Dipti Rai1, R.K. Pandey2, Ajay Kumar Maurya1,C. Rai2, Dilip Kumar2* and Manju Tiwari1
1Centre of Food Science and Technology, Banaras Hindu University, Varanasi – 221 005, India.
2Department of Animal Husbandry and Dairying, Banaras Hindu University, Varanasi, India.
ABSTRACT
The study was designed to evaluate the effect of cereals viz. oats, sorghum and amaranth on the storage quality parameters of synbiotic yoghurt. The products were developed by incorporating optimum level of oats (3.0%), sorghum (1.0%), amaranth (1.6%) and oats (3.0%), separately and were aerobically packaged in low-density polyethylene cups and assessed for various storage quality parameters under refrigerated (4±1°C) conditions for 28 days of storage. The products were evaluated for various physico-chemical, microbiological and sensory parameters at regular intervals of 0, 7, 14, 21 and 28 days. Microbial population (log cfu/g) of both Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus were in the same range in all yoghurt samples while cereals flour supplementation increased the count of Lactobacillus plantarum. The average pH of samples decreased from 4.5 to 4.3 during 28 days storage period. DPPH-radical-scavenging activity (77.97%) in cereal enriched yoghurt (synbiotic yoghurt) was significantly higher than probiotic (71.4%) and control sample (57%). Total phenolic was highest in synbiotic yoghurt in comparison with other yoghurt samples during the entire storage period. Supplementation of cereal flour along with probiotic bacteria Lactobacillus plantarum thus improved the functionality of yoghurt.
Keywords: Cereals, yoghurt, probiotic, synbiotic, DPPH-radical-scavenging activity.
INTRODUCTION
Yogurt is one of the dairy products, which continue to increase in sales due to diversiûcation in the range of yogurt-like products, including reduced fat content yogurts, probiotic yogurts, yogurt shakes, drinkable yogurts, yogurt mousse, yogurt ice-cream etc (Fiszman and Salvador, 1999). Yogurt is the most common fermented dairy products consumed around the world. As the popularity of yogurt products continues to grow, manufacturers are continuously investigating value-added ingredients such as prebiotics and probiotics to entice health-conscious consumers (Allgeyer et al., 2010). Probiotics are live microorganisms which, when administered in adequate amounts, confers health benefit to the host (Araya et al., 2002). Lactobacillus and Bifidobacteria species are the most common probiotics. Besides the probiotic properties, the antioxidative ability of lactic acid bacteria, including yogurt starters, has been reported (Kullisaar et al., 2002, 2003; Lin et al., 2000). The antioxidative activity of some Lactobacillus strains used as food components and probiotics may have a substantial impact on human health (Lin et al., 2000; Oxman et al., 2000). Probiotic microorganisms are delivered to target site using fermented food as a vehicle. However, these foods are not able to promote the growth of target organisms in the large colon due to the rapid absorption of fermentable substrates in the small intestine (Michida et al., 2006). Indigestible carbohydrates, fibres can selectively mediate the growth of beneficial colonic bacteria. These compounds, which are present in various cereal grains, are known as prebiotics and selectively stimulate the activity or growth of beneûcial bacteria in the colon. Current sources of prebiotics include cereals, such as wheat and barley, soybeans, chicory, sago starch (Schima et al., 2012). Cereals have higher content of certain essential vitamins, prebiotic dietary ûber, and minerals than milk, but have lesser quantities of readily fermentable carbohydrates (Charalampopoulos et al., 2002). In the previous studies, cereals were evaluated as good substrates for the growth of probiotic strains (Marklinder et al., 1994; Charalampopoulos et al., 2002) and cereal extracts were found to enhance acid and bile tolerance. But study on potential of whole cereal especially sorghum and amaranth in enhancing the functionality of yoghurt is limited.
