinetobacter junii, A. radioresistens: Acinetobacter radioresistens, -: Negative Reaction, +: Positive Reaction, V: Variable Reactions, S: Sensitive, R: Resistant, OF: Oxidation-fermentation.
The disk-diffusion method was used for the antimicrobial susceptibility testing. The test isolates were inoculated in peptone water broth and incubated at 37°C for 2-3 hours. Then turbidity of inoculated broth was compared with 0.5 McFarland standard. Lawn culture was made by streaking the swab evenly in 3-planes onto the surface of the petri dish containing Mueller-Hinton Agar. The isolates were tested by using the following antibiotic discs (Hi-Media) ampicillin-sulbactam (A/S, 10/10 mcg), piperacillin-tazobactam (PIT, 100/10 mcg), ceftazidime (CAZ, 30 mcg), cefepime (CP, 30 mcg), cefotaxime (CTX, 30 mcg), amikacin (AK, 30 mcg), gentamicin (GEN, 10 mcg), ciprofloxacin (CIP, 5 mcg), levofloxacin (Le, 5 mcg), imipenem (IMP, 10 mcg), meropenem (MRP, 10 mcg), polymyxin B (PB, 300 unit), doxycycline (DO, 30 mcg), tetracycline (TC, 30 mcg) and trimethoprim-sulfamethoxazole (TS, 1.25/23.75 mcg). After the application of antimicrobial discs, the MHA plates were kept for overnight incubation at 37°C in ambient air for 16-18 hours and after overnight incubation, the zone diameters (including the 6mm disc) were measured with a ruler on the under-surface of the petri dish and interpreted as sensitive, intermediate and resistant according to Clinical and Laboratory Standards Institute standards (CLSI 2018) guidelines. Quality Assurance Every culture medium utilized in this investigation underwent sterility and performance testing. Using the E. coli ATCC 25922 control strains, the AST’s quality was ensured.13
Statistical Analysis
Descriptive statistical methods like frequency and percentage distribution and graphical presentation were used for the analysis of categorical variables in the study. The Chi-square test was used to test the association between antibiotic resistance and the clinical profile of patients.
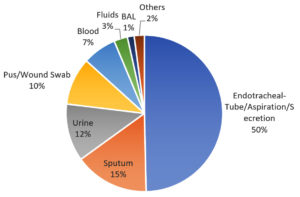
A total number of 168 Acinetobacter species, such as 143(85%) A. baumannii, 12 (7%) A. calcoaceticus, 09 (5%) A. lwoffii, 03 (2%) A. haemolyticus, 01 (1%) A. junii were isolated from various clinical specimens. Among the 143 A. baumannii, most of the isolates 95 (66%) were obtained from the respiratory samples, such as endotracheal tube/secretions, sputum and bronchoalveolar lavage, followed by urine 17 (12%) pus/wound swab14 (10%), blood 10 (7%), fluids 4(3%) and other samples3(2%) (Figure 1). A remarkably higher percentage, 76% (109) of A. baumannii isolates were found in ICU compared with general wards24% (34). The average age of the patients was (54.36 ± 16.80) years, A. baumannii infection was more common in patients in the age group 61-86 and 41-60 years 55 (38%) and less common in the 19-40 years age group 33 (23%). There was a higher incidence of infection among the males observed at 86 (60%)as compared with females at 57 (40%).
The majority of A. baumannii isolates from patients who had underlying/diagnosed diseases such as aspiration pneumonia/pneumonia 45 (35%), cerebrovascular accident/haemorrhagic shock 44 (30.7%), respiratory failure 35 (24%), accelerated HTN/HTN 26 (18%), and less common were septicemia 12 (8.4), acute kidney injury/chronic kidney diseases 11 (7.7%) and trauma/burns 8 (5.5%). The most common pre-disposing factors observed in patients with mechanical ventilation/endotracheal tube 83 (58%), urinary catheter 76 (53%), extended hospital stay (> 7 days) 70 (49%), and less common factors were diabetes mellitus 26 (18%), surgical site infections and COPD 14 (9.7%), central venous catheter and cancer 03 (2%) (Figure 2).
The antibiotic susceptibility testing showed higher antibiotic resistance to cefotaxime 135 (94%), ceftazidime 133 (93%), cefepime 132 (92%), imipenem 131 (92%), meropenem 130 (90%), amikacin 126 (88%), ciprofloxacin 126 (88%), trimethoprim-sulfamethoxazole 126 (88%), gentamicin 125 (87%), levofloxacin 125 (87%), piperacillin-tazobactam 117 (81%), ampicillin-sulbactam 105 (73%), tetracycline 11 (64%) (applied to urine samples only) and the resistance was low to doxycycline 56 (39%) Polymyxin B 11 (8%) (Figure 3). Of 143 A. baumannii isolates, 123 (86%) were multidrug-resistant (MDR) and 122 (85%) extensively resistant (XDR); the isolates resistant to at least one agent in three or more antimicrobial categories- penicillin’s, cephalosporins, aminoglycosides, β-lactam combination, fluoroquinolones, and carbapenems.
