ISSN: 0973-7510
E-ISSN: 2581-690X
Extensive use of hexavalent chromium in various industrial processes and improper treatment technology for remediation has laid serious public health problems. The present study was conducted to isolate chromium-reducing bacterial consortium from chromium contaminated industrial soils/effluent. Bacterial consortium SN6 having five bacterial strains led to biotransform 99.9% of 3 mM Cr (VI) under favourable conditions i.e. 35°C and pH 8.0 within 32 h. SN6 have efficiency to reduce Cr(VI) with different metals i.e. Zn, Cu, Ni, Ca, Co. V. radiata L. was utilized to evaluate phytotoxicity of hexavalent chromium detoxification. Characterization of cell associated reduced products was analysed by SEM-EDAX, XRD, FT-IR, and total chromium concentration was estimated by ICP-AES spectroscopy. SN6 reasonably tolerates and reduces hexavalent chromium under wide variety of investigational environment. That shows promise for its possible use in reclamation of chromate containing industrial wastewaters.
Cr (VI) reduction, Consortium, Biotransformation, Phytotoxicity, Detoxification
High concentration of Cr(VI) in the environment due to industrial wastes is of great concern. Uncontrolled release of such wastes from electroplating, alloy production, leather tanning, wood preservation, dye and pigment industries caused contamination of soil-water ecosystems (Ackerley et al., 2006). The degree of toxicity of chromium ions is on its oxidation state. Amongst different oxidation sates, Cr(VI) and Cr(III) are the most common and thermodynamically stable forms. Chromium in its +6 form is rapidly transported through biological membranes and inside the cells interacts with proteins and nucleic acids which increase its toxicity (Srinath et al., 2001). Cr(VI) is an irritant, corrosive of skin and respiratory tract; it is responsible for lung carcinoma, also acts as a teratogen (Thacker et al., 2005). In contrast, low solubility and mobility of Cr(III) restrict its biological availability which makes it 100 times less toxic than Cr(VI) (Gonzalez et al., 2003).
Biotransformation and detoxification of hexavalent chromium by biological means is an inexpensive as well as a healthy option for environment among several accessible traditional approaches. These biological approaches can be used to decontaminate the affected environments like ground water, contaminated industrial zones etc. Several bacteria from the genera Pseudomonas (McLean and Beveridge, 2001; Huang et al., 2016), Brucella (Thacker et al., 2007), Bacillus (Xu et al., 2015), Arthrobacter sp. SUK 1201 (Dey et al., 2013), Providencia sp. (Thacker et al., 2006), Escherichia, Micrococcus, Staphylococcus (Kathiravan et al., 2010) have been isolated from chromium contaminated or uncontaminated environments. The mechanism of Cr(VI) reduction by these microorganisms are mainly of two types biotransformation and bioabsorption, which depends on species (Camargo et al., 2003). Chromium contaminated sites have higher chances to have chromium tolerant bacteria and they may play an important role either individually or synergistically to remediate the Cr(VI) from the contaminated environment.
The present investigation describes the Cr(VI) reduction by newly isolated native bacterial consortium SN6, enriched from the chromium contaminated site. Factors influencing Cr(VI) reduction by SN6, were studied and the products of the process were characterized by SEM-EDAX, XRD, FT-IR and ICP-AES. Reduction in toxicity was investigated by phytotoxicity study on Vigna radiata L. The results of present investigation will be a new report in knowing the importance of bacterial consortium in bioremediation and detoxification of toxic compounds.
Sample collection, isolation and identification of chromium resistance bacterial consortium
Sub-surface (8 cm below) soil samples from chrome plating industries and industrial zone of Lambha, Ahmedabad, India were collected in sterile plastic bags. The soil sample was characterized to know the presence of metallic contaminants as shown in Table: 1. One gm of soil sample was added to 250 mL conical flasks having 100 mL of sterile Luria-Bertani (LB) broth (tryptone: 10, yeast extract: 5, and sodium chloride:10 g/L, pH 7.2±0.1, incubation at 37°C) amended with 3mM of K2Cr2O7. Native population was repeatedly transferred to the fresh medium amended with 3 mM K2Cr2O7 for acclimatization. LB agar medium amended with 1mM K2Cr2O7 was utilized for isolation of pure culture of native bacterial mixture. Colonies with different morphological characters, grams staining and Cr(VI) resistant level were isolated and stored at 4°C for further identification.
Table (1):
Soil characterization for metallic contaminants.