A combination of probiotic and prebiotic in a single food is shown to improve the survival of probiotic bacteria during the storage of the product and also during the passage along the intestinal tract. Several studies have shown that the growth and viability of probiotics could be increased in yogurt or fermented milk products in the presence of prebiotics such as resistant starch, inulin, fructooligosaccharides, polydextrose, and oligofructose (Ranadheera et al. 2010, Ningegowda et al. 2012, Zare et al., 2011, Patil 2014)
The present study focuses on characterization of antioxidative functionality of probiotic and cereal enriched yogurt samples during refrigerated storage for 28 days and evaluation of chemical composition and sensory characteristics of cereal enriched (synbiotic), probiotic and control yoghurt samples.
Materials and Methods
Yogurt preparation
Fresh, pasteurized milk containing 1.5% fat was used for the preparation of yogurt. The milk was pasteurized at 63°C for 30 min at this stage 7% skim milk powder was added to increase the SNF (%) of milk, followed by cooling to 42p C before inoculation, an appropriate temperature for incubation of yoghurt culture. The milk was divided into 3 batches; in the first batch namely control 10g/L yoghurt starter culture was added, in the second batch Lactobacillus plantarum (1%) was inoculated along with 10g/L yoghurt starter bacteria. Last batch was supplemented with 1% sorghum, 1.61% amaranth and 3% oats flour along with same quantity yoghurt starter and probiotic bacteria used in previous groups. The level of cereals in cereals enriched yoghurt formulation was optimized by using Response surface methodology on the basis of textural, sensory and antioxidant characteristics. The preparation was mixed thoroughly and kept for incubation at 37°C for 16-18 hrs. After incubation, yogurt samples were stored at 4°C for 28 days. Samples were drawn at weekly intervals up to fourth week.
Determination of Viability
The colony counts of Lactobacillus delbrueckii subsp. bulgaricus (NCDC 199) and Streptococcus thermophilus (NCDC 285) were determined as described elsewhere (Dave and Shah, 1996; Tharmaraj and Shah, 2003). The count of S. thermophilus, L. bulgaricus and L. plantarum in to the yoghurt samples were evaluated on 1st, 7th, 14th, 21st and 28th day. One ml sample was taken from each yogurt sample for serial dilution. Serial tenfold dilution was prepared in a solution of 0.9% NaCl (w/v) and 0.1% (w/v) bactopeptone and suitable dilutions were placed on appropriate media. L. bulgaricus were enumerated on Lactobacilli MRS agar, when the incubation is carried out at 42°C for 72 h. S. thermophilus were enumerated on ST agar under aerobic incubation at 37°C for 24h. L. plantarum was enumerated on selective medium (LPSM), under anaerobic incubation at 37°C for 48 h (Bujalance et al., 2006).
Physico-chemical Analysis
In yoghurt samples chemical properties like pH, titratable acidity (AOAC, 2000), moisture, protein and fat (AOAC, 1997) content of yoghurt samples were recorded. The samples were analyzed once for pH, titratable acidity and in 7 days for a period of 28 days. The data from each experiment were analyzed by analysis of variance (ANOVA) using SPSS 17 software.
Measurement of DPPH free radical–scavenging activity
The DPPH radical scavenging activity of yoghurt samples was determined according to the method used by Gulcin et al. (2010) with some modifications. A 0.1 mM DPPH radical solution in 95% ethanol was prepared.1 ml of ethanolic DPPH solution (8mg/ml) was mixed with 0.2 gram of yogurt sample, vortexed well and incubated for a 12 hours at room temperature. In blank 0.2ml distilled water was used instead of sample. The samples were centrifuged for 10 min at 4000 rpm, and the absorbance of samples was measured at 517 nm using UV-visible spectrophotometer. The antioxidant activity was expressed as percentage (%) DPPH scavenging = [(absorbance of blank-absorbance of sample)/ (absorbance of blank) ×100].
