ISSN: 0973-7510
E-ISSN: 2581-690X
https://dx.doi.org/10.22207/JPAM.13.1.23 | © The Author(s). 2019
The margarine constituents were examined by Gas Chromatography-Mass Spectrometry (GC-MS), it contained 41 compounds, fifteen of them were identified as the major compound, Hexadecanoic acid, methyl ester (22.39%), Methyl stearate (14.92%), methyl elaidate (13.80%), Methyl tetradecanoate (10.74%), Capric acid, methyl ester (8.34%) Lauric acid (4.52%) methyl octanoate (2.70%), linoleic acid methyl ester (2.57%), Methyl 11-octadecenoate (1.90%), methyl caproate (1.77%), Methyl pentadecanoate (1.72%), Methyl (8E,11E)-8,11-octadecadienoate (1.70%), Heptadecanoic acid, methyl ester, (1.46%),trans-13-Octadecenoic acid, methyl ester (Oleic acid) (1.20%), Methyl palmioleate (1.00%). The effect of goat margarine, against four different pathogenic bacteria Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, and Bacillus cereus, were carried out by using a disc diffusion technique, the highest antibacterial activity was detected against Salmonella typhimurium and the lowest one against Escherichia coli. The antibacterial activity of the antibiotics, (Ciprofloxacin, Tetracycline Ceftriaxone, Chloramphenicol and Gentamycin), were tested by the disc diffusion technique, and by measuring zones of inhibition, shows that there were differences, among all antibiotic the highest activity of antibiotic against bacteria was due to the action of ciprofloxacin. Ceftriaxone and Tetracycline antibiotic give lowest activity. Among the bacteria the highest inhibition zone by antibiotic against Salmonella typhimurium, and the lowest one against Escherichia coli
Goat margarine; GC-MS; antibiotics; pathogenic bacteria; disc diffusion method.
Traditional goat margarine can be made by separation the fat from butter. The structure of margarine contain of carbon, hydrogen and oxygen, those atoms were difference bonded together (saturated and unsaturated). Goat milk contain mostly unsaturated fatty acids (monounsaturated (MUFA), polyunsaturated fatty acids (PUFA)), which all are known to be beneficial for human health, especially for cardiovascular conditions1,2,3, essential fatty acids have nutritional importance it is a source of omega-3 and omega-64,5,6,7 the goat margarine have less amount of trans- acids. The extracts of goat margarine are mainly, oleic acid have activity against bacterial8 Linoleic acid methyl ester prevent body against microorganism9, trans-13-Octadecenoic acid, methyl esteract against inflammation, prevent against cancer, act as cosmetic, prevent skin from many decrease, anemia and act as insects ide10. Hexadecanoic acid, methyl ester act as Antibacterial and antifungal, methyl octanoate and n-hexa-decanoic acid have higher activity against bacteria11,12,13,14. Margarine come from goat milk lipid have best activity against allergic, inflammation, it have high nutritive value15. Caproic and caprylic acids, they have possess antimicrobial activity, total fat content have higher activity for prevention bacteria.
The present study was to know the goat margarine chemical Compound and the activity of it against pathogenic bacteria attacked human, compare it with the antibiotics.
Goat milk collected from domesticated goat, piece of cloth, yogurt, refrigerator and milk pudding machine, Gas Chromatography-Mass Spectrometry (GC-MS).
Microorganism
All the microorganisms used in this work were obtained from the National Research Centre (Khartoum) Sudan. The identification of bacterial was carried out by conventional biochemical methods according to the standard micro-biological techniques These microbes were, Escherichia coli, Pseudomon aaeruginosa, Salmonella typhimurium and Bacillus cereus.
Manufacture of margarine
Traditional method
Milk filtered from any impurities by piece of cloth, coagulation the milk by yogurt After the milk reaches the level of good coagulation, put in the refrigerator for at least 4 hours to cool down The coagulated milk is placed in a special machine or can be placed in a tightly sealed container and is dipped for about an hour with the hands, but in the machinefor milk pudding it takes about 45 minutesthen collect the butter from above the milk and washed with cold water then heat and collect margarine.
Sample Preparation
2g of the goat margarine was mixed thoroughly with 7ml of alcoholic sodium hydroxide (Noah). The mixture was then shake for 5 minutes .The content of the test tube was left to stand overnight.1 ml of Super saturated sodium chloride (NaCl) was added and shaken, added2ml of normal hexane and then the contents were shake thoroughly for three minutes. Then the n-hexane layer (the upper layer of the test tube) was formed, 5 µl from the n-hexane extract was diluted with 5 ml of diethyl ether. filtered the mixture and dried with 1g of anhydrous sodium sulphate, and 1µl of the diluted sample was injected in the GC.MS instrument.
