ISSN: 0973-7510
E-ISSN: 2581-690X
The capability of thermophilic microorganisms to flourish at high temperatures makes their enzyme systems ideal for various biotechnological applications. Based on the proteolytic and amylolytic activities, two thermophilic bacteria were isolated from hot springs in Jazan, Saudi Arabia. The antibiotic and heavy metals susceptibility patterns of the bacterial isolates were performed. According to the metabolic fingerprint, the bacterial isolates were identified as Brevibacterium linens and Bacillus subtilis. Moreover, the impact of temperature, substrate concentration, and some metal ions on the production of proteases and amylases from the bacterial strains was investigated. The maximum protease production was achieved at 50°C while the greatest amylase production was recorded at 30°C for both strains at a constant pH of 7.5. The highest enzyme production was recorded at 5% skimmed milk for protease of B. linens and 10% for B. subtilis while 0.6% starch was the optimum substrate concentration of amylase production of the two strains. Furthermore, Ca2+ showed a simulative influence on protease production from the two strains whereas Mg2+ and Mn2+ demonstrated minor effect. On the other hand, Ca2+, Mg2+ and Mn2+ demonstrated a positive effect on the amylase production from both strains.
Thermophiles, Hot springs, Hydrolytic enzymes, Antibiotics, Heavy metals.
Bacteria are ubiquitous and highly diverse prokaryotes that can survive in adverse habitats. Most of bacteria present in the environment are still unexplored and hence remain obscure for their ecological functions. Thermophilic bacteria are microbes that mostly inhabit hot springs, live and survive in temperatures between 45 and 122oC. Thermophiles including bacterial and archaeal species are found in various geothermally heated regions of the earth such as hot springs and deep-sea hydrothermal vents. In Saudi Arabia, there are about ten geothermal springs with varying deep temperatures of and different flow rates. They are distributed in Gizan and Al-Lith areas. Of these, Ain Khulab, Ain Khulab Quwa, Ain Mijara Quwa, Ain ad Damad, Ain al Wagrah and Ain al Wagrah Dam are located in Gizan meanwhile Ain al Harra, Ain Jumah, Ain Markub, and Ain ad Darakah are located in Al-Lith area.1,2 Hot springs in Gizan are of volcanic origin and used as places for bathing and recreation by citizens and tourists.
Microbial communities inhabiting aquatic environments vary according to the physiochemical parameters including temperature, salinity, pH and nutrient loads. Extremophiles are microbes that can live and reproduce in harsh environments. It is well known that the enzymes of an organism are adapted to function optimally at or near its growth conditions, and so the range of extremes at which life is found defines the range of conditions at which enzyme activity might be detected3,4.The phenotypic and genotypic characterization of thermophilic bacteria has been done for many geothermal areas in different parts of the world including Turkey5, Italy6, Bulgaria7, Greece8, China9, India10, and Morocco.11
Several reports on the isolation of thermophilic bacteria from hot springs in Saudi Arabia have been published. In 2012, Khiyami and coauthors12 have identified about 15 thermo-aerobic bacteria from Jazan and Al-Lith geothermal springs where Bacillus cereus, Bacillus licheniformis, Bacillus thermoamylovorans, Pseudomonas aeruginosa and Enterobacter sp. were the dominant strains. Another study was conducted with Khalil (2011) who isolated thirteen thermophilic bacteria from hot springs in Saudi Arabia. Based on the biochemical characterization, all the isolates were lipase positive, 11 isolates showed amylase activity, while only 3 of them showed cellulase activity.3
It is possible to consider thermophiles as sources of industrially relevant thermostable enzymes. Many industrial processes require enzymes such as amylase, cellulase, xylanase, pectinase, protease, and lipase that are operationally stable at high temperatures13,14. Such these enzymes are usually produced by Gram-positive bacteria and fungi. These organisms secrete enzymes directly into the fermentation broth which offers major advantages in their downstream processing. Gram-positive Bacillus species are among the bacterial champions in enzyme production. Proteases are a group of enzymes that perform protein catabolism by hydrolysis of peptide bond. They are among the most important class of industrial enzymes that have various applications including detergent, leather preparation, peptide synthesis, textile industry, food industry, pharmaceutical industry as well as in bioremediation process15,16,17. Thermostable proteases are of greater advantage in applications because they remain active at high temperatures. Bacillus spp. of hot-springs has gained commercial significance as source of thermostable enzymes18. Amylases are one of the main enzymes used in industry. Their wide range of application in nutritional, detergent, alcohol, textile, food, paper, cosmetic and pharmaceutical industries increases their significance. Amylases are characterized by their ability to hydrolyze starch to generate glucose, maltose, a mixture of malto-oligosaccharides, and various á-limit dextrin-containing á (1-6) bonds19. Microorganisms are the predominant source of amylase production due to optimum growth requirements, accessibility, efficient, ecofriendly, and cost-effective as compared to other resources such as animal and plant20.