Sr. No. |
Metal |
Concentration in the soil sample. mg/kg of soil |
|---|---|---|
1 |
Chromium |
4219 |
2 |
Nickel |
200 |
3 |
Cadmium |
369 |
4 |
Copper |
588 |
5 |
Arsenic |
93 |
6 |
Lead |
53 |
Genomic DNA of all the bacteria present in consortium-SN6 was extracted followed by amplification of 16S rRNA gene by PCR using universal eubacterial primers 8F and 1492R. The purified PCR product of nearly 1.5 kb from each strain was sequenced by BDT v3.1 Cycle sequencing kit on ABI 3730xl Genetic Analyzer. The resulting sequences were analyzed using BLAST tool, using the Clustal | | program; most similar sequences were obtained from the database, aligned for similarity analysis. The phylogenetic tree was constructed using neighbour joining method in MEGA 6.0 software (Balapure et al., 2014).
Determination of Minimum Inhibitory Concentration (MIC)
The MIC means that lowest metal [Cr(VI) in the present case] concentration that restricts the growth of all individual bacteria from consortium-SN6 completely, was determined in the LB medium. Individual bacterial isolates, as well as consortium SN6, were inoculated in the LB broth spiked with different concentration of hexavalent chromium as K2Cr2O7. After one week incubation at 37°C, cell growth was measured at OD600 (He et al., 2011).
Growth and Cr (VI) reduction at aerobic/microaerophilic condition
To verify suitable condition for growth and Cr(VI) reduction, consortium-SN6 was inoculated to LB broth spiked with 3mM hexavalent chromium as K2Cr2O7 and incubated at 37°C under shaking (in a rotary shaker 120 rpm) as well as microaerophilic (static) condition. Optical density of the cell culture and Cr(VI) reduction were checked periodically. Cr(VI) reduction was calculated along with abiotic control to eliminate the Cr(VI) reduction by media components if any. All the experimental sets performed in triplicates at a time. The resulting data was averaged and S.D. (n=3) was determined.
Effect of metal ions on growth of SN6 and Cr (VI) reduction
The efficiency of consortium-SN6 to reduce Cr(VI) in the presence of various metal ions was studied. Consortium-SN6 was inoculated in fresh medium containing 3mM of Cr(VI) along with chloride salts of Hg, Zn, Ca, Ag, Ni, Cu, Co, and Cd at 1mM concentration separately. After 48 h of incubation, the cell density and residual chromium was measured by U.V-Vis spectrophotometer OD600 and DPC method respectively (APHA 2012).
Effect of temperature and pH on Cr (VI) reduction
The effect of temperature and pH on Cr(VI) reduction by consortium-SN6 was assessed with the LB medium spiked with 3mM Cr(VI) and inoculated with 10% fresh inoculum. Temperature and pH examined from 33-40°C with an interval of two degree centigrade and 4.0-10.0 pH with an interval of one respectively. pH of the LB medium was kept 7.0 (varied for the pH test with 1N NaOH or 1N HCl before sterilization) and incubated at 37°C (changed when temperature optimized). Remaining chromium concentration was measured time to time by 1-5 diphenyle carbazide method as described below.
Effect of inoculum size and chromate concentration on Cr (VI) reduction
The inoculum size and chromate concentration were investigated from 4-16% and 0.5 mM to 7 mM respectively. Hexavalent chromium reduction was examined under microaerophilic (static) condition in 100 mL LB broth amended with suitable concentration of Cr(VI) (3mM- inoculum size) and inoculated with appropriate cells of bacterial suspension (10% inoculum-chromate concentration experiment), incubated at 35°C. Left over Cr(VI) concentration was examined time to time by 1-5 diphenyle carbazide spectrophotometric method as described in the section no. 2.7.
Analysis of Cr (VI) and total chromium
Cr(VI) was estimated by 1,5-diphenyle carbazide method (DPC). Culture broth was separated from the cells by centrifugation. Cell pellets and supernatant were separated and used for further analysis. 0.2 mL of 0.25% DPC reagent (prepared in acetone) was added to 1 mL of resultant supernatant under acidic condition. After colour development (for 10-15 min) under ambient condition, O.D. was measured at 540 nm against the Cr(VI) standard, prepared in the nutrient medium to eliminate the interference (APHA, 2012). The total chromium concentration [Cr(VI) and Cr(III)] in cultured media was estimated as described in APHA, 2012 by inductively coupled plasma spectroscopy. Trivalent chromium concentration was calculated by subtracting the hexavalent chromium concentration from total chromium concentration.
