ISSN: 0973-7510
E-ISSN: 2581-690X
Trichoderma species are potential fungal bio-control agents used against a wide range of soil borne plant pathogens. In the present study a total of 20 Trichoderma isolates viz., AT1, AT2, AT3, AT4, AT5, AT6, AT7, BT1, BT2, BT3, BT4, BT5, BT6, BT7, BT8, BT9, BT10, BT11, BT12, BT13 were isolated from vegetable fields of Kashmir valley and their efficacy was tested by using various biochemical tests. Thirteen isolates of Trichoderma viz., AT1, AT2, AT3, AT4, AT5, AT6, AT7, BT1, BT7, BT8, BT10, BT11 and BT12 were found to be positive for ammonia production. Similarly twelve isolates viz., AT2, AT3, AT7, BT1, BT3, BT4, BT7, BT8, BT10, BT11, BT12 and BT13 were found to be positive as far as the chitinase activity is concerned. In the IAA production assay maximum IAA was produced by isolate BT9 (6.605 µg mL-1) followed by BT6 (5.278 µg mL-1), while minimum IAA was produced by isolate AT1 (1.538 ìg mL-1). Only five isolates viz., AT1, AT2, AT3, AT4 and AT5 metabolized lactose and sucrose while seven isolates viz., AT1, AT2, AT3, AT4, AT5, AT6 and AT7 were found to metabolize maltose. Hydrocyanic acid (HCN) was observed to be produced by only three isolates of Trichoderma viz., AT3, AT5 and AT7. Trichoderma isolate AT3 qualifying most of the biochemical tests were morphological characterized as Trichoderma harzianum.
Trichoderma, Biochemical Characterization, Morphological Characterization.
The practices of integrated agricultural management, where chemicals are either replaced or minimized by by-products, are the most suitable option (Cavalcante et al., 2008). Biological control agents (BCAs) are by-products based on microorganisms that cause harmful alterations to plant pathogens by chemical or physical processes (Vinale et al., 2008). BCA differ from chemical agents in that to be effective, they need to grow and successfully colonize and therefore they need to be applied in high and frequent quantities (Lo et al., 1998). Fungi from the genus Trichoderma spp. have a long history of successful control of plant diseases (Benitez et al., 2004). Trichoderma play an important role not only in controlling the plant diseases but also in increasing growth and yield of the plants (Chadha et al., 2015), so different biochemical attributes leading to its effective use against various pathogens are of paramount importance. There is a vast number of biochemical being released by Trichoderma species to bring about suppression of a huge number of phytopathogens and these biochemicals may be released constitutively or may be induced by the pathogen presence (Aarti and Meenu, 2015; Rawat and Tewari, 2011) .
What we observe and define as biocontrol may be the culmination of a number of different mechanisms working synergistically to achieve disease control. Our knowledge of the complexity of these systems is currently limited by our ability to perceive them, and a great deal of research will have to be undertaken in order to fathom exactly what is taking place during the biocontrol process. As with so many other aspects of science, basic knowledge about the mechanisms involved in the biocontrol process will be of immense value to that intent on developing new methods for utilizing biocontrol agents. With this aim present study was undertaken to study the various biochemicals involved in the process of plant growth promotion by Trichoderma species.
Sample Collection
Rhizospheric soil samples were collected from various commercially grown chilli fields and kitchen gardens of district Anantnag (Bangidar, BagiWanpoh, Danter, Harnag) and Baramulla (Delina, Arampora, Palhalan and Johama) of Kashmir valley. Twenty (20) samples from each district were randomly collected, out of which 10 were taken from commercially grown chilli fields and 10 from local kitchen gardens.
Biochemical Characterization
Trichoderma species isolated from soil samples were characterized for carbohydrate metabolization (Bakker and Schipper, 1987), phosphate solubilization (Pikovskaya, 1948), ammonia (Bakker and Schipper, 1987), chitinase (Okay et al., 2008), HCN (Bakker and Schipper, 1987) and IAA production (Brick et al., 1991).
Morphological Characterization
Distinct cultural and morphological characteristics were observed for identification, and the plates were stored at 4°C. Cultural and morphological characteristics which were studied include colony growth rate, colony colour, reverse colour, colony edge, mycelial form, conidiophore branching, conidial colour and presence or absence of chlamydospores (Shahid et al., 2013).