A. baumannii has become an important hospital pathogen in recent years. It is notorious for developing antibiotic resistance to most widely administered antimicrobials and has alarmingly high rates of AMR.4 The present study was conducted to evaluate the antibacterial resistance pattern and clinical profile of patients. Out of 143 isolates of A. baumannii, a significantly higher percentage (76%) of A. baumannii isolates were found in ICU as compared with general wards (24%) which is similar to other studies.14,15A study from Pune which is showed quite contrasting results such as 62% and 38% ICU and wards respectively.12
Our study results showed that in ICU the majority of patients acquired aspiration pneumonia/pneumonia usually which is after hospital admission and was attributed to the use of mechanical ventilation. Few studies from Maharashtra, India and Turkey, have also reported similar findings.3,16 Patients with cerebrovascular accident/hemorrhagic shock were 30.7% observed in our study which is consistent with other studies conducted by Konca C et al. and Ryu et al.16,17 In the present study, respiratory failure was documented in 24% patients, whereas in other studies reported 16.3% and 3.2% respectively Ryu et al. and Mathai A.S. et al., which is not following with present study.17,18 In our observation 18% of patients were suffering from hypertension and septicemia (8.4%) while, acute kidney injury, chronic kidney diseases (7.7%), trauma or burns (5.5%)were evident.
The incidence of A. baumannii isolates was more common in the patients who had catheterization (53%) in our study, almost analogous to the study by Tripathi et al.3 We observed extended hospital stay in 49% of patients in our study which is not matching with the study reported by Tripathi et al.3 The previously published studies have accounted for the usage of central-line in 21.5% and 51% of the patients but our results showed less percentage (2%) of usage.3,17 One of the recent studies showed surgical site infections 37.5% which is higher as compared with our result (9.7%).3The remaining findings like prosthesis, COPD, cancer and diabetes mellitus of our study were consistent with previously published results.3,17the majority of the A. baumannii isolates were obtained from the respiratory samples (66%), which is parallel to the results of studies previously conducted,1,9,16 but as compared with other studies this sample size is higher.19, 20, 21In previously published results reported that 37% of A. baumannii isolates were from blood samples, but in our study, a smaller number of isolates were from blood.12,16,21 Few studies conducted in Iran and Karnakata reported 46% and 30%, respectively, of A. baumannii isolates from pus/wound samples which were similar to other studies also with slight variation in the number, but in our study very less number (14) of isolates from pus/wound samples.20,22 Next to this 12% of the samples was from UTIs, and the remaining 3% and 2% were body fluids and others, respectively. These results were consistent with other studies.12,20,21
The high proportions of resistance patterns to 3rd and 4th generation cephalosporins such as cefotaxime 94%, ceftazidime 93%, and cefepime 92%, the results following recent studies conducted by Banerjee T et al., Raut et al. and Rajkumari et al.15,23,24 This study showed a relative abundance of carbapenems resistance; imipenem 92%, and meropenem 90% which is consistent with the previous study conducted in Iran but our results were highest than previous studies.15,23 The increasing frequency of resistance patterns of aminoglycosides showed amikacin at 88%, and gentamicin at 87%, these findings concord with previous studies conducted in Varanasi and Iran.15,23Trends of resistance patterns fluoroquinolones; ciprofloxacin 88%, levofloxacin 87%, penicillins/β-lactamase inhibitors 77%, folate pathway inhibitors 88% and tetracycline 64%.15,24 There were little variations in antibiotic resistance patterns rate when compared with other studies conducted by Khoshnood et al., M. Moosavian et al. and Pattanaik A. et al.1,20, 22
The trends of resistance patterns of doxycycline 39% Polymyxin B 8% were observed low which is in concord with recent studies and observed raised compared with previous studies.1, 4,14,24A. baumannii have a natural MDR phenotype and high proportions of MDR and XDR isolates were found in our study, and increasing and high proportions of MDR and XDR are also been reported globally.9 The association between antibiotic resistance and the clinical profile of patients was found significant as shown in Table 2.