Method of analysis
Gas Chromatography-Mass Spectrometry (GC/MS) Conditions
The identification of goat margarine constituents were carried out by using Gas chromatography with mass spectrometry, model (GC/MS-QP2010-Ultra)’ Simadzu Company, Japans with serial number 020525101565SA and capillary column (Rtx-5ms-30m׳0.25 mm׳0.25 µm). injection of the sample by using split mode, the carrier gas was helium passed with flow rate 1.61 ml/min, the rate of temperature was began from 6°C with flow rate 10c/min to 30°C as end temperature degree with 3 minutes hold time, the injection port temperature was 30°C, the temperature of the ion was 200c and the interface temperature was 25°C. The goat margarine sample was analyzed by using scan mode in the range of m/z 40-500 charges to ratio and the total run time was 27 minutes. The identification of the compound by comparing their retention index and mass fragmentation patents with those are found in the library, the National Institute of Standards and Technology (NIST).
Disc diffusion method
For microbial sensitivity test using the paper disc diffusion method, to screen the antibacterial activity of goat margarine and performed by using Muller Hinton agar. Sterile 5 mm diameter paper disc soaked with the margarine was placed gently on the media, which had been freshly inoculated with each of the organisms. The plates were incubated for 24 hours at 37°C. The results were recorded by measuring the zone inhibition by goat margarine, also used the same method for microbial sensitivity test of the antibiotic17.
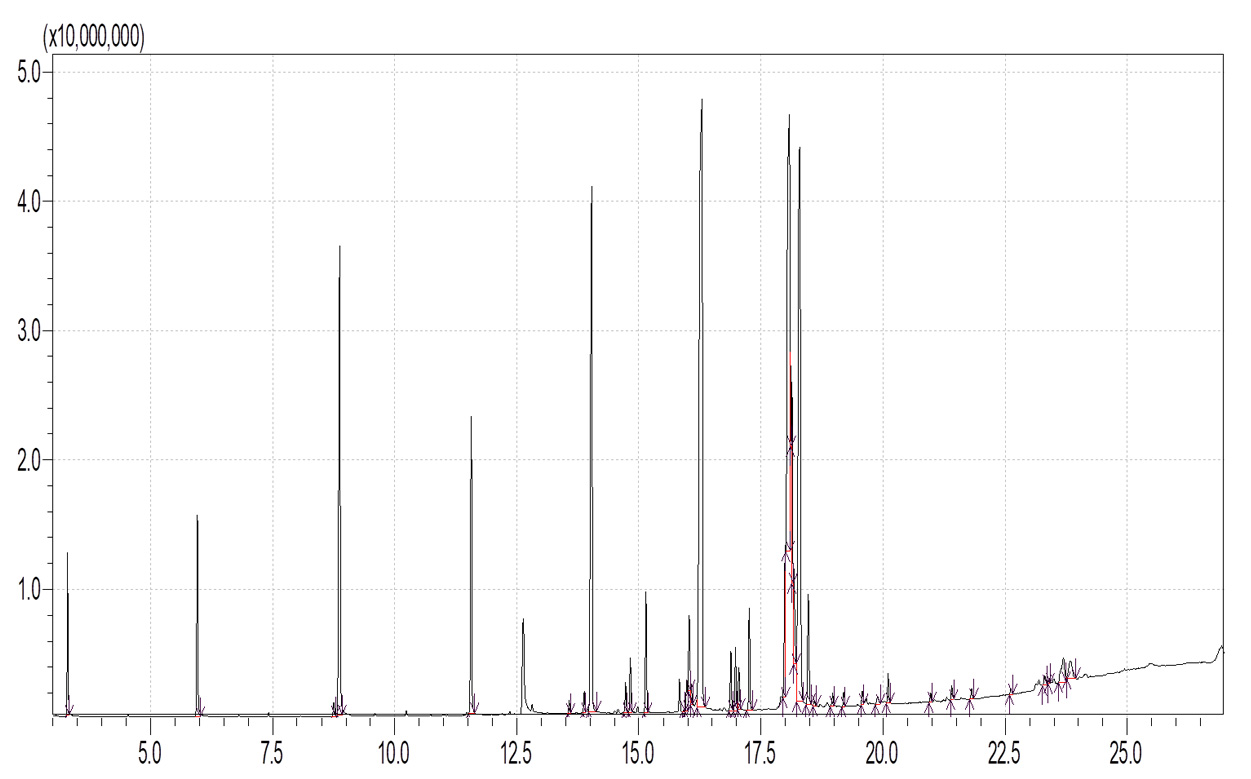
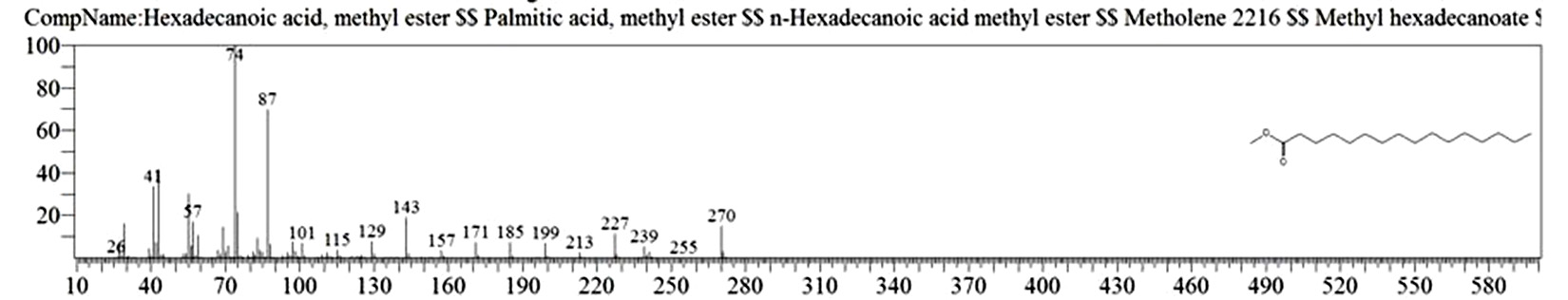
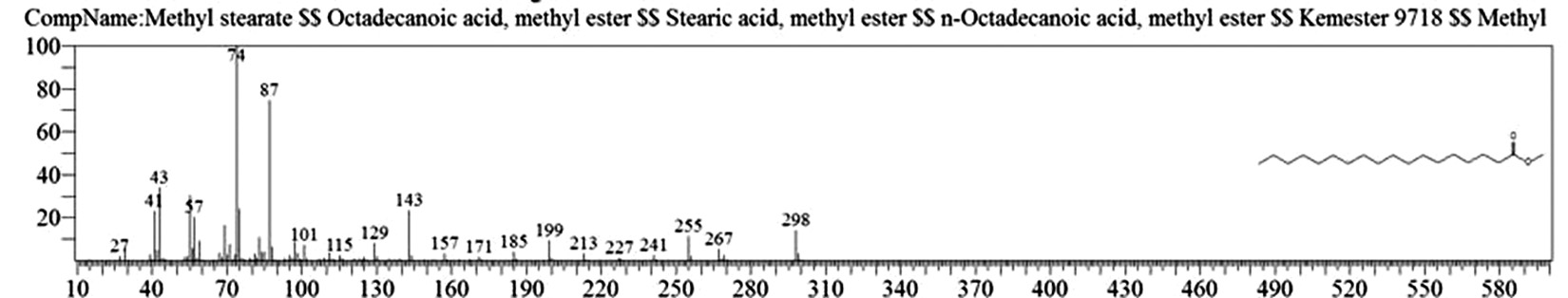
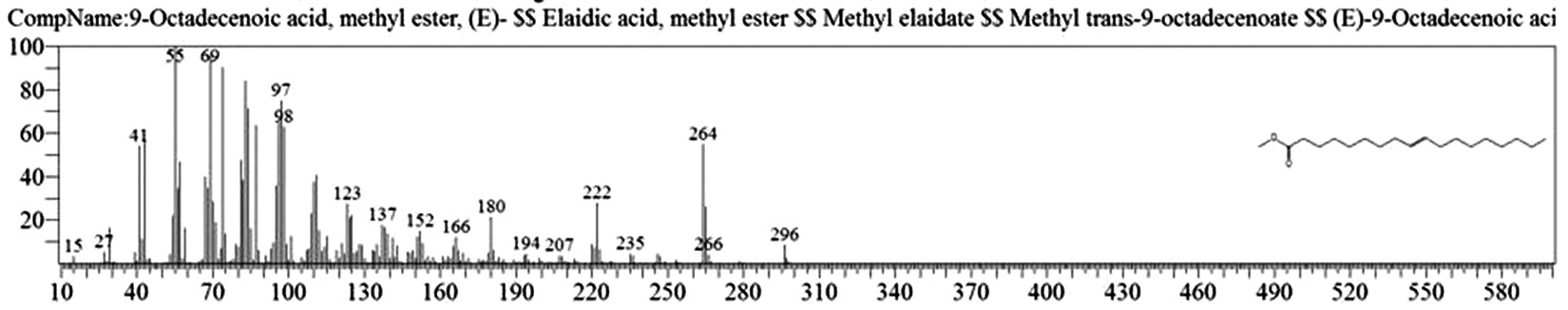