Isolation of Trichoderma species from vegetable fields
A total of 20 (7 from Anantnag and 13 from Baramulla) Trichoderma isolates (plate 1) were isolated from 40 randomly collected rhizospheric soil samples from various commercially grown chilli fields and kitchen gardens of district Anantnag and Baramulla of Kashmir valley by using Trichoderma specific medium. Nomenclature given to these Trichoderma isolates, the collection sites and source of soil samples is detailed in Table 1. Similarly Trichoderma species were isolated from chilli rhizosphere by Wani et al. (2014) and the technique used for isolation of Trichoderma species is in agreement with chaudhari et al. (2011) and Khandelwal et al. (2012).
Table (1):
Details of Trichoderma isolates isolated from rhizospheric soils of chilli fields.
| District | Location | Isolate name | Source |
|---|---|---|---|
| Anantnag | Danter | AT1 | Kitchen garden |
| Bangidar | AT2 | Commercial field | |
| Bangidar | AT3 | Commercial field | |
| Harnag | AT4 | Commercial field | |
| Harnag | AT5 | Kitchen garden | |
| Bagi-Wanpoh | AT6 | Commercial field | |
| Bagi-Wanpoh | AT7 | Commercial field | |
| Baramulla | Delina | BT1 | Kitchen garden |
| Delina | BT2 | Kitchen garden | |
| Delina | BT3 | Kitchen garden | |
| Arampora | BT4 | Commercial field | |
| Arampora | BT5 | Commercial field | |
| Arampora | BT6 | Commercial field | |
| Arampora | BT7 | Commercial field | |
| Palhalan | BT8 | Commercial field | |
| Palhalan | BT9 | Commercial field | |
| Palhalan | BT10 | Commercial field | |
| Johama | BT11 | Kitchen garden | |
| Johama | BT12 | Kitchen garden | |
| Johama | BT13 | Kitchen garden |
Screening of Trichoderma isolates for ammonia production, chitinase activity, HCN production and phosphate solubilisation
During ammonia production test thirteen isolates (AT1, AT2, AT3, AT4, AT5, AT6, AT7, BT1, BT7, BT8, BT10, BT11 and BT12) were found to be positive and seven isolates (BT2, BT3, BT4, BT5, BT6, BT9 and BT13) were found negative for ammonia production (Table 2). These findings are in agreement with the earlier reports (Aarti and Meenu, 2015). Similar findings were reported by Chadha et al. (2015) in Mucor hiemalis, Aspergillusnigerand Fusarium moniliforme. Ammonia production by T. harzianum, KT6, SE6, KT28 and BRT11 has widely been documented (Rawat and Tewari, 2011) as means to offer nitrogen to plant and culminate the pathogens in the vicinity as a result of its toxicity. The production of lytic enzymes by Trichoderma species is known as one of the major mechanisms for biocontrol activity against phytopathogenic fungi (Lorito et al., 1994). Chitinase activity is one of the important beneficial character exhibited by Trichoderma species as chitinases are known to contribute to the biocontrol properties of Trichoderma atroviride (Limon et al., 1999; Woo et al., 1999; Viterbo et al., 2001). This might be due to the reason that chitinases attack directly on the fungal structural components (Sela-Buurlage et al., 1993), while checking chitinase activity twelve isolates (AT2, AT3, AT7, BT1, BT3, BT4, BT7, BT8, BT10, BT11, BT12 and BT13) were found to be positive and eight isolates (AT1, AT4, AT5, AT6, BT2, BT5, BT6 and BT9) were found negative (Table 2). This is in agreement with earlier reports (Sharaf et al., 2012). Lytic enzymes like chitinases and b-1,3-glucanases, proteases and cellulases are potential mechanism associated with the ability of Trichoderma to control phytopathogens (Harighi et al., 2007).
Table (2):
Screening of various isolates of Trichoderma species for ammonia production, chitinase activity, HCN production and Phosphate solubilisation.