Determination of total phenol content
The total phenolic content of the samples was determined by the Folin-Ciocalteau method described by Cliffe et al. (1994) with some modification. 5 grams per 50 mL of sample was filtered with whatman no.1 paper 0.5 mL of the sample was added to 2.5mL of 0.2 N up to 25 mL using distilled water. The above solution was then kept for incubation for 2 hours at room temperature (30±2°C). Absorbance was measured at 760 nm using 1 cm cuvette UV- 1800 spectrophotometer (Shimadzu Corporation, Japan).Gallic acid was used to prepare standard curve. Each experiment was performed in triplicates The total phenolic content was expressed in mg of Gallic acid equivalent (GAE)/g of extract.
Sensory Evaluation
Sensory evaluation of yoghurt samples was performed by panel of 10 semi-trained panellists (5 male and 5 female, aged 20-30 years) from the Centre of Food Science and Technology, Banaras Hindu University, Varanasi, India, including students, faculty members and other residents of Banaras Hindu University, Varanasi, India. Panel booths were illuminated uniformly with special day light bulbs for evaluation of colour and appearance. Yoghurt samples were served in a labelled plastic cups. Sensory evaluation was done at 25°C and 60% relative humidity. The judges were asked to score for the sensory attributes viz. colour and appearance, flavour, body and texture and overall acceptability on a 9-point Hedonic scale.
Statistical Analysis
Duncan multiple range test was performed for data obtained from microbial analysis to measure the test of significance between samples stored at different temperatures by post hoc test using SPSS 17.0 software (SPSS Italia, Bologna, Italy).
Results and Discussion
Microbial growth in yoghurt after production and during storage
The addition of cereal flour had no significant effect (P<0.05) on the viability of the yogurt starter of S. thermophilus and L. bulgaricus on first day which is in accordance with previous study by Vasiljevic et al. (2007). Viable counts of S. thermophilus in all yogurt samples, declined from 7.9 to 7.4 log cfug-1 (P<0.05) and the viable cell counts of L. bulgaricus in yoghurt samples declined from 7.7 to 6.5 log cfug-1 (P<0.05) approximately over a period of 28 days (Table. 1). Supplementation with cereal flour slightly improved viability of L. bulgaricus. Zare et al. (2011) also reported that addition of lentil flour increased the viability of Lactobacillus.
- plantarum count increased on day 1 in both probiotic and synbiotic yoghurt. Supplementation with 1% sorghum, 1.61% and 3% oats significantly (P<0.05) improved the viability of L. plantarum in synbiotic yoghurt samples during storage (Table. 1). Addition of cereals could either act as an additional nutrient or modify the unfavourable environmental inûuences, resulting in improved probiotic viability (Desai et al., 2004; Madhu and Prapulla, 2012). On the day 1 of freshly prepared yoghurt L. plantarum count in probiotic and synbiotic yohurt sample was 8.43 log cfu g-1and 9.26 log cfu g-1 respectively and in both the yoghurt samples count of L. plantarum decreased over the 28-day storage period. The steady fall in the microbial viability was observed on day 14 in all the yoghurt samples and the decline remained so thereafter in up to day 28. The decrease in bacterial growth is a result of the reduced amount of sugars remaining in the yogurt, leaving bacteria with far less nutrients to consume and promote growth (Agil and Hosseinian, 2012).
Nevertheless, the synbiotic yoghurt samples still showed higher cfug-1 (P<0.05) in comparison with the probiotic and control yoghurt samples at the end of storage (Table. 1). The viable cell counts of probiotic bacteria i.e. L. plantarum by the end of 28 days of storage was above 7 log cfug-1, and thus, the yogurt developed could be considered as a probiotic product (Bevilacqua et al., 2013).