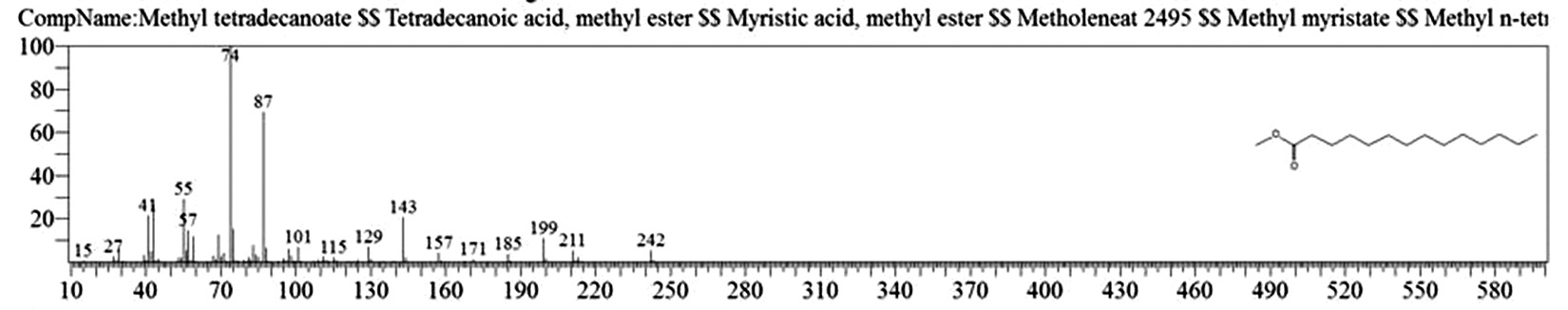
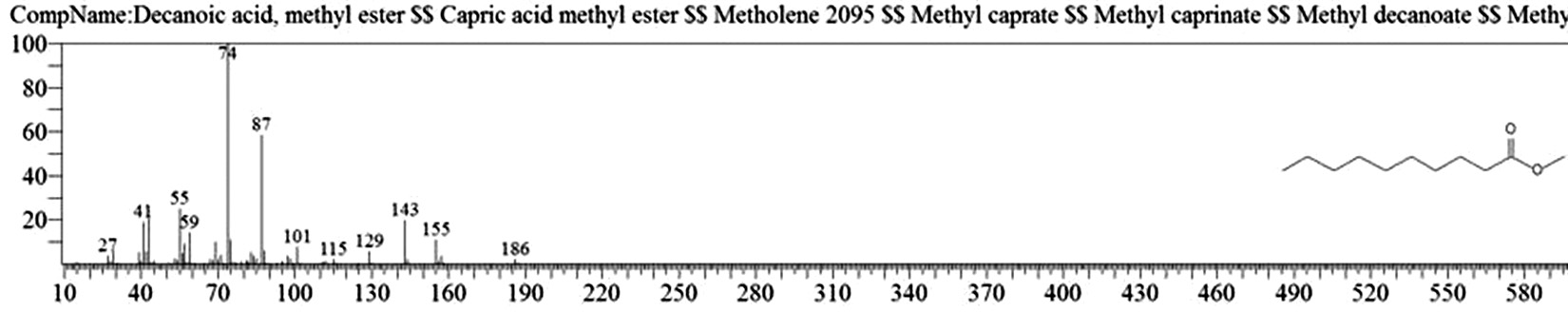
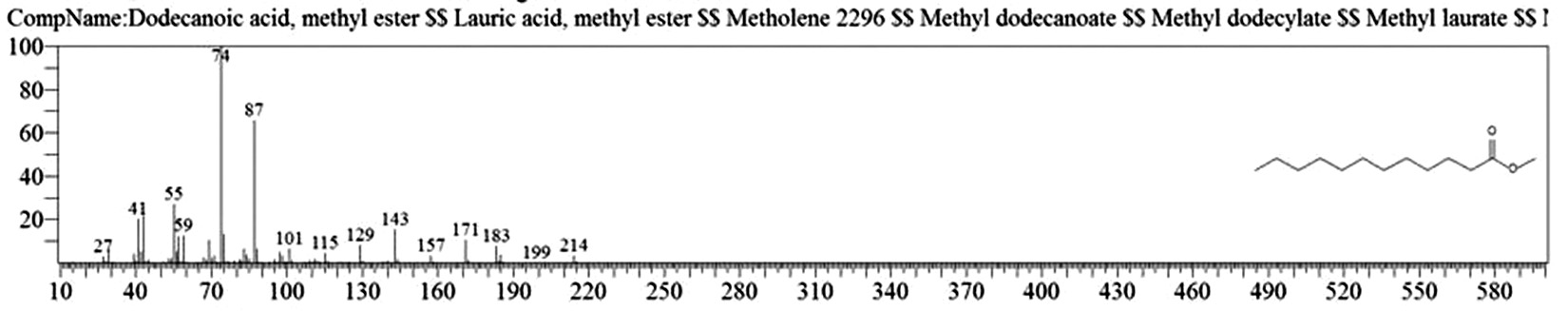
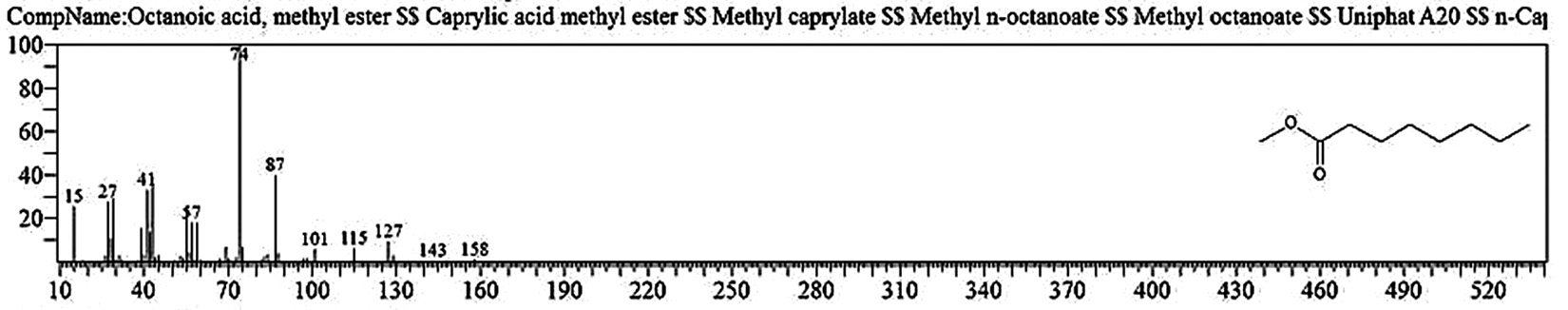
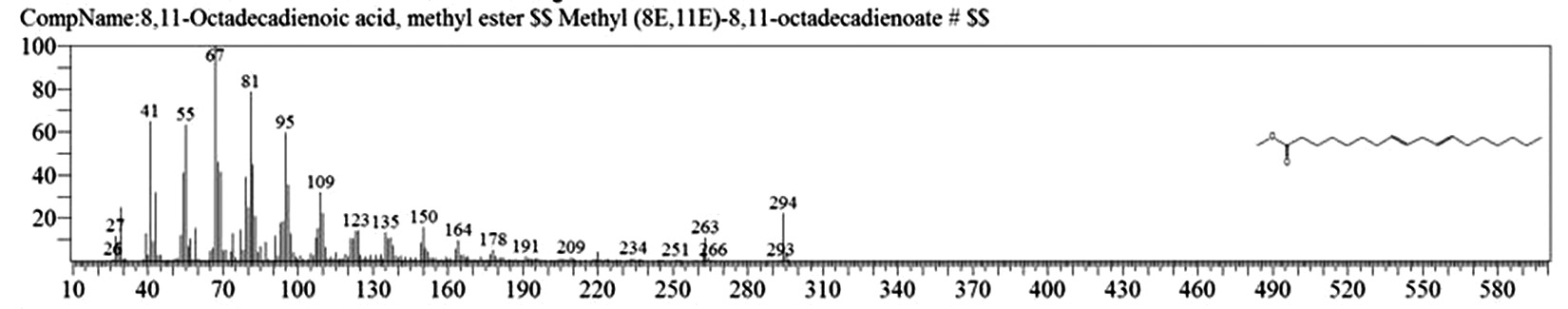
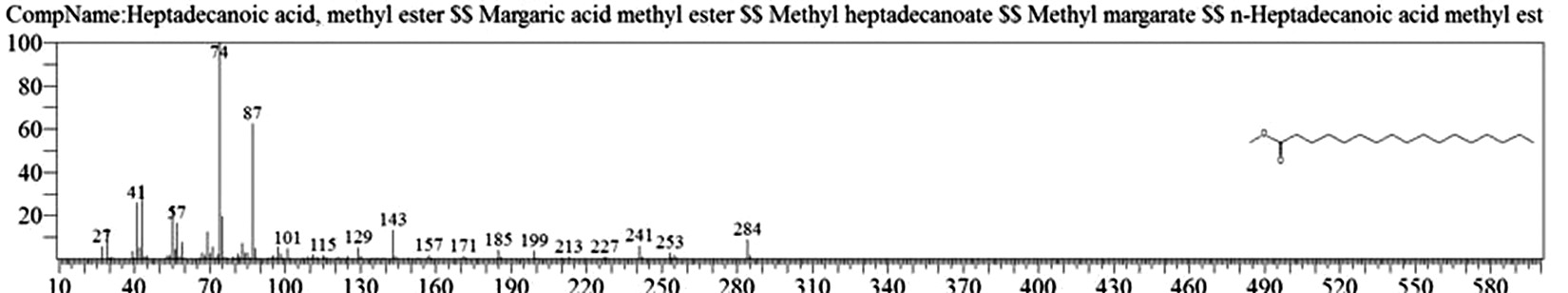
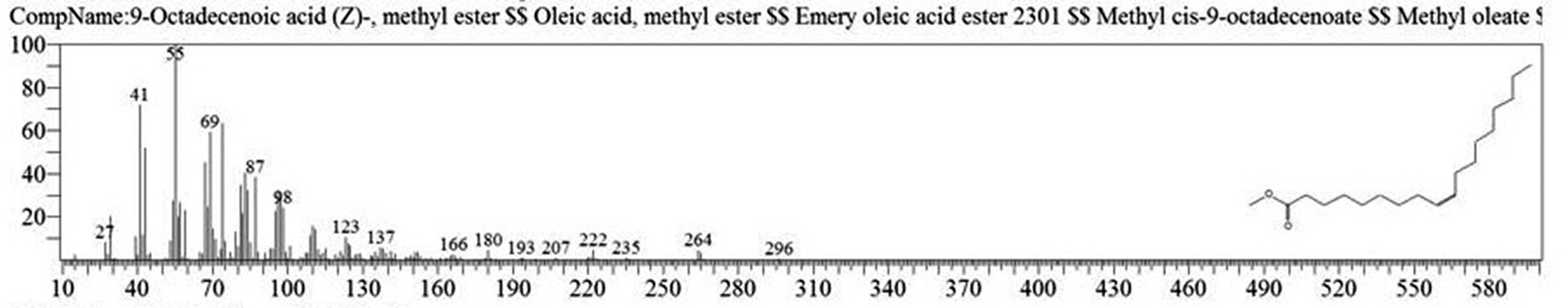
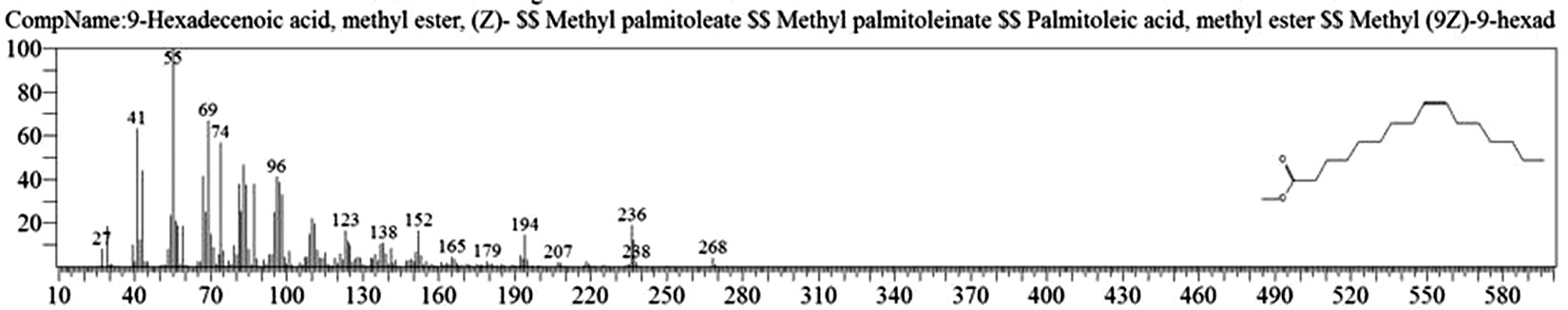
Table 1. and Fig. 1 shows that the chemical constituents of the goat margarine are given in in the order of the retention times 41 constituents were identified, 15 of them constituted as major compound such as Hexadecanoic acid, methyl ester (22.39%), fig. 2. Methyl stearate (14.92%), fig.3. 9-Octadecenoic acid (Z)-, methyl ester (13.80%), fig. 4. Methyl tetradecanoate (10.74%), fig. 5. Capric acid, methyl ester (8.34%). fig. 6. Lauric acid, methyl ester (4.52%), fig. 7. Methyl octanoate (2.70%), fig. 8. Linoleic