S. No. |
Isolates |
Ammonia production |
Chitinase activity |
HCN production |
Phosphate solubilisation |
|---|---|---|---|---|---|
1 |
AT1 |
+ |
– |
– |
– |
2 |
AT2 |
+ |
+ |
– |
– |
3 |
AT3 |
+ |
+ |
+ |
– |
4 |
AT4 |
+ |
– |
– |
– |
5 |
AT5 |
+ |
– |
+ |
– |
6 |
AT6 |
+ |
– |
– |
– |
7 |
AT7 |
+ |
+ |
+ |
– |
8 |
BT1 |
+ |
+ |
– |
– |
9 |
BT2 |
– |
– |
– |
– |
10 |
BT3 |
– |
+ |
– |
– |
11 |
BT4 |
– |
+ |
– |
– |
12 |
BT5 |
– |
– |
– |
– |
13 |
BT6 |
– |
– |
– |
– |
14 |
BT7 |
+ |
+ |
– |
– |
15 |
BT8 |
+ |
+ |
– |
– |
16 |
BT9 |
– |
– |
– |
– |
17 |
BT10 |
+ |
+ |
– |
– |
18 |
BT11 |
+ |
+ |
– |
– |
19 |
BT12 |
+ |
+ |
– |
– |
20 |
BT13 |
– |
+ |
– |
– |
HCN production is also an important trait found in various soil micro-organisms as it indirectly promotes plant growth by controlling some soil borne diseases (Kremer and Souissi, 2001; Siddiqui et al., 2006), while screening for HCN only three isolates (AT3, AT5 and AT7) were found positive and rest were found negative. This is in agreement with earlier reports (Aarti and Meenu., 2015). The metabolite HCN production by fungal isolates SE6, KT28 and BRT11 has widely been reported as a possible mechanism of disease control (Rawat and Tewari 2011). Similar results were obtained by Ngoma et al. (2013) in case of bacterial isolates. While in case of phosphate solubilisation test, none of the isolate was found to be positive. Trichoderma species are widely known for releasing phosphatases into soil and release the otherwise unavailable phosphate forms from tricalcium phosphate primirily (Saraf et al., 2013). Phosphate solubilizing efficiency of different isolates of T. harzianum was observed by Tallapragada and Gudimi (2011) on Sperber‘s medium with modifications and it was observed that many of the isolates of Trichoderma isolates do not solubilize phosphate.
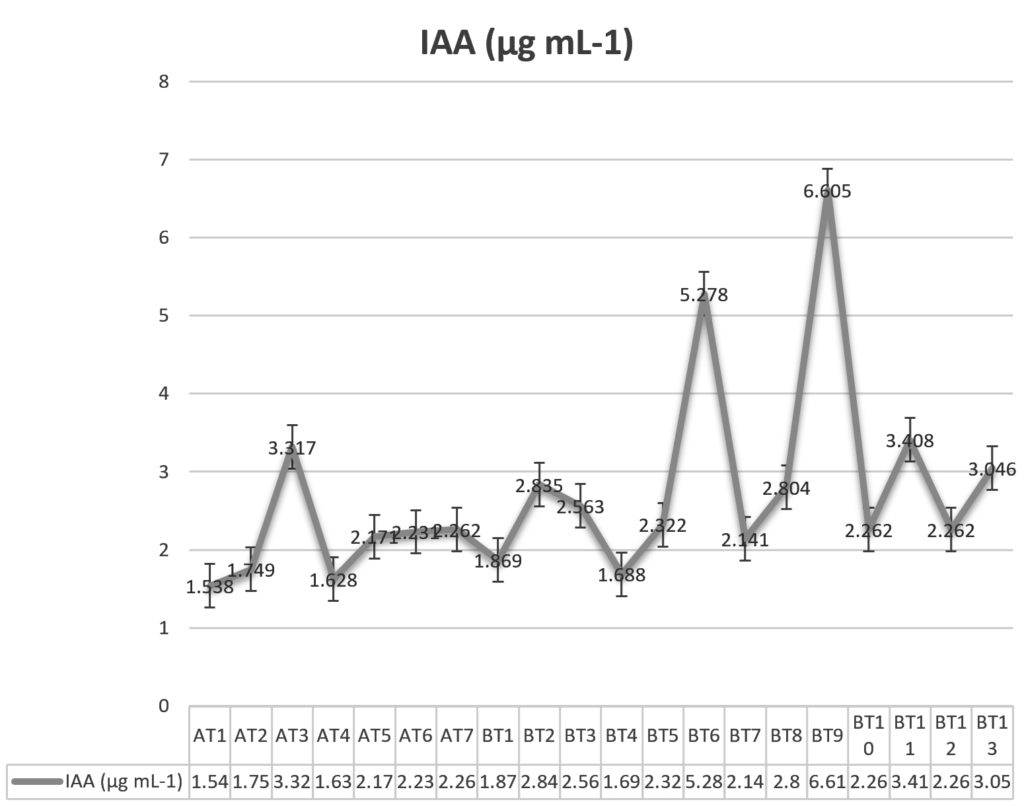
Production of IAA by various isolates of Trichoderma specie
Microbial synthesis of the phytohormone auxin (indole-3-acetic acid/indole acetic acid/IAA) has been known for a long time. It is reported that 80% of microorganisms isolated from the rhizosphere of various crops possess the ability to synthesize and release auxins as secondary metabolites (Patten and Glick, 1996). During IAA production test all the isolates were found to produce IAAhowever their production amount varied considerably. Maximum IAA was produced by isolate BT9 (6.605 ìg mL-1) followed by BT6 (5.278 ìg mL-1), BT11 (3.408 ìg mL-1) and AT3 (3.317 ìg mL-1) while minimum IAA was produced by isolate AT1 (1.538 ìg mL-1) (Fig. 1). Resende et al. (2014) also reported the production of IAA by various isolates of Trichoderma and the amount of IAA produced varied from as low as 1.21ìg mL-1 and as high as 2.18 ìg mL-1and thus supporting our findings. Similar findings were recorded by Aarti and Meenu (2015), Gravel et al. (2007) and Badawi et al. (2011) in Trichoderma species.