Characterization of reduced products
Cell pallets of bacterial consortium SN6 with or without reduced products were examined by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDAX), X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), and Inductive coupled plasma spectroscopy respectively.
The cell pellets was spread on the carbon tape and dried under vacuum and examined at 50kV under ESEM EDAX XL-30, Philips for SEM analysis. Analysis of required elements was performed by EDAX. XRD was recorded on a Philips, Xpert MPD X-ray diffractometer using CuKa radiation at a scanning speed of 2° min-1. FT-IR (Perkin Elmer, Spectrum GX spectrophotometer) analysis of the dried cells associated with or without reduced product was recorded using KBr phase from 400 to 4000 cm-1 (mid-infrared region).
Detoxification study
Cr(VI) being a toxic compound, has several detrimental effects on all life forms. To evaluate the efficacy of SN6 for detoxification of Cr(VI) was checked by phytotoxicity on Vigna radiata L. Farm soil taken from a local organic farm having physical characteristics: 60.9% sand; 21.64% slit; 17.46% clay; 20.18% moisture; 4.8% organic matter (estimated by loss in weight after ignition at 600°C for 2 h (Ali et al., 2004) and a pH of 7.9 (measured by adding 100 gm of soil in 100 mL distilled water (1:1 ratio) as described in methods of soil analysis part 2 McLean 1982). The soil was sieved with 2-mm sieve and filled in the plastic trays (315 mm × 210 mm × 110 mm). The soil was rehydrated with tap water spiked with 3mM Cr(VI) for control; SN6 treated supernatant for test and tap water for blank to 60% of the water-holding capacity of the soil. The seeds of Vigna radiata L. were sowed at equal distance in the respective trays in duplicates (35 Nos.). The experiment was carried out for 14 days in control, test and blank trays. Percentage of seed germination, root length, shoots length, wet, and dry weight of seedlings was monitored at the end of the experiment.
Bacteria with the ability to grow under the harsh environmental condition, detoxification of xenobiotic compounds and degradation of a structurally inert compound in a mutualistic manner made them omnipresent. Present study describes a native consortium SN6, isolated and screened from a chromate contaminated soil, exploited to know the Cr(VI) reduction capacity and its detoxification along with characterization of reduced product.
Screening and identification of SN6
Isolation of bacterial consortium resistant to a large amount of Cr(VI) is extremely essential for Cr(VI) reduction from effluents of various industries to decrease its toxicity. Thirty consortiums were developed from the contaminated soil samples. Bacterial consortium-SN6 indicating promising growth pattern and Cr(VI) reduction under a short period of time was selected for the further studies. SN6 is comprised of five different bacterial isolates (Fig. 1) identified as Alcaligenes sp. SN6A (NCBI Accession No. KX281149), Providencia sp. SN6B (KX281150), Providencia sp. SN6C (KX281151), Enterococcus sp. SN6D (KX281152), and Pseudomonas sp. SN6E (KX281153). As per our best knowledge, Cr(VI) reduction by a native bacterial population from the heavy metal and/or chromium contaminated soil is explored by very few researchers. Earliest reported Cr(VI) reduction by a native consortium of SRB was by Fude et al., (1994). Sulphate reducing bacterial consortium was able to accumulate and transform Cr(VI) on the bacterial cell surface. Addition of uranium and zinc increased Cr(VI) accumulation in the SRB III consortium stated by Fude et al., (1994). Arias et al., (2003) focused on community shifts during time course experiments of Cr(VI) reduction. In presence of Cr(VI) non sulfidogenic bacterial community grows well followed by sulfidogenic bacterial growth after Cr(VI) reduction, concluded by Arias et al., (2003). Pinon-Castillo et al., (2010) reported bacterial consortia comprising of Enterobacter Sp. and Halomonas chromatireducens isolated from an industrial sediment capable of reducing Cr(VI). Present investigation might be a first report of a native bacterial microaerophilic consortia screened from the Lambha, Ahmedabad, Gujarat, India. However SN6 comprising individual isolates have been isolated by different researchers but a native consortium has not been reported till date best of our knowledge. Thacker et al., (2006) isolated Providencia sp. reducing 100 mg/L of Cr(VI) in 30h, from industrially contaminated site. Kang et al., (2017) reported Cr(VI) reduction at the rate of 0.41 mg/L/h under optimized condition by Pseudomonas sp. Sayel et al., (2012) reported Enterococcus strain for the first time for Cr(VI) reduction i.e. 150 mg/L under aerobic condition in about 50 h. Alcaligenes spp. was isolated from the mine seepage water from Orissa, India, by Dey and Paul, (2010).