Due to the ability to produce thermostable enzymes, thermophilic microorganisms have gained great magnitude in pharmaceutical and industrial applications. The aim of the present study is the characterization of some thermophilic bacteria isolated from hot springs in Jazan, KSA in addition to the investigation of the optimum conditions for amylases and proteases production.
Samples collection and isolation of bacteria
The hot springs chosen for the present study were Bani Malik and Al Khawbah. The first is located in Bani Malik mountains at a distance of about 150 km northeast of Jazan city (43° 042 E, 17° 332 N) while the second is located in Al Harth governorate at a distance of about 50 km southeast of Jazan city (43° 152 E, 16° 562 N). Water samples were collected from Bani Malik hot spring and Al-Khobah hot spring , Jazan, KSA. Samples were assembled in 500 mL glass screw cap bottles and brought to the laboratory. Serial dilution was made and 100 µL of each dilution was inoculated into Nutrient Agar plates in triplicates. After the incubation of NA plates at 37 °C for 24 hrs the total bacterial count was recorded as CFU mL-1 as described earlier21. The developed colonies were repeatedly streaked on NA plates for the isolation of pure cultures. The purified colonies were transferred into NA slants and kept in refrigerator for further study.
Identification of the bacterial isolates
The bacterial isolates were Gram stained and observed under a high power magnifying lens in light microscope. Then the bacterial isolates were identified using GEN III MicroPlate™ test panel of the Biolog System (Biolog, Inc., Hayward, USA). The test panel provides a “Phenotypic Fingerprint” of the microorganism which can then be used to identify them to a species level. The GEN III MicroPlates™ enable testing of Gram-negative and Gram-positive bacteria in the same test panel. The test panel contains 71 carbon sources and 23 chemical sensitivity assays. This test analyzes the ability of the cell to metabolize all major classes of compounds, in addition to determining other important physiological properties such as pH, salt and lactic acid tolerance, reducing power, and chemical sensitivity. Fresh overnight cultures of the isolates were tested as recommended by the manufacturer. The bacterial suspensions were prepared by removing bacterial colonies from the plate surface with a sterile cotton swab and agitating it in 5 mL of 0.85% saline. 150 µL of the suspension was dispensed into each well of a Biolog GEN III microplate. The plates were read in the MicroStation semi-automated reader after 20 h and results interpreted by the identification system’s software (GEN III database, version 5.2.1).
Characterization of the bacterial isolates
Antibiotics susceptibility test
A single bacterial colony was picked using a sterile loop and streaked on the nutrient agar plate. A filter paper disk saturated with the following antibiotics (Mast Diagnostics, Bootle, UK); amikacin (30µg), gentamicin (10µg), cefepime (30µg), ticarcillin (75µg), piperacillin (100µg), imipenem (10µg), colistin (10µg), rifampicin (5µg), penicillin G (10 units µg), erythromycin (15µg), cephalothin (30µg), clindamycin (2µg), cotrimoxazole (25µg) and ampicillin (10µg) were dispensed onto the media plate separately. Using a sterilized forceps, each disc was placed on the nutrient agar medium to ensure that the disc are attached and fixed on the agar. Nutrient agar medium containing antibiotic discs were incubated overnight at 35 °C22.
Heavy metals susceptibility test
Analytical grade salts of CdCl2, CuSO4, HgCl2, AgNO3, Pb(NO3)2, ZnSO4 and K2Cr2O7 (Merck) were used to prepare 0.1 M stock solutions. Metal solutions were sterilized by using 0.45 µm pore-size polysulfone sterile filters (Whatman). Susceptibility tests were conducted using well diffusion method according to Essa et al. (2005)23. The metal solutions of Cr, Ag, Cd, and Hg were used at the concentrations 25, 50 and 100 mg/l while metal solutions of Pb, Cu and Zn were used at the concentrations 50, 100 and 150 mg/L. To each NA plate, 0.5 mL of metal solution was added in a central well of 1 cm in diameter and 4 mm in depth. Plates were then incubated for 24 hrs at 37 °C to allow diffusion of the metal into the agar. Then the plates were inoculated in radial streaks with the tested bacterial isolates. Plates were incubated at 37 °C for 48 h. After incubation, the area of inhibition zone (mm) was millimeter24.
Screening of bacterial isolates for protease and amylase production
Skim milk nutrient agar media were used for protease qualitative screening for several colonies using streaking method15. Colony forming transparent zone due to the partial hydrolysis of milk casein was selected. Regarding the screening of amylase, starch agar plates were inoculated with the bacterial isolates and incubated at 37 oC for 48 hours. Then the Petri dishes were flooded with iodine solution. The clear zone surrounding the colony was measured in millimeter25.The bacterial isolates K2 (isolated from Bani Malik hot spring) and K10 (isolated from Al-Khobah hot spring) were chosen for study based on their elevated proteolytic and amylolytic activities.