Table 1. Effect of cereal supplementation on the viability of yoghurt starter cultures (L. delbruekii ssp. bulgaricusand S. thermophilus) and probiotic organisms L. Plantarum.
| Cultures
Samples |
Period of storage, day (log cfu/ml) | ||||
| 1 | 7 | 14 | 21 | 28 | |
| Lactobacillus plantarum | |||||
| Probiotic | 8.46cB | 8.4cB | 8.07bB | 7.82aB | 7.8aB |
| Synbiotic | 9.45dA | 9.32cA | 9.29cA | 9.21bA | 9.02aA |
| Streptococcus thermophilus | |||||
| Control | 7.87dA | 7.83dA | 7.76cB | 7.56bB | 7.48aA |
| Probiotic | 7.88dA | 7.83cA | 7.82cA | 7.55bA | 7.43aB |
| Synbiotic | 7.94dA | 7.87cA | 7.82cA | 7.6bA | 7.46aB |
| Lactobacillus bulgaricus | |||||
| Control | 7.76eA | 7.49dB | 7.24cB | 6.88bB | 6.57aB |
| Probiotic | 7.72eA | 7.48dB | 7.23cB | 6.84bB | 6.55aB |
| Synbiotic | 7.75eA | 7.62dA | 7.43cA | 7.16bA | 6.85aA |
Values are mean ± SE of 3 replicates .
Values bearing different small superscripts (a, b, c, d) in a column differ significantly (Duncan test, P<0.05).
Values bearing different capital superscrits (A, B, C) in a row differ significantly (Duncan test, P<0.05).
Where A= control yoghurt, B= probiotic yoghurt, C= synbiotic (cereal enriched) yoghurt.
Effect of cereals flour supplementation on the antioxidant properties of yogurt samples
The DPPH-scavenging activity of yogurt samples is shown in Table. 2. The synbiotic (cereal enriched) yoghurt had significantly (P<0.05) higher antioxidant potential compared to probiotic and control yoghurt sample. Higher antioxidant activity of synbiotic yoghurt is because of presence of bound phenolics in the cereals (Agil & Hosseinian, 2012). On the day 1% DPPH radical inhibition in synbiotic yoghurt sample was 77.97 % when compared to that of control which had DPPH scavenging activity of 57.11%. The higher antioxidant potential of probiotic and synbiotic yoghurt sample indicate that the metabolic end products of Lactic acid bacteria, resulting from the utilization of fibres of grains, might be contributing to the higher antioxidant potential. (Ningegowda et al., 2012). The % DPPH activity of the control, probiotic and synbiotic yoghurt sample decreased from 57.11% to 49.13%, 71.49% to 61.88% and 77.97% to 69.56% respectively during the 28 days of storage period. Gad et al. (2010) had reported that the antioxidant power of yogurt supplemented with 10 % date palm syrup was 43.3-33.5 mg Fe2+/100 mL at 12 days storage.
Total phenolic content of synbiotic and probiotic yoghurt was significantly (P<0.05) higher compared to the control yoghurt sample (Fig. 1). The increased total phenolic content in the synbiotic and probiotic yoghurt could be due to the fermentative activity of the probiotics (Ningegowda et al., 2012) and also due to the presence of bound phenolics in the cereals (Agil and Hosseinian, 2012). Total phenolic content of all the yoghurt samples was found to decrease during 28 days storage period. This could be due to loss in fermentative activity of lactic acid bacteria during storage. The total phenolics in control yoghurt, probiotic yoghurt and synbiotic yoghurt sample decreased from 18.07 mg GAE per 100 mL to 8.62 mg GAE per 100 mL, 27.48 mg GAE per 100 mL to 19.21 mg GAE per 100 mL and 40.12 mg GAE per 100 mL to 25.75 mg GAE per 100 mL of yoghurt respectively during 28 days of storage period. Gad et al. (2010) also reported that total phenols content of yoghurt decrease during storage.
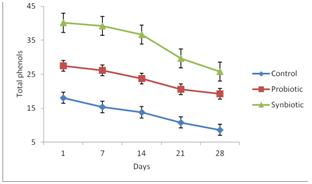
Fig. 1.Effect of storage on total phenolic content of yogurt samples.