Carbohydrate metabolization by various isolates of Trichoderma species
During these tests, it was found that all the isolates metabolized glucose. Similarly all the isolates metabolized Fructose except isolates BT5 and BT6. It was found that only five isolates (AT1, AT2, AT3, AT4 and AT5) metabolized lactose and sucrose while seven isolates (AT1, AT2, AT3, AT4, AT5, AT6 and AT7) were found to metabolize maltose (Table 3). Kubicek et al. (2003) also detected the species-specific metabolic properties of Trichoderma. The carbon sources supported best growth in all species detected were: d-mannitol, N-acetyl-d-glucosamine, l-erythritol, glycerol, fructose, fucose, l-arabinose, d-galactose, and xylitol and thus authenticatingour findings further even though there were some isolates lacking the ability to metabolize lactose and sucrose may be as a result of certain enzymatic complications. Similar findings were observed by Monga (2001) and Mehta et al. (2012).
Table (3):
Cultural and morphological characteristics of Trichoderma isolates.
| Name of the isolate | Colony color | Colony edge | Common characters among all isolates |
|---|---|---|---|
| AT1 | Dark green | Wavy | Conidial color: Green Conidiophore
branching: Highly branched regular Mycelial form Floccose to Arachnoid Reverse color : Colorless Colony growth rate (cm/day) : 8-9 in 3 days Mycelial color : Watery white |
| AT2 | Dark green | Wavy | |
| AT3 | Dark green | Wavy | |
| AT4 | Dark green | Wavy | |
| AT5 | Dark green | Wavy | |
| AT6 | Dark green | Wavy | |
| AT7 | Dark green | Wavy | |
| BT1 | Light green | Wavy | |
| BT2 | Light green | Wavy | |
| BT3 | Light green | Smooth | |
| BT4 | Light green | Smooth | |
| BT5 | Light green | Smooth | |
| BT6 | Light green | Wavy | |
| BT7 | Light green | Wavy | |
| BT8 | Light green | Wavy | |
| BT9 | Light green | Smooth | |
| BT10 | Light green | Smooth | |
| BT11 | Light green | Smooth | |
| BT12 | Light green | Smooth | |
| BT13 | Light green | Smooth |
Cultural and morphological characterization of Trichoderma isolate (AT3)
Although cultural and morphological characteristics of all the isolates were studied, our main focus was on the potential Trichoderma isolate (AT3). The characteristics which were studied include colony growth rate, colony colour, reverse colour, colony edge, mycelial form, conidiophore branching, conidial colour and presence or absence of chlamydospores (Table 4). After studying these characteristics Trichoderma isolate (AT3) was found to resemble Trichoderma harzianum.
Table (4):
Carbohydrate metabolisation by various isolates of Trichoderma species.