Factors affecting the production of protease and amylase from thermophilic bacteria
Effect of temperature on both of protease and amylase production was studied at different temperatures (30, 37 and 50 °C). Also, the effect of substrate concentration on enzymes production was studied. For protease production; the skim milk substrate concentrations 5, 10 and 20% were added to nutrient agar. For amylase production; the starch substrate concentrations were 0.2, 0.4 and 0.6%. The effect of trace elements on both enzymes production was studied. Calcium, magnesium and manganese trace elements were used at concentrations 20, 40,80 mg/L.
Bacteriological analysis of water samples
The total bacterial count of water samples from Bani Malek and Al-khoba hot springs was carried out. The bacterial count was 2000 CFU mL-1 in Bani Malek sample and 300 CFU mL1 in Alkhoba sample. Based on the proteolytic and amylolytic activities, two bacterial isolates with maximum potential of starch and protein degradation were chosen; K2 from Bani Malek hot spring and K10 from Al-khoba hot spring.
Antibiotics and heavy metals resistance profile
The obtained results (Table 1 and Figure 1) showed the antibiotic resistance pattern of the two bacterial isolates. The first isolate (K10) recorded a clear sensitivity against all the tested antibiotics with different degrees. Meanwhile the other isolate (K2) demonstrated resistance against ticarcillin, colistin, penicillin, cephalothin, clindamycin and ampicillin. Regarding heavy metals resistance profile, data shown in Table (2) clarified that both isolates exhibited high resistance against chromium (100 µg/mL), zinc and copper (150 µg/mL). At the same time, the bacterial isolate K10 demonstrated a marked resistance against mercury (100 µg/mL), silver (100 µg/mL) and lead (150 µg/mL). On the contrary, isolate K2 exhibited sensitivity against the same heavy metals.
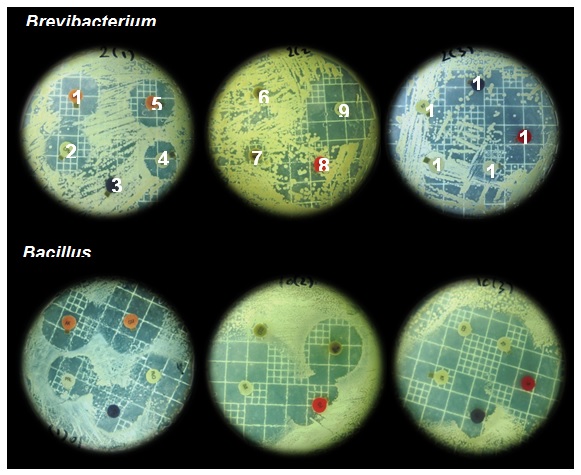
Fig. 1. Shows the effect of some antibiotics on the isolates as shown in Table 2. Where 1: Amikacin (AK), 2:Cefepime (CPM), 3:Ticarcillin (TC), 4:Piperacillin (PRL), 5:Gentamicin(GM), 6:Colistin (CL), 7:Riampicin (RIF), 8:PenicillinG (PG), 9:Imipenem (IMI), 10:Cephalothin (KF), 11:Clindamycin (CD), 12:Cotrimoxazole (TS), 13:Erythromycin (E), 14:Ampicillin (AP).
Table (1):
Physiological profile of the bacterial strains (K2 & K10) isolated from Bani Malek and Al-khoba hot springs, Jazan, KSA using Biolog System (Biolog, Inc., Hayward, USA)
Properties |
K2 |
K10 |
Properties |
K2 |
K10 |
|---|---|---|---|---|---|
Dextrin |
– |
+ |
Glycyl-l-Proline |
– |
+ |
D-Maltose |
+ |
+ |
L-Alanine |
– |
+ |
D-Trehalose |
– |
+ |
L-Arginine |
– |
+ |
D-Cellobiose |
– |
+ |
L-Aspartic Acid |
– |
+/- |
Gentiobiose |
– |
+ |
L-Glutamic Acid |
– |
+ |
Sucrose |
– |
+ |
L-Histidine |
– |
+ |
D-Turanose |
– |
+ |
L-Pyroglutamic Acid |
– |
– |
Stachyose |
– |
– |
L-Serine |
– |
+/- |