acid methyl ester (2.57%), fig. 9, Methyl 11-octadecenoate (1.90%), fig. 10. Hexanoic acid, methyl ester (1.77%), fig. 11. Methyl pentadecanoate (1.72%), fig. 12. Methyl(8E,11E)-8, 11-octadecadienoate (1.70%), fig.13. Heptadecanoic acid, methyl ester (1.46%) fig. 14. Oleic acid (1.20%), fig. 15. 9-Hexadecenoic acid, methyl ester, (Z)- (1.00%), fig. 16, these compound were found active during infla-mmation, act during oxidationas antioxidant, hypocholesterolemic highly active against pest, insect and nematode8,18,19. Table 2. show that the antibacterial activity of the goat margarine against four different pathogenic organisms Escherichia coli, Pseudomona aeruginosa, Salmonella typhimurium and Bacillus cereus, (the highest concentration of the goat margarine is (100 mg/ml) and the lowest one is (12.5 mg/ml) there was differences effect among bacteria, the highest inhibition zone by goat margarine was detected against Salmonella-typhimurium and the lowest inhibition zone against Escherichia coli, in lower concentration (12.5) there was no result. these results conformity with those obtained in previous studies16,19,20 Table 3. Shows that the inhibition zones (in mm) for the antibiotic there was differences among them, highest activity of antibiotic against bacteria was due to the action of ciprofloxacin among all antibiotic and the lowest inhibition detected by Ceftriaxone and Tetracycline antibiotic, the highest inhibition zone among the bacteria by antibiotic against Salmonellatyphimurium and the lower inhibition zone is Escherichia coli, there was no high different between antibiotic, (Gentamycin, Chloram-phenicol, Ceftriaxone and Tetracycline) and goat margarine against bacteria.