Change in pH and Titratable acidity during Storage
In all the yoghurt samples, the pH decreased from 4.56 to approximately 4.30 (P<0.05) over the 28 day storage period (Table. 2). Higher reduction in pH of synbiotic yoghurt depicts that bacteria are significantly more active in the presence of cereals increasing the acidity and thereby lowering the pH. In case of probiotic yoghurt higher reduction in pH is attributed to the presence of additional probiotic bacteria which is leading to production of more acid compared to control. The lowest pH value was observed on 21 day in all the yoghurt samples. These decreases might be attributed to the utilization of residual carbohydrates by viable microorganism and production of lactic acid, small amount of CO2 and formic acid from lactose (Patil, 2014).
The variations in the TTA (% lactic acid) profile of control, probiotic and synbiotic yogurt samples during refrigeration over a period of 28 days was shown in Table 2. There was a sharp increase (P<0.05) in the TTA levels in all the yoghurt samples till the day 21 and thereafter it decreased slightly on the day 28. The titratable acidity of control yoghurt increased from 0.76% to 0.90%, from 0.78% to 0.95% in samples of probiotic yoghurt and that in control yoghurt increased from 0.86% to 0.90%. Titrable acidity was found to increase more in multigrain bio-yoghurt compared to other yoghurt samples, thus depicting the production of lactic acid in the presence of husk. The ascending trend of TTA corresponds to the sharp decline in the pH values of the yogurt samples during storage. Similar findings have been reported by Agil et al. (2012).
Table 2. Changes in DPPH scavenging activity and pH of yogurt samples immediately after fermentation and during 28 days storage
Values are mean ± SE of 3 replicates.
Values bearing different small superscripts (a, b, c, d) in a column differ significantly (Duncan test, P<0.05).
Values bearing different capital superscrits (A, B, C) in a row differ significantly (Duncan test, P<0.05).
Where A= control yoghurt, B= probiotic yoghurt, C= synbiotic (cereal enriched) yoghurt.
Sensory Evaluation
Synbiotic yoghurt recorded lowest sensory scores (Fig. 2). Fernandez (1997) also reported that addition of corn fibres reduces the overall acceptability of yoghurt. This was perhaps due to the masking of natural appearance, flavour and colour of yoghurt by cereals. Table. 3 shows the sensory score of all the yoghurt samples.
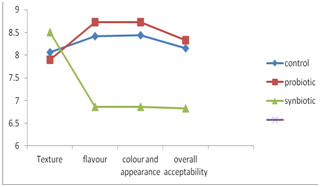
Fig. 2. Sensory evaluation of control, probiotic & synbiotic yoghurt.
Table 3. Average sensory score of yoghurt samples.
Yoghurt |
Texture |
Flavour |
Colour and appearance |
Overall acceptability |
Control |
8.06±0.07a |
8.42±0.19a |
8.44±0.10a |
8.15±0.30a |
Probiotic |
7.89±0.07b |
8.73±0.29b |
8.83±0.28b |
8.33±0.50b |
Synbiotic |
8.50±0.33c |
6.86±0.50c |
7.0±0.50c |
6.83±0.28b |
9 point hedonic scale was used with 1: dislike extremely and 9: like extremely.
Values are mean ± SE of 3 replicates.
Values bearing different small superscripts (a, b, c, d) differ significantly (Duncan test, P<0.05).
Where A= control yoghurt, B= probiotic yoghurt, C= synbiotic (cereal enriched) yoghurt.
Evaluation of physico-chemical composition of synbiotic, probiotic and control yoghurt samples
Chemical composition (pH, acidity, moisture, protein and fat) of synbiotic, probiotic and control yoghurt are presented in Table. 4. Addition of oats, sorghum and amaranth had increased acidity, protein and fat while decreased moisture content and pH of yoghurt. Damian et al. (2014) also reported increase in acidity of yoghurt supplemented with pea fibre. The increase in acidity of synbiotic yoghurt could be due to the stimulatory effect of cereals flour on the growth of lactic acid bacteria.
Table 4. Chemical composition of control, probiotic and cereal enriched yoghurt samples
Values are mean ± SE of 3 replicates.