Table (2):
Association of Clinical Profile of patients and Antibiotic-resistance in A. baumannii isolates.
| Hospital associated infections | Clinical Profile of patients | A. baumannii n=143 (%) | β-lactam combinations | Cephalosporins | Aminoglycosides | Fluoroquinolones | Carbapenems | Lipopeptides | Tetracyclines | Folate pathway antagonists |
|---|---|---|---|---|---|---|---|---|---|---|
| Ventilator associated pneumonia | Aspiration pneumonia/ pneumonia | 45(31) | 38 | 42 | 37 | 39 | 40 | 5 | 18 | 38 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | – | <0.001 | ||
| COPD | 14 (9.7) | 10 | 12 | 12 | 12 | 12 | 1 | 7 | 11 | |
| p-value | 0.090 | 0.006 | 0.006 | 0.006 | 0.006 | 0.001 | 1 | 0.029 | ||
| Respiratory Failure | 35 (24) | 32 | 35 | 35 | 35 | 35 | 1 | 13 | 34 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | – | <0.001 | ||
| Mechanical Ventilation | 83 (58) | 77 | 82 | 80 | 80 | 81 | 7 | 39 | 78 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.745 | <0.001 | ||
| Septicemia | CVA/Hemorrhagic Shock | 44 (30.7) | 42 | 44 | 44 | 44 | 44 | 3 | 21 | 43 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | – | <0.001 | ||
| Septicemia | 12 (8.4) | 10 | 12 | 9 | 9 | 11 | 0 | 2 | 9 | |
| p-value | 0.039 | <0.001 | 0.146 | 0.146 | 0.006 | 0.000 | 0.039 | 0.146 | ||
| Central line | 03 (2.0) | 3 | 3 | 3 | 3 | 3 | 0 | 0 | 3 | |
| p-value | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 | ||
| Accelared HTN/HTN | 26 (18) | 22 | 24 | 24 | 23 | 23 | 5 | 14 | 24 | |
| p-value | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | – | <0.001 | ||
| UTI/CAUTI | CKD/AKI | 11 (7.7) | 7 | 9 | 8 | 9 | 9 | 3 | 2 | 9 |
| p-value | 0.549 | 0.065 | 0.227 | 0.065 | 0.065 | 0.227 | 0.065 | 0.065 | ||
| Catheterization | 76 (53) | 66 | 73 | 70 | 71 | 73 | 7 | 43 | 69 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Surgical Site Infection | Post-surgical/SSI | 14 (9.7) | 9 | 12 | 13 | 12 | 12 | 2 | 7 | 11 |
| p-value | 0.212 | 0.006 | 0.001 | 0.006 | 0.006 | 0.006 | 1 | 0.029 | ||
| Trauma/Burns | 8 (5.5) | 7 | 8 | 7 | 8 | 7 | 0 | 3 | 7 | |
| p-value | 0.070 | <0.001 | 0.070 | <0.001 | 0.070 | <0.001 | 0.727 | 0.070 | ||
| Diabetes mellitus | 26 (18) | 23 | 24 | 25 | 24 | 26 | 3 | 12 | 24 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Prosthesis | 05 (3.5) | 4 | 5 | 5 | 5 | 5 | 0 | 1 | 5 | |
| p-value | 0.187 | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0 | 0.031 | ||
| Others | Extended Hospital stay (> 7 days) | 70 (49) | 59 | 65 | 63 | 64 | 65 | 8 | 29 | 61 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0 | <0.001 | ||
| Cancer | 03 (2.0) | 2 | 3 | 3 | 2 | 2 | 0 | 2 | 2 | |
| p-value | 1.000 | 0.250 | 0.250 | 1.000 | 1.000 | 0.250 | 1 | 1.000 |
(If p-value < 0.05 is significant by Chi–Square Test).
In our study, remarkably high antibiotic resistance to classes of antibiotics was observed in A. baumannii isolates, especially MDR and XDR strains. The emergence of MDR and XDR A. baumannii is a serious global threat to public health. Currently, there is no effective drug to fight against multidrug resistance isolates, apart from polymyxins. To address the infections caused by antibiotic-resistant A. baumannii, appropriate antibiotic administration in a clinical setting is essential. Moreover, local and national surveillance data, stringent infection control, and antimicrobial stewardship are required. Molecular methods have been developed for the accurate identification of Acinetobacter species. The speed, accuracy, ability to detect outbreaks, and interpretation of changing trends in technology of molecular methods are enabling further scope of research.
ACKNOWLEDGMENTS
The authors would like to thank Members of the Microbiology Research Laboratory at Index Medical College Hospital & Research Centre, Indore, India for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HS and RK conceptualized the study. HS, RK and PKG designed the study. PKG and LS performed the experiments and wrote the manuscript. HS and RK guided and revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the ethics committee of Index Medical College, Hospital & Research Centre affiliated with Malwanchal University, Indore (MP) (MU/Research/EC/Ph.D/2019/44(a)).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Moosavian M, Ahmadi K, Shoja S, Mardaneh J, Shahi F, Afzali M. Antimicrobial resistance patterns and their encoding genes among clinical isolates of Acinetobacter baumannii in Ahvaz, Southwest Iran. MethodsX. 2020;7:101031.
Crossref - Roy S, Chowdhury G, Mukhopadhyay AK, Dutta S, Basu S. Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection. Front Med. 2022;9:793615. doi: 10.3389/fmed.2022.793615.