Table (1):
Chemical constituents of goat margarine identified by GC-MS
ID# |
Name |
Ret.Time |
Area |
Area% |
|---|---|---|---|---|
1. |
Hexanoic acid, methyl ester |
3.300 |
16420096 |
1.77 |
2. |
Octanoic acid, methyl ester |
5.957 |
25031032 |
2.70 |
3. |
4-Decenoic acid, methyl ester, Z- |
8.753 |
1973471 |
0.21 |
4. |
Decanoic acid, methyl ester |
8.871 |
77338694 |
8.34 |
5. |
Dodecanoic acid, methyl ester |
11.566 |
41934099 |
4.52 |
6. |
Tridecanoic acid, 12-methyl-, methyl ester |
13.570 |
1724913 |
0.19 |
7. |
Methyl Z-11-tetradecenoate |
13.881 |
2696851 |
0.29 |
8. |
Methyl tetradecanoate |
14.031 |
99602220 |
10.74 |
9. |
Pentadecanoic acid, methyl ester |
14.727 |
3919991 |
0.42 |
10. |
Tetradecanoic acid, 12-methyl-, methyl ester |
14.825 |
7091336 |
0.76 |
11. |
Pentadecanoic acid, 14-methyl-, methyl ester |
15.144 |
15978254 |
1.72 |
12. |
7,10-Hexadecadienoic acid, methyl ester |
15.919 |
291271 |
0.03 |
13. |
7-Hexadecenoic acid, methyl ester, (Z)- |
15.982 |
4681016 |
0.50 |
14. |
9-Hexadecenoic acid, methyl ester, (Z)- |
16.030 |
9266601 |
1.00 |
15. |
11-Hexadecenoic acid, methyl ester |
16.069 |
2138576 |
0.23 |
16. |
Hexadecanoic acid, methyl ester |
16.289 |
207249077 |
22.39 |
17. |
Hexadecanoic acid, 15-methyl-, methyl ester |
16.881 |
7748042 |
0.84 |
18. |
Hexadecanoic acid, 14-methyl-, methyl ester |
16.977 |
8482084 |
0.91 |
19. |
cis-10-Heptadecenoic acid, methyl ester |
17.046 |
5044403 |
0.54 |
20. |
Heptadecanoic acid, methyl ester |
17.261 |
13576564 |
1.46 |
21. |
9,12-Octadecadienoic acid (Z,Z)-, methyl ester |
17.995 |
23805616 |
2.57 |
22. |
9-Octadecenoic acid (Z)-, methyl ester |
18.081 |
127920245 |
13.80 |
23. |
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- |
18.105 |
4085216 |
0.44 |
24. |
11-Octadecenoic acid, methyl ester |
18.135 |
17578257 |
1.90 |
25. |
trans-13-Octadecenoic acid, methyl ester |
18.172 |
11153676 |
1.20 |
26. |
Methyl stearate |
18.290 |
138367840 |
14.92 |
27. |
8,11-Octadecadienoic acid, methyl ester |
18.468 |
15794339 |
1.70 |
28. |
9,11-Octadecadienoic acid, methyl ester, (E,E)- |
18.590 |
775753 |
0.08 |
29. |
10-Nonadecenoic acid, methyl ester |
18.966 |
2042381 |
0.22 |
30. |
Nonadecanoic acid, methyl ester |
19.195 |
2051960 |
0.22 |
31. |
5,8,11,14-Eicosatetraenoic acid, methyl ester, (all-Z)- |
19.577 |
1780403 |
0.19 |
32. |
11-Eicosenoic acid, methyl ester |
19.887 |
1881376 |
0.20 |
33. |
Eicosanoic acid, methyl ester |
20.104 |
3864392 |
0.42 |
34. |
Heneicosanoic acid, methyl ester |
20.974 |
1297791 |
0.14 |
35. |
Methyl 6,9,12,15,18-heneicosapentaenoate |
21.406 |
1940477 |
0.21 |
36. |
Docosanoic acid, methyl ester |
21.812 |
1495112 |
0.16 |
37. |
Tricosanoic acid, methyl ester |
22.618 |
873501 |
0.09 |
38. |
Vinyl decanoate |
23.314 |
2191100 |
0.24 |
39. |
Tetracosanoic acid, methyl ester |
23.393 |
317580 |
0.03 |
40. |
Sulfurous acid, pentadecyl pentyl ester |
23.693 |
8958125 |
0.97 |
41. |
Octanoic acid, 4-pentadecyl ester |
23.831 |
6844877 |
0.74 |
Table (2):
Inhibition zoon (in mm) for different concentrations of goat margarine
| Microorganism | Concentration of the goat margarine (μg/disc) | |||
|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | |
| Salmonella typhimurium | 0 | 12 | 13 | 15 |
| Pseudomonas aeruginosa | 0 | 11 | 13 | 14 |
| Escherichia coli | 0 | 10 | 11 | 11 |
| Bacillus Cereus | 0 | 12 | 14 | 14 |
Table (3):
Inhibition zoon (in mm) for different antibiotics
| Bacterial test strains (No. tested) | Antibiotics concentration in (μg/disc)m | ||||||
|---|---|---|---|---|---|---|---|
| Ceftriaxone | Tetracycline | Chloramphenicol | Gentamycin | Ciprofloxacin | |||
| Salmonella typhimurium | 12 | 9 | 18 | 15 | 25 | ||
| Pseudomonas aeruginosa | 14 | 12 | 12 | 10 | 26 | ||
| Escherichia coli | 10 | 10 | 13 | 11 | 14.9 | ||
| Bacillus cereus | 6 | 11 | 10 | I3 | 21 | ||
Ciprofloxacin (5μg), Gentamycin (10 μg), Chloramphenicol (10μg), Tetracycline (25μg) and Ceftriaxone (30μg),
This work comes to conclude that the goat margarine contain non-polar components had potent antibacterial activity against pathogen bacteria, and have no high different between it and synthetic antibiotic in the activity against microorganism,
The authors would like to express their deepest thanks to the laboratories of Chemistry and Microbial products “National Centre for Research, Khartoum Sudan” for their help in analyzing the samples.