| S. No. | Isolates | Carbohydrate metabolisation | ||||
|---|---|---|---|---|---|---|
| Glucose | Fructose | Lactose | Sucrose | Maltose | ||
| 1 | AT1 | + | + | + | + | + |
| 2 | AT2 | + | + | + | + | + |
| 3 | AT3 | + | + | + | + | + |
| 4 | AT4 | + | + | + | + | + |
| 5 | AT5 | + | + | + | + | + |
| 6 | AT6 | + | + | – | – | + |
| 7 | AT7 | + | + | – | – | + |
| 8 | BT1 | + | + | – | – | – |
| 9 | BT2 | + | + | – | – | – |
| 10 | BT3 | + | + | – | – | – |
| 11 | BT4 | + | + | – | – | – |
| 12 | BT5 | + | – | – | – | – |
| 13 | BT6 | + | – | – | – | – |
| 14 | BT7 | + | + | – | – | – |
| 15 | BT8 | + | + | – | – | – |
| 16 | BT9 | + | + | – | – | – |
| 17 | BT10 | + | + | – | – | – |
| 18 | BT11 | + | + | – | – | – |
| 19 | BT12 | + | + | – | – | – |
| 20 | BT13 | + | + | – | – | – |
ACKNOWLEDGMENTS
The financial assistance from the University Grants Commission, New Delhi through a research project (No. 43-15/2014 (SR) that supported this study is gratefully acknowledged.
- Aarti, T and Meenu, S. Role of volatile metabolites from Trichoderma citrinoviride in biocontrol of phytopathogens. International Journal of Research in Chemistry and Environment., 2015; 5: 86-95.
- Badawi, F.S.F., Biomy, A.M.M and Desoky, A.H. Peanut plant growth and yield as influenced by co-inoculation with Brady-rhizobium and some rhizo-micro-organisms under sandy loam soil conditions. Annual Journal of Agricultural Science., 2011; 56: 17-25.
- Bakker, A.W. and Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas species mediated plant growth stimulation. Soil Biology and Biochemistry., 1987; 19: 451-457.
- Bakker, A.W. and Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas species mediated plant growth stimulation. Soil Biology and Biochemistry. 1987; 19: 451-457.
- Benítez, T., Rincon, A.M., Limon, M.C. and Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol, 2004; 7: 249-260.
- Brick, J.M., Bostock, R.M. and Silvertsone, S.E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Applied Environmental Microbiology. 1991; 57: 535-538.
- Brick, J.M., Bostock, R.M. and Silvertsone, S.E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Applied Environmental Microbiology. 1991; 57: 535-538.
- Cavalcante, R.S., Lima, H.L.S., Pinto, G.A.S., Gava, C.A.T. and Rodrigues, S. Effect of moisture on Trichoderma conidia production on corn and wheat bran by solid state fermentation. Food Bioprocess Technol. 2008; 1: 100-104.
- Chadha, N., Prasad, R. and Varma, A. Plant promoting activities of fungal endophytes associated with tomato roots from central himalya, India and their interaction with Piriformosporaindica. International Journal of Pharma and Bio Sciences. 2015; 6: 333 – 343.
- Chaudhari, P. J., Shrivastava, P and Khadse, A. C. Substrate evaluation for mass cultivation of Trichoderma viride. Asiatic Journal of Biotechnology Resources. 2011; 2: 441-446.
- Gravel, V., Antoun, H. and Tweddel, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil biology and Biochemistry. 2007; 39: 1968-1977.
- Harighi, M.J., Zamani, M.R. and Motallebi, M. Evaluation of antifungal activity of purified chitinase 42 from Trichoderma atroviride PTCC5220, Biotechnol. 2007; 6: 28-36.
- Khandelwal, M., Datta, S., Mehta, J., Naruka, R., Makhijani, K., Sharma, G., Kumar, R. and Chandra, S. Isolation, characterization & biomass production of Trichoderma viride using various agro products- a biocontrolagent. Advances in Applied Science Research. 2012; 3: 3950-3955.
- Kremer, R. J. and Souissi, T. Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Current Microbiology. 2001; 43: 182-186.
- Kubicek, C.P., Bissett, J., Druzhinina, I., Gradinger, C.K. and Szakacs, G. Genetic and metabolic diversity of Trichoderma: a case study on South-East Asian isolates. Fungal Genetics and Biology. 2003; 38: 310–319.
- Limon, M.C., Pintor-Toro, J.A. and Benitez, T. Increased antifungal activity of Trichoderma harzianum transformants that overexpress a 33-KDa chitinase. Phytopathology. 1999; 89: 254-261.
- Lo, C.T., Nelson, E.B., Hayes, C.K. and Harman, G.E. Ecological Studies of Transformed Trichoderma harzianum Strain 1295-22 in the Rhizosphere and on the Phylloplane of Creeping Bentgrass. Phytopathology. 1998; 88: 129-136.
- Lorito, M., Hayes C.K., Di Pietro A., Woo S.L. and Harman G.E. Purification, characterization, and synergistic activity of a glucan-b-1,3-glucosidase and a N-acetyl-b-glucosaminidase from Trichoderma harzianum, Phytopathol. 1994; 84: 398-406.