Crossref - Tripathi PC, Gajbhiye SR, Agrawal GN. Clinical and antimicrobial profile of Acinetobacter spp.: An emerging nosocomial superbug. Adv Biomed Res. 2014;3:13:13.
Crossref - Donadu MG, Mazzarello V, Cappuccinelli P, et al. Relationship between the Biofilm-Forming Capacity and Antimicrobial Resistance in Clinical Acinetobacter baumannii Isolates: Results from a Laboratory-Based In Vitro Study. Microorganisms. 2021;9(11):2384.
Crossref - Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243-250.
Crossref - Vijayakumar S, Gopi R, Gunasekaran P, et al. Molecular Characterization of Invasive Carbapenem-Resistant Acinetobacter baumannii from a Tertiary Care Hospital in South India. Infect Dis Ther 2016;5(3):379-387.
Crossref - Qi L, Li H, Zhang C, et al. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii. Front Microbiol. 2016;7:483.
Crossref - Eze EC, Chenia HY, El Zowalaty ME. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Resist. 2018;11:2277-2299.
Crossref - Odsbu I, Khedkar S, Khedkar U, Nerkar SS, Tamhankar AJ, Lundborg CS. High Proportions of Multidrug-Resistant Acinetobacter spp. Isolates in a District in Western India: A Four-Year Antibiotic Susceptibility Study of Clinical Isolates. Int J Environ Res Public Health. 2018;15(1):153.
Crossref - Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. London: Churchill Livingstone; 1996. p. 131-45.
- Koneman, Elmer W., and Procop, Gary W. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 7th ed. United States: Wolters Kluwer Health; 2017:253-309.
- Gupta N, Gandham N, Jadhav S, Mishra RN. Isolation and identification of Acinetobacter species with special reference to antibiotic resistance. J Nat Sci Biol Med. 2015;6(1):159-162.
Crossref - CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. CLSI standard M02. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
- Shenkutie AM, Yao MZ, Siu GK, Wong BKC, Leung PH. Biofilm-Induced Antibiotic Resistance in Clinical Acinetobacter baumannii Isolates. Antibiotics. 2020;9(11):817.
Crossref - Banerjee T, Mishra A, Das A, Sharma S, Barman H, Yadav G. High Prevalence and Endemicity of Multidrug Resistant Acinetobacter spp. in Intensive Care Unit of a Tertiary Care Hospital, Varanasi. India. J Pathog. 2018;2018:9129083.
Crossref - Konca C, Tekin M, Geyik M. Susceptibility Patterns of Multidrug-Resistant Acinetobacter baumannii. Indian J Pediatr. 2021;88(2):120-126.
Crossref - Ryu SY, Baek WK, Kim HA. Association of biofilm production with colonization among clinical isolates of Acinetobacter baumannii. Korean J Intern Med. 2017;32(2):345-351.
Crossref - Mathai AS, Oberoi A, Madhavan S, Kaur P. Acinetobacter infections in a tertiary level intensive care unit in northern India: epidemiology, clinical profiles and outcomes. J Infect Public Health. 2012;5(2):145-52.
Crossref - Mostafavi SN, Rostami S, Nokhodian Z, et al. Antibacterial resistance patterns of Acinetobacter baumannii complex: The results of Isfahan Antimicrobial Resistance Surveillance-1 Program. Asian Pac J Trop Med. 2021;14(7):316-322.
Crossref - Pattanaik A, Banashankari GS. Characterisation of Acinetobacter with special reference to carbapenem resistance and biofilm formation. Trop J Path Micro. 2019;5(6):386-395.
Crossref - Basatian-Tashkan B, Niakan M, Khaledi M, et al. Antibiotic resistance assessment of Acinetobacter baumannii isolates from Tehran hospitals due to the presence of efflux pumps encoding genes (adeA and adeS genes) by molecular method. BMC Res Notes. 2020;13(1):543.
Crossref - Khoshnood S, Sadeghifard N, Mahdian N, et al. Antimicrobial resistance and biofilm formation capacity among Acinetobacter baumannii strains isolated from patients with burns and ventilator-associated pneumonia. J Clin Lab Anal. 2023;37(1):e24814.
Crossref - Raut S, Rijal KR, Khatiwada S, et al. Trend and Characteristics of Acinetobacter baumannii Infections in Patients Attending Universal College of Medical Sciences, Bhairahawa, Western Nepal: A Longitudinal Study of 2018. Infect Drug Resist. 2020;13:1631-1641.
Crossref - Rajkumari S, Pradhan S, Sharma D, Jha B. Prevalence and Antibiogram of Acinetobacter Species Isolated from Various Clinical samples in a Tertiary Care Hospital, Bharatpur, Chitwan, Nepal. JCMS Nepal. 2020;16(1):26-32.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.