Values bearing different small superscripts (a, b, c, d) differ significantly (Duncan test, P<0.05).
Conclusion
This study showed that supplementation of cereals flour resulted in significantly higher viable count of L. plantarum in comparison with non supplemented sample. Furthermore supplementation also enhanced the antioxidant potential of yoghurt and hence the nutritional value of the yoghurt. Due to its high fibre and protein content, and on the basis of the microbial property investigated, the results suggest that cereals flour could be potentially considered as a source of prebiotic ingredient for use in L. plantarum fermented products.
References
- Agil, R., Gaget, A., Gliwa, J., Avis, T.J., Willmore, W.G., Hosseinian, F. Lentils enhance probiotic growth in yogurt and provide added benefit of antioxidant protection. LWT – Food Science and Technology, 2012; 50: 45-49.
- Agil, R., Hosseinian, F. Dual Functionality of Triticale as a Novel Dietary Source of Prebiotics with Antioxidant Activity in Fermented Dairy Products. Plant Foods Human Nutrition, 2012; 67: 88-93.
- Allgeyer, L.C., Miller, M.J., Lee, S.Y. Sensory and microbiological quality of yogurt drinks with prebiotics and probiotics. Journal of Dairy Science, 2010; 93: 4471-4479.
- AOAC. Official Methods of Analysis of AOAC International, 16th ed., Vol. 2, 1997 International, Gaithersburg MD.
- AOAC. Official Methods of Analysis, 17th ed., Association of Official Analytical Chemistry. 2000; Arlington, Virginia, USA.
- Araya, M., Morelli, L., Reid, G., Sanders, M.E., Stanton C., Pineiro, M., Embarek, P.B. (2002) Guidelines for the evaluation of probiotics in food Joint FAO/WHO Working Group Report Drafting Guidelines Eval. Probiotics Food, London (Ontario,Canada),2002; 30: 1–11.
- Bevilacqua, A., Campaniello, D., Corbo, M.R., Maddalena, L. Sinigaglia, M. Suitability of Bifidobacterium spp. and Lactobacillus Plantarum as probiotics intended for fruit juices containing citrus extracts. Journal of Food Science, 2013; 78: M1764-M1771.
- Bujalance, C., Jimenez-Valera, M., Moreno, E., Ruiz-Bravo, A. A selective differential medium for Lactobacillus plantarum. Journal of Microbiological Methods. 2006; 66: 572–575.
- Charalampopoulos, D., Wang, R., Pandiella, S.S., Webb, C. Application of cereals and cereal components in functional foods: a review. International Journal of Food Microbiology, 2002a; 79: 131–141.
- Charalampopoulos, D., Wang, R., Pandiella, S.S., Webb, C. Growth studies of potentially probiotic lactic acid bacteria in cereal-based substrates. Journal of Applied Microbiology, 2002b; 92: 851–859.
- Cliffe, S., Fawer, M.S., Maier, G., Takata, K., Ritter, G. Enzyme assays for the phenolic content of natural juices. Journal of Agricultural and Food Chemistry, 1994, 42: 1824–1828.
- Damian, C., Olteanu, A. Influence of dietary fiber from pea on some quality characteristics of yoghurts. Journal of Agroalimentary Processes and Technologies. 2014; 20: 156-160.
- Dave, R.I., Shah, N.P. Effectiveness of ascorbic acid as an oxygen scavenger in improving viability of probiotic bacteria in yoghurts made with commercial starter cultures. International Dairy Journal, 1996; 7: 435–443.
- Desai, A.R., Powell, I.B., Shah, N.P. Survival and activity of probiotic lactobacilli in skim milk containing prebiotics. Journal of Food Science, 2004; 69: 57–60.
- Fernandez-Garcia, E., McGregor, J.U. Fortification of sweetened plain yogurt with insoluble dietary fiber Zeitschrift für Lebensmittel-Untersuchung und –Forschung. 1997; 204: 433-437.