pH 6 |
+ |
+ |
Lincomycin |
– |
– |
pH 5 |
– |
+ |
Guanidine HCl |
+ |
– |
D-Raffinose |
– |
– |
Niaproof 4 |
– |
– |
D-Lactose |
– |
– |
Pectin |
+/- |
+ |
D -Melibiose |
– |
+ |
D-Galacturonic Acid |
– |
– |
β-Methyl-D-Glucoside |
– |
+ |
L-Galactonic Acid Lactone |
– |
+ |
D-Salicin |
– |
+ |
D-Gluconic Acid |
– |
+ |
N-Acetyl-d-Glucosamine |
– |
+ |
D-Glucuronic Acid |
– |
– |
N-Acetyl-β-d-Mannosamine |
– |
– |
Glucuronamide |
+/- |
– |
N-Acetyl-d-Galactosamine |
– |
– |
Mucic Acid |
– |
+ |
N-Acetyl Neuraminic Acid |
– |
– |
Quinic Acid |
+/- |
– |
1% NaCl |
+ |
+ |
D-Saccharic Acid |
– |
+ |
4% NaCl |
+ |
+ |
Vancomycin |
– |
– |
8% NaCl |
ND |
+ |
Tetrazolium Violet |
– |
– |
α-d-Glucose |
– |
+ |
Tetrazolium Blue |
– |
– |
D-Mannose |
– |
+ |
p-HydroxyPhenylacetic Acid |
+ |
– |
D-Fructose |
+ |
+ |
Methyl Pyruvate |
– |
+/- |
D-Galactose |
– |
– |
D-Lactic Acid Methyl Ester |
– |
– |
3-Methyl Glucose |
– |
– |
L-Lactic Acid |
– |
+ |
D-Fucose |
– |
– |
Citric Acid |
– |
+ |
L-Fucose |
– |
– |
α-Keto-Glutaric Acid |
– |
– |
L-Rhamnose |
– |
– |
d-Malic Acid |
+ |
– |
Inosine |
– |
– |
L-Malic Acid |
– |
+ |
1% Sodium Lactate |
+ |
+ |
Bromo-Succinic Acid |
– |
– |
Fusidic Acid |
– |
– |
Nalidixic Acid |
– |
– |
D-Serine |
– |
– |
Lithium Chloride |
+ |
+ |
D-Sorbitol |
– |
+ |
Potassium Tellurite |
– |
+ |
D-Mannitol |
– |
+ |
Tween 40 |
– |
– |
D-Arabitol |
– |
– |
γ-Amino-Butryric Acid |
– |
– |
myo-Inositol |
– |
+ |
α-Hydroxy Butyric Acid |
– |
– |
Glycerol |
– |
+ |
β-Hydroxy-d,l-Butyric Acid |
– |
– |
D-Glucose- 6-PO4 |
– |
– |
α-Keto-Butyric Acid |
+/- |
– |
D-Fructose- 6-PO4 |
+/- |
+/- |
Acetoacetic Acid |
+/- |
+/- |
D-Aspartic Acid |
– |
– |
Propionic Acid |
– |
– |
D-Serine |
– |
– |
Acetic Acid |
– |
– |
Troleandomycine |
– |
– |
Formic Acid |
– |
– |
Rifamycine SV |
– |
+ |
Sodium bromate |
– |
+ |
Minocycline |
– |
– |
Sodium butyrate |
+ |
+ |
Table (2):
The antibiotic sensitivity test of the bacterial strains (K2 & K10) isolated from Bani Malek and Al-khoba hot springs, Jazan, KSA
Antibiotic |
Bacterial isolate K2 |
Inhibition zone (mm) |
Bacterial isolate K10 |
Inhibition zone (mm) |
|---|---|---|---|---|
Amikacin |
S |
24 |
S |
26 |
Cefepime |
S |
25 |
S |
25 |
Ticarcillin |
R |
0 |
S |
20 |
Piperacillin |
S |
18 |
S |
21 |
Gentamicin |
S |
25 |
S |
32 |
Colistin |
R |
0 |
S |
30 |
Riampicin |
S |
10 |
S |
26 |
Penicillin |
R |
0 |
S |
26 |
Imipenem |
S |
35 |
S |
50 |
Cephalothin |
R |
0 |
S |
50 |
Clindamycin |
R |
0 |
S |
20 |
Cotrimoxazole |
S |
10 |
S |
30 |
Erythromycin |
S |
20 |
S |
35 |
Ampicillin |
R |
0 |
S |
22 |
Identification of the bacterial isolates
The bacterial isolates (K2 & K10) were Gram-positive rod-shaped cells. According to the metabolic fingerprints (Table 3), the isolate K2 from Bani Malik hot spring was identified as Brevibacterium linens meanwhile the K10 isolate from Al-khoba hot spring was identified as Bacillus subtilis.