The author declares that there are no conflict of interest.
- Chilliard, Y., Ferlay, A., Dietary lipids and forages interactions on cow and goat milk fatty acid composition and sensory properties. Reprod. Nutr. Dev., 2004; 44: 467–492.
- Cordain, L.; Eaton, L.S.B; Sebastian, A; Mann, N.; Lindeberg, S; Watkins, B.A.; O keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr., 2005; 81,341-354. Damiבn
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008; 233: 674-688.
- Williams C.M. Dietary fatty acids and human health. Annales de Zootechnie, 2000; 49: 165-180.
- Lock A.L., Bauman D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids, 2004; 39: 1197–1206.
- Wahle K.W., Heys S.D., Rotondo D. Conjugated linoleic acids: Are they beneficial to human health? Progress in Lipid Research, 2004; 43: 553–587.
- Tilahun Zenebe, Nejash Ahmed, Tadele Kabeta and Girma Kebede. Review on Medicinal and Nutritional Values of Goat Milk, Academic Journal of Nutrition, 2014; 3(3): 30-39.
- Awa, E.P.; Ibrahim, S.; Ameh, D.A. GC/MS analysis and antimicrobial. Activity of diethyl ether fraction of methanolic extract from the stem bark of Annona senegalensis Pers. Int. J. Pharm. Sci. Res., 2012; 3: 4213–4218.
- Yu, F.R.; Lian, X.Z.; Guo, H.Y.; McGuire, P.M.; Li, R.D.; Wang, R.; Yu, F.H. Isolation and characterization of methyl ester ad derivatives from Euphorbia kansui (Euphorbiaceae) and their in hibitory effects on the human SGC-7901 cells. J. Pharm. Pharm. Sci., 2005; 8: 528–535.
- Krishnamoorthy, K.; Subramaniam, P. Phyto-chemical pro ling of leaf, stem, and tuber parts of Solena amplexicaulis (Lam.) Gandhi Using GC-MS. Int. Sch. Res. Not., 2014.
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inuammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des., 2012; 80: 434–439.
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res., 2010; 4: 191–195.
- Rahuman, A.A.; Gopalakrishnan, G.; Ghouse, B.S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia, 2000; 71: 553–555.
- Jan midrkal, TerezakarloVa, VladimirFilip, markיtaZבruboVa and iveta HradkoVa. Antimicrobial Properties of 11-Cyclohexylundecanoic Acid, Czech J. Food Sci., 2009; 6: 463–469.
- Hanifah, R., Arief, I. I. and Budiman, C. Antimicrobial activity of goat milk yoghurt with addition of aprobiotic Lactobacillus acidophilus IIA – 2B4 and roselle (Hibiscus sabdariffa L) extract. International Food Research Journal, 2016; 23(6): 2638-2645.
- Van Immerseel, F., J. De Buck, F. Boyen, L. Bohez, F. Pasmans, J. Volf, M. Sevcik, I. Rychlick. F. Haesebrouck and R. Ductaelle. Medium chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enteric serovaren-teritidis. Applied and Environmental Microbiology, 2004; 70: 3582-3587..
- Meena, M.R., and Sethi, V. Antimicrobial activity of essential oils from spices. Journal of Food Science Technology, 1994; 31: 68-70.
- Chen, J.J.; Duh, C.Y.; Chen, I.S. Cytotoxic chromenes from Myriactis humilis. Planta Med., 2005, 71: 370–371.
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia, 2009; 80: 123–126.
- Mustapha N. Abubakar and Runner R. T. Majinda. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicine, 2016; 3: 3.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.