- Fiszman, S.M., Salvador A. Effect of gelatin on the texture of yogurt and of acid heat induced milk gels. LWT., 1999; 208: 100–105.
- Gad, A.S., Kholif, A.M., Sayed, A.F. Evaluation of the nutritional value of functional yogurt resulting from combination of date palm syrup and skim milk. American Journal of Food Technology, 2010; 5: 250–259.
- Gulcin, I.E., Kirecci, E., Akkemik, F., Topal, Hisar, O. Antioxidant and antimicrobial activities of an aquatic plant: duckweed (Lemna minor L.). Turkish Journal of Biology, 2010; 34: 175-188.
- Kullisaar, T., Songisepp, E., Mikelsaar, M., Zilmer, K., Vihalemm, T., Zilmer, M. Antioxidative probiotic fermented goat’s milk decreases oxidative stress-mediate atherogenicity in human subjects. British Journal of Nutrition, 2003; 90: 449–456.
- Kullisaar, T., Zilmer, M., Mikelsaar, M., Vihalemm, T., Annuk, H., Kairane, C., Kilk, A. Two antioxidative Lactobacilli strains as promising probiotics. International Journal of Food Microbiology, 2002; 72: 215-224.
- Lin, M.Y., Chang, F.Y. Antioxidative effect of intestinal bacteria Biûdobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Digestive Disease & Sciences, 2000; 45: 1617–1622.
- Madhu, A.N., Prapulla, S.G. In vitro fermentation of prebiotics by Lactobacillus plantarum CFR 2194: selectivity, viability and effect of metabolites on ²-glucuronidase activity. World Journal of Microbiology & Biotechnology, 2012; 28: 901–908.
- Marklinder, I., Lonner, C. Fermented oatmeal soup – inûuence of additives on the properties of a nutrient solution for enteral feeding. Food Microbiology,1994; 11 505–513.
- Michida, H., Tamalampudi, S., Pandiella, S.S., Webb, C., Fukuda, H., Kondo, A. Effect of cereal extracts and cereal ûber on viability of Lactobacillus plantarum under gastrointestinal tract conditions. Biochemical Engineering Journal, 2006; 28: 73-78.
- Ningegowda, A.M., Amrutha, N., Prapulla, S.G. Characterization and Antioxidant Property of Probiotic and Synbiotic Yogurts. Probiotics & Antimicrobial Proteins, 2012; 4: 90-97.
- Oxman, T., Shapira, M., Diver, A., Klein, R., Avazov, N., Rabinowitz, B. A new method of long-term preventive cardioprotection using Lactobacillus. American Journalof Physiology, 2000; 27: H1717–H1724.
- Patil, S.R. Effect of blackgram (Phaseolus Mungo) husk on microbial, physiochemical and sensory attributes of synbiotic yogurt. International Journal of Scientific Engineering and Research, 2014; 2: 2347-3878.
- Ranadheera, R.D.C.S, Baines, S.K. Adams, M.C. Importance of food in probiotic efficacy. Food Research International, 2010; 43: 1–7.
- Schima, A.R.R., Salina, H.F., Masniza, M., Atiqah, A.H. Viability of Lactic acid bacteria in homemade yogurt containing Sago Starch Oligosaccharides. International Journal of Basic Applied Sciences, 2012; 12: 58-62.
- Tharmaraj, N., Shah, N.P. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Biûdobacteria, Lactobacillus casei, Lactobacillus rhamnosus, Propionibacteria. Journal of Dairy Science, 2003; 86: 2288–2296.
- Vasiljevic, T., Kealy, T., Mishra, V.K. Effects of ²-glucan addition to a probiotic containing yogurt. Journal of Food Science, 2007; 72: 405–411.
- Zare, F., Boye, J.I., Orsat, V., Champagne, C., Simpson, B.K. Microbial, physical and sensory properties of yogurt supplemented with lentil ûour. Food Research International, 2011; 44: 2482–2488.