Table (3):
The heavy metals resistance profile of Brevibacterium linens and Bacillus subtilis where the sensitivity was calculated by measuring the diameter of clear zone (mm)
| Isolate | Heavy metals | Concentration (µg/ml) | ||
|---|---|---|---|---|
| 25 | 50 | 100 | ||
| K2 | HgCl2 | 5 | 8 | 13 |
| K10 | 0 | 0 | 0 | |
| K2 | CdCl2 | 13 | 17 | 19 |
| K10 | 18 | 20 | 21 | |
| K2 | AgNO3 | 9 | 17 | 21 |
| K10 | 0 | 0 | 0 | |
| K2 | K2Cr2O7 | 0 | 0 | 0 |
| K10 | 0 | 0 | 0 | |
| Isolate | Heavy metals | Concentration (µg/ml) | ||
| 50 | 100 | 150 | ||
| K2 | Pb(NO3)2 | 7 | 10 | 12 |
| K10 | 0 | 0 | 0 | |
| K2 | ZnSO4 | 0 | 0 | 0 |
| K10 | 0 | 0 | 0 | |
| K2 | CuSO4 | 0 | 0 | 0 |
| K10 | 0 | 0 | 0 | |
Optimization of protease and amylase production
In the present study, the production of proteases and amylases of B. linens and B. subtilis was assayed under various temperatures, substrate concentrations in addition to calcium, magnesium and manganese levels. The obtained data (Table 4) showed that protease production of the bacterial strains on skim milk agar was increased with the raise of temperature reaching the maximum rate at 50oC for the two strains. At the same time, 5% skim milk was the optimum concentration for the enzyme production. Regarding the impact of Ca, Mg & Mn on the protease production, the enzyme production was augmented via increasing calcium concentration in the cultures for both strains up to 80 ppm. However the enzyme production of B. subtilis was decreased with increasing of magnesium and maximum protease production was recorded at 20 ppm. In case of Mn, the maximum protease production was achieved at 40 ppm for B. linens and 20 ppm for B. subtilis.
Table (4):
Effect of temperature, substrate concentration in addition to calcium, magnesium and manganese concentrations on the production of protease and amylase enzymes from Brevibacterium linens and Bacillus subtilis. Experiments were carried out in triplicate and diameters of the halo zones were measured by millimeter
| Factors | Brevibacterium linens | Bacillus subtilis | Factors | Brevibacterium linens | Bacillus subtilis | |
|---|---|---|---|---|---|---|
| Protease production | Amylase production | |||||
| Temperature | 30°C | 36 | 37 | 30°C | 17 | 25 |
| 37°C | 38 | 37 | 37°C | 10 | 13 | |
| 50°C | 40 | 41 | 50°C | 8 | 11 | |
| Substrate | 5% | 37 | 39 | 0.2% | 8 | 13 |
| 10% | 35 | 37 | 0.4% | 18 | 28 | |
| 20% | 35 | 37 | 0.6% | 15 | 37 | |
| CaCl2 | 20 ppm | 35 | 33 | 20 ppm | 11 | 20 |
| 40 ppm | 38 | 35 | 40 ppm | 11 | 28 | |
| 80 ppm | 40 | 40 | 80 ppm | 13 | 15 | |
| MgSO4 | 20 ppm | 40 | 40 | 20 ppm | 8 | 18 |
| 40 ppm | 39 | 35 | 40 ppm | 8 | 20 | |
| 80 ppm | 39 | 35 | 80 ppm | 20 | 29 | |
| MnCl2 | 20 ppm | 35 | 40 | 20 ppm | 13 | 16 |
| 40 ppm | 38 | 35 | 40 ppm | 25 | 23 | |
| 80 ppm | 38 | 35 | 80 ppm | 25 | 25 | |
Data in Table (4) demonstrated that the amylase production was steadily decreased on starch agar via rising the temperature and the maximum enzyme production was recorded at 30oC for both strains. At the same time, 0.4% & 0.6% starch were the optimum concentrations for the amylase production from B. linens and B. subtilis, respectively. Regarding the impact of Ca on the amylase production, the maximum enzyme production was recorded at 80 ppm for B. linens and 40 ppm for B. subtilis. In case of Mg and Mn, the amylase production was increased for both strains via increasing Mg and Mn up to 80 ppm.
Geothermal areas which are favorable habitats for thermophilic organisms are limited to restricted sites all over the world. In Saudi Arabia there are about 10 geothermal springs that are distributed in Jazan and Al-Lith areas. The microorganisms in geothermal springs are fastidious and exist in limited number. The total count of bacteria in hot spring water was low and ranged between 3 x 10-2 CFU mL-1 in Alkhoba sample and 2 x 10-3 CFU mL-1 in Bani Malek sample. A similar total count trend of thermophilic bacteria in water samples collected from geothermal springs in Jazan, Saudi Arabia were recorded12. Also, a very low number of thermophilic bacteria were recorded in four hot springs in Morocco11. The low density of the bacterial populations in hot springs could be attributed to the adverse conditions of these environments or due to the application of culture-dependent identification approaches that identify only a small portion of the total microbial communities26.
Although the bacterial isolate (K10) exhibited sensitivity against all the tested antibiotics, the isolate (K2) demonstrated resistance against some antibiotics. Moreover, both thermophilic strains showed resistance against different heavy metals. The positive correlation between heavy metals resistance and antibiotic resistance could be ascribed to the co-existence of heavy metals and antibiotics in the environment. Some studies have clarified the relation between the abundance of antibiotic resistance genes and the elevated concentrations of antibiotic and heavy metals in environments27,28. At the same time, low levels of heavy metals could play a role in the emergence of antibiotic resistance in the resistant bacteria or induce antibiotic resistance in sensitive bacteria29.30.