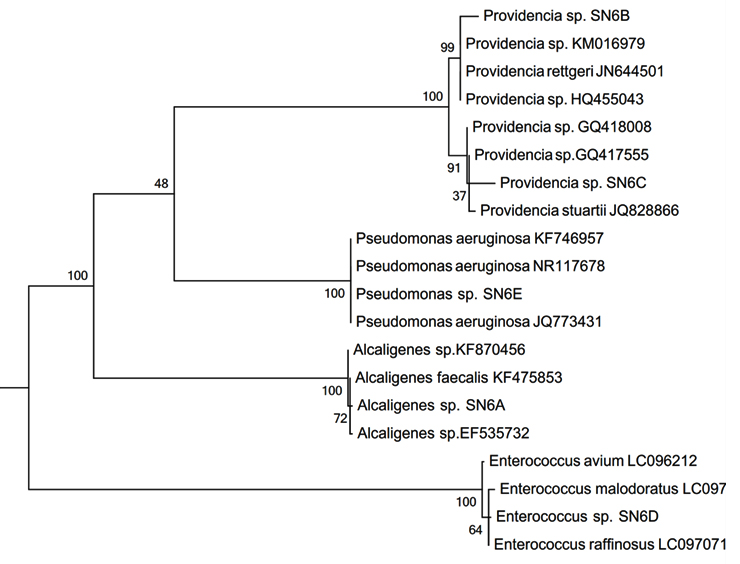
Fig. 1. Phylogenetic tree of consortium-SN6
Determination of Minimum Inhibitory Concentration (MIC)
MIC for Cr(VI) showed that Enterococcus sp. SN6D has maximum MIC i.e. 7 mM whereas Providencia sp. SN6B has minimum MIC (4mM) amongst isolated cultures from bacterial consortium-SN6. Interestingly the minimum inhibitory concentration of SN6 was 12 mM, considerably higher than the individual cultures (Table: 2). This could be because of the synergistic effect of diverse organisms present in consortium-SN6. Tolerance of Cr(VI) resistant microorganisms isolated from chromium contaminated sites ranges between 0.05 to 8 mM (Mishra et al., 2012).
Table (2):
Chromate resistance of consortium-SN6 and bacteria comprising it.
| Culture Code | Cr(VI) concentration (mM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| SN6A Alcaligenes sp. | + | + | + | + | ||||||||
| SN6B Providencia sp. | + | + | + | |||||||||
| SN6C Providencia sp. | + | + | + | + | ||||||||
| SN6D Enterococcus sp. | + | + | + | + | + | + | ||||||
| SN6E Pseudomonas sp. | + | + | + | + | + | |||||||
| Consortium-SN6 | + | + | + | + | + | + | + | + | + | + | + | |
Table (3):
Results of Phytotoxicity study.
Germination (%) |
Root length (cm) |
Shoot length (cm) |
Wet weight (cm) |
Dry weight (cm) |
|
|---|---|---|---|---|---|
Blank |
95.4 |
11.35 |
14.09 |
2.3 |
0.30 |
Control |
40.71 |
2.11 |
1.73 |
0.68 |
0.14 |
Test |
82.85 |
10.0 |
13.6 |
2.1 |
0.2 |
Growth and Cr (VI) reduction at static/shake flask condition
Growth and Cr(VI) reduction potential of SN6 was studied at static as well as shake flask condition. The growth of SN6 didn’t showed much difference at static and shake flask condition, but static flask showed greater reduction of chromium compare to shake flask condition (Fig. 2). Total reduction of 3mM Cr(VI) within 48 h at static condition was achieved by consortium-SN6 however at shake flask condition it reduces less than 50% within 48 h whereas the optical density of culture suspension was increased up to 48 h. As described by Bachate et al., (2013) with Bacillus firmus TE7, incomplete reduction of Cr(VI) at shake flask condition was a result of decreased uptake of Cr(VI) under the shake flask. Similar results were also observed in Pseudomonas fluorescence LB300 (Ohtake et al., 1987). The efficiency of the treatment to reduce Cr(VI) in static flask could be due to the use of Cr(VI) as a terminal electron acceptor or it might be due to redox mediator by bacteria comprising SN6. Abiotic Cr(VI) reduction was negligible resulting in conclusion that the Cr(VI) reduction was SN6 mediated.