- Mehta, J., Khandelwal, M., Datta, S., Naruka, R, Makhijani, K., Sharma, G., Kumar, R. and Chandra, S. Isolation, characterization and biomass production of Trichoderma viride using various agro-products. Advances in Applied Science Research. 2012; 3: 3950-3955.
- Monga, D. Effect of carbon and nitrogen sources on spore germination, biomassproduction and antifungal metabolites by species of Trichoderma and Gliocladium. Indian Phytopathology. 2001; 54: 435-4337.
- Ngoma, L., Esau, B. and Babalola, O. Isolation and characterization of beneficial indigenous endophytic bacteria for plant growth promoting activity. African Journal of Biotechnology. 2013; 12: 4105-4114.
- Okay, S., Ozdal, M. and Kurbnoglu, E.B. Characterisation, antifungal activity and cell immobilization of a chitinase from Serratia marcescens MO-1. Turkish Journal of Biology. 2008; 37: 639-644.
- Okay, S., Ozdal, M. and Kurbnoglu, E.B. Characterisation, antifungal activity and cell immobilization of a chitinase from Serratia marcescens MO-1. Turkish Journal of Biology. 2008; 37: 639-644.
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologiya. 1948; 17: 362-370.
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologiya. 1948; 17: 362-370.
- Rawat, R. and Tewari, L. Effect of abiotic stress on phosphate solubilisation by biocontrol fungus Trichoderma species. Current Microbiology. 2011; 62: 1521-1526.
- Resende, M.P., Jakobu, I.C.M.C., Santos, L.C.R.D., Soares, M.A., Pereira, F.D., Souchie, E.L. and Silava, F.G. Phosphate solubilization and phytohormone production by endophytic and rhizosphere Trichoderma isolates of guanandi (CalophyllumbrasilienseCambess). Afarican Journal of Microbiology Research. 2014; 8: 2616-2623.
- Saraf, M., Thakkar, A., Pandya, U., Joshi, M. and Parikh, J. Potential of plant growth promoting microorganisms as biofertilizers and biopesticides and it’s exploitation in sustainable agriculture. Journal of Microbiology and Biotechnology. 2013; 3: 54-62.
- Sela-Buurlage, M.B., Ponstein, A.S., Bres-Vloemans, S.A., Melchers, L.S., Van den Elzen, P.J.M and Comelissen, B.J.C. Only specific tobacco (Nicotiana tabacum) chitinases and â-1, â-3 glucanase exhibit antifungal activity. Plant Physiology. 1993; 101: 857-863.
- Sharaf, E.F., El-Sarrany, A.Q and El-Deeb, M. Biorecycling of shrimp shell by Trichoderma viride for production of antifungal chitinase. African Journal of Microbiology. 2012; 6: 4538-4545.
- Siddiqui, I.A., Shaukat, S.S., Sheikh, I.H. and Khan, A. Role of cyanide production by Pseudomonas fluorescens CHAO in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World Journal ofMicrobiology and Biotechnology. 2006; 22: 641-650.
- Tallapragada, P., and Gudimi, M. Phosphate solubility and biocontrol activity of Trichoderma harzianum. Turk. J. Bio. 2011; 35: 593-601.
- Vinale, F., Sivasithamparam, K., Ghisalberti, E.L., Marra, R., Woo, S.L. and Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008; 40: 1-10.
- Viterbo, A., Haran, S., Friesem, D., Ramot, O. and Chet, I. Antifungal activity of a novel endochitinase gene (chit36) from Trichoderma harzianum Rifai TM, FEMS Microbiology Letters 2001; 200: 169-174.
- Wani, S.A., Mohiddin, F.A., Hamid, B., Rizvi, G., Bhat, K.A., Hamid, A., Alam, A., Baba, Z.A., Padder, S.A. and Bhat, M.A. Incidence of Fusarium wilt of chilli (Capsicum annum L.) in Kashmir valley and its management by Trichoderma species. Mycopath. 2014; 12: 1-8.
- Woo, S.L., Donzelli, B., Scala, F., Mach, R., Harman, G. E., Kubicek, C.P., Del Sorbo, G. and Lorito, M. Disruption of the ech42 (endochitinase-encoding) gene affects biocontrol activity in Trichoderma harzianum P1. Molecular Plant-Microbe Interactions. 1999; 12: 419-429.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.