Based on the metabolic fingerprint, the bacterial isolates K2 and K10 were identified as Brevibacterium linens and Bacillus subtilis, respectively. In fact, Brevibacterium is a unique genus of the Brevibacteriacea family located in the suborder Micrococcineae, order Actinomycetales, and class Actinobacteria. Brevibacterium is a Gram-positive bacterium that can tolerate high salt concentrations (8–20%), and is capable of growing in a broad pH range31. B. subtilis is a Gram-positive bacterium that is found in soil and the gastrointestinal tract of ruminants and humans. B. subtilis can form endospore allowing it to tolerate extreme environmental conditions. It can secrete proteins into the medium which is considered as a major advantage. This allows the accumulation of the native product with high yield and relatively in a pure form. Moreover, B. subtilis has no known pathogenic interaction with human or animals32.
Proteases production by microorganisms is affected by the medium compositions in addition to the environmental conditions. Thus, it is important to explore the influencing factors to attain the maximum protease production. Cultivation temperature affects protein synthesis by influencing rate of biochemical reactions within the cell and consequently inducing or repressing enzyme production33. The present study showed that the optimum temperature of protease production was 50oC and 5% skimmed milk at a constant pH of 7.5 for B. linens and B. subtilis. Meanwhile, the optimum amylase production was recorded at 30oC and 0.4 – 0.6 % starch for the two strains. Previous studies have reported a similar finding on a Bacillus sp. with maximum protease production at 45 oC34,35. At the same time, it was reported a lower optimum temperature of 30°C for protease production by Bacillus sp. The obtained data showed that 5% skim milk was the optimum concentration for protease production of the two strains and the increase above that level led to a decrease of protease yield36. Protease is an inducible enzyme which needs a substrate to encourage the microbial cell to produce it. Consequently, the increase of skim milk concentration in the fermentation medium led to increase of protease production until the skim milk concentration reduced the dissolved oxygen in fermentation medium resulting in a feedback inhibition of the protease production37. Regarding the influence of metal ions on the protease production and activity, Ca2+ showed a positive effect on proteases of both strains meanwhile Mg2+ and Mn2+ demonstrated a minor effect. These results indicated that protease requires metal ions as cofactors. These findings correlate with the observations of Kunamneni et al.38 and Sayem et al.39 who found that the activity of protease from Bacillus subtilis and Bacillus licheniformis was accelerated by the addition of Mg2+, Ca2+ and Mn2+. It could be suggested that these metal ions could play a vital role in protecting the enzyme against thermal denaturation in addition to their task for maintaining the active conformation of the enzyme at higher temperatures40.
This study clarified that maximum amylase production was recorded at 30 oC for both strains and the production rate was reduced by increasing the temperature. These findings are in accordance with those reported who recorded that 30oC was the optimum temperature for the production of amylase from Bacillus subtilis41. Similarly, It was showed that the maximum yield of amylase produced from Brevibacterium linens was achieved at 32.5 oC42. The decrease of the amylase production by increasing the temperature above 30 oC could be attributed to the loss of moisture in the substrate which adversely affects the metabolic activities of the microbes leading to reduced growth and decline in enzyme production43. The obtained data showed that 0.4 – 0.6% starch was the optimum concentration for amylase production. Excess of starch in the medium could be responsible for feedback inhibition of theamylase production. The current study showed that Ca2+, Mg2+ and Mn2+ have a positive effect on the amylase production and activity of both strains. These results are in harmony with previous studies who recorded that the activities of amylases of Bacillus subtilis and Bacillus amyloliquefaciens were stimulated in the presence of some metal ions such as Ca2+, Ba2+, Mg2+, Li2+, Fe2+ and Mn2+44,45. It is also well established that calcium has a vital task in the thermal stabilization of a-amylases46,47.
Thermophilic bacteria and their hydrolytic enzymes have become a chief research subject due to their valuable biotechnological applications. The current study highlighted the capability of the thermophilic bacteria Brevibacterium linens and Bacillus subtilis for the production of amylolytic and proteolytic enzymes. The optimum conditions of the production of these enzymes were achieved at 50oC for both strains at 5% skimmed milk for protease of B. linens and 10% for B. subtilis while 0.6% starch recorded the highest amylase production for both strains. At the same time, Ca2+, Mg2+ and Mn2+ demonstrated a positive impact on the production rate of these enzymes. Further studies are in progress for the isolation and purification of protease and amylase from different thermophilic bacteria to evaluate their application in different commercial fields.
ACKNOWLEDGMENTS
The authors gratefully acknowledge all the staff of the Biology Department, Faculty of Science, Jazan University, KSA especially Dr. Hassien Elnasheri.
- Al-Dayel, M. Geothermal resources in Saudi Arabia. Geothermics, 1988; 17(2-3): 465 – 476.
- Rehman, S., Shash, A. Geothermal resources of Saudi Arabia–country update report. Proceedings World Geothermal Congress, 2005.
- Khalil,A. Isolation and characterization of three thermophilic bacterial strains (lipase, cellulose and amylase producers) from hot springs in Saudi Arabia. African Journal of Biotechnolog, 2011; 10(44): 8834-8839.
- Panda, M.K., Sahu, M.K., Tayung, K. Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. Iran J Microbiol, 2013; 5(2): 159–165.
- Adiguzel, A.,Ozkan, H.,Baris, O.,Inan, K.,Gulluce, M., Sahin, F. Identification and characterization of thermophilic bacteria isolated from hot springs in Turkey. J. Microbiology Method, 2009; 79: 321- 328.
- Maugeri, T. L., Gugliandolo, C., Caccamo, D.,Stackebrandt, E. A Polyphasic Taxonomic Study of Thermophilic Bacilli from Shallow, Marine Vents. Systematic and Applied Microbiology, 2001; 24: 572-587.
- Derekova, A., Mandeva, R., Kambourova, M. Phylogenetic diversity of thermophilic carbohydrate degrading Bacilli from Bulgarian hot springs. World J. Microbiology and Biotechnology, 2008; 24: 1697-1702.
- Sievert, S.M., Ziebis, W., Kuever, J., Sahm, K. Relative abundance of Archaea and Bacteria along a thermal gradient of a shallowwater hydrothermal vent quantified by rRNA slot-blot hybridization. Microbiology, 2000; 146: 1287-1293.
- Lau, M.C., Aitchison, J.C., Pointing, S.B. Bacterial community composition in thermophilic microbial mats from five hot springs in central Tibet. Extremophiles, 2009; 13: 139-149.
- Verma, A., Gupta, M., Shrikot, P. Isolation and characterization of thermophilic bacteria in natural hot water springs of Himachal Pradesh (India). Bioscan, 2014; 9:947-952.
- Aanniz, T., Ouadghiri, M., Melloul, M., Swings, J., Elfahime, E., Ibijbijen, J.,Amar, M. Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Brazilian Journal of Microbiology, 2015; 46(2):443-453.
- Khiyami, M.A., Serour, E.A., Shehata, M.M., Bahklia, A.H. Thermo-aerobic bacteria from geothermal springs in Saudi Arabia. African Journal of Biotechnology, 2012; 11(17):4053-4062.
- Haki, G.D., Rakshit S.K. Developments in industrially important thermostable enzymes: a review. Bioresour Technol, 2003; 89:17–34.
- Bouzas, T.M., Barros-Velázquez, J., Villa, T.G. Industrial applications of hyperthermophilic enzymes: a review. Protein Pept Lett, 2006; 13:645–651.
- El-Gayar, K.E., Zaghloul, T. I.,Haroun, M.A, Saeed, H.M. Purification of alkaline protease from hydrolyzed chicken feather waste using recombinant B. subtilis strain. Scientific journal of King Faisal university, KSA, 2012; 13(1): 1433.
- Al Abboud, M.A. Isolation and Identification of protease producing bacterial strain from Jazan province, KSA. Journal of Jazan University – Applied Sciences Branch, 2014; 3(1).
- Mienda, B.S., Yahya, A., Galadima, I.A., Shamsir, M.S. An overview of microbial proteases for industrial applications. Res J Pharm Biol Chem Sci, 2014; 5: 388-396.
- Pednekar, P., Jain, R., Mahajan G. Anti-infective Potential of Hot-spring Bacteria. J Glob Infect Dis, 2011; 3(3): 241–245.
- Yang, C.H., Liu, W.H. Purification and properties of a maltotriose-producing a-amylase from; Thermobifidafusca. Enzyme MicrobTechnol, 2004; 35(2): 254–60.
- Paul,D. Microorganisms and a-amylase: A concise review. Innovare journal of sciences, 2016; 4(4).
- Essa, A. M. M.,Khallaf M. K. Antimicrobial potentiality of consolidation polymers impregnated with copper nanoparticles. BMC Microbiology, 2016; 16:144.
- Agwa, O.K., Uzoigwe, C.I., Wokoma, E.C. Incidence and antibiotic sensitivity of Bacillus cereus isolated from ready to eat foods sold in some markets in Portharcourt, Rivers state, Nigeria. Asian J. Microbiol. Biotech. Env. Sc, 2012; 14 (1) : 13-18.
- Essa A.M.M. Macaskie L.E. and Brown N.L. 2005. A new method for mercury removal. Biotechnology Letters 27(21): 1649-55.
- Essa, A.M.M. Effect of continuous mercury stress on mercury reducing community of some characterized bacterial strains. Afr J Microbiol Res, 2012; 6: 1255-1261.
- Qadeer, M.A., Aurangzeb, M., Igbal, J. Production of raw starch hydrolyzing enzymes by mould cultures. Proceeding of the International Symposium on Biotechnology for Energy, Malik, K. A.; Nagvi, S.H. M. and Aleem, M.I. H. (eds.) Faisalabad, Pakistan,1989; pp119-128.
- Ranjard, L., Poly, F., Nazaret, S. Monitoring complex bacterial community using culture-independent molecular techniques: application to soil environment. Res Microbiol, 2000; 151: 167-177.
- Knapp, C.W., McCluskey, S.M., Singh, B.K., Campbell, C.D., Hudson, G., Graham, D.W. Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived Scottish soils. PLoS ONE, 2011; 6: 27300.
- Zhu, Y.G., Johnson, T.A., Su, J.Q., Qiao, M., Guo, G.X., Stedtfeld, R.D., Hashsham, S.A., Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA.2013; 110: 3435–3440.
- Martinez, J.L., Rojo, F. Metabolic regulation of antibiotic resistance. FEMS Microbiol. Rev, 2011; 35: 768–789.
- Chen, S., Li, X., Sun, G., Zhang, Y., Su, J., Ye, J. Heavy metal induced antibiotic resistance in bacterium LSJC7. International journal of molecular sciences, 2015; 16(10):23390-23404.
- Motta, A.S., Brandelli, A. Properties and antimicrobial activity of the smear surface cheese coryneform bacterium Brevibacterium linens. European Food Research and Technology, 2008; 227(5): 1299-1306.
- Errington, J., Mountain, A: Is Bacillus an alternative expression system? In: Protein Production by Biotechnology.T.J.R. Harris (ed.), Elsevier Science Publishers Ltd., England, 2008; 1–14.
- Bakermans, C., Nealson, K.H. Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobactercryopegella. Journal of Bacteriology, 2004; 186: 2340-2345.
- Sepahy, A.A., Jabalameli, L. Effect of culture conditions on the production of an extracellular protease by Bacillus sp. isolated from soil sample of Lavizan Jungle Park. Enzyme Research, 2011; Article ID: 219628.
- Olajuyigbe, F.M. Optimized production and properties of thermostable alkaline protease from Bacillus subtilis SHS-04 grown on groundnut (Arachishypogaea) meal. Advances in Enzyme Research, 2013; 1(4):112.
- Gouda, M.K. Optimization and purification of alkaline proteases produced by marine Bacillus sp. MIG newly isolated from Eastern Harbour of Alexandria. Polish Journal of Microbiology, 2006; 55: 119-126.
- Chu, W.H. Optimization of extracellular alkaline protease production from species of Bacillus. Journal of industrial microbiology & biotechnology, 2007; 34(3): 241-245.
- Kunamneni, A., Poluri, E.,Davuluri, S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS Pharm Sci Tech, 2003; 4:1-9.
- Sayem, S.A., Alam, M.J., Hoq, M.M. Effect of temperature, pH and metal ions on the activity and stability of alkaline protease from novel Bacillus licheniformis MZK03. Proceedings-Pakistan Academy of Sciences, 2006; 43(4):257.
- Sharma, K.M., Kumar, R., Panwar, S., Kumar, A. Microbial alkaline proteases: Optimization of production parameters and their properties. J Genetic Engineering and Biotechnology, 2017.
- Nanganuru, H., Korrapati, N., Mutyala, S. Studies on the production of á-amylase by Bacillus subtilis. IOSR J. Eng, 2012; 2(5): 1053-1055.
- Shabbiri, K., Adnan, A., Noor, B.,Jamil, S. Optimized production, purification and characterization of alpha amylase by Brevibacterium linens DSM 20158, using bio-statistical approach. Annals of microbiology, 2012; 62(2):523-532.
- Prakasham,R.S., Rao, Ch.S., Rao,R.S. Sarma P.N. Enhancement of acid amylase production by an isolated Aspergillus awamori. Journal of applied microbiology, 2007; 102(1): 204–211.
- Demirkan, E. Production, purification, and characterization of alpha-amylase by Bacillus subtilis and its mutant derivate. Turkish Journal of Biology, 2011; 35(6): 705-712.
- Deb, P., Talukdar, S.A., Mohsina, K., Sarker, P.K., Sayem, S.A. Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus, 2013; 2(1):154.
- Gupta, R., Gigras, P., Mohapatra, H., Goswami, V.K., Chauhan,B. Microbial a-amylases: a biotechnological prospective. Process Biochem, 2003; 38: 1599–1616.
- Vengadaramana, A. Industrial Important Microbial alpha-Amylase on Starch-Converting Process. Scholars Academic Journal of Pharmacy (SAJP), 2013; 2(3):209-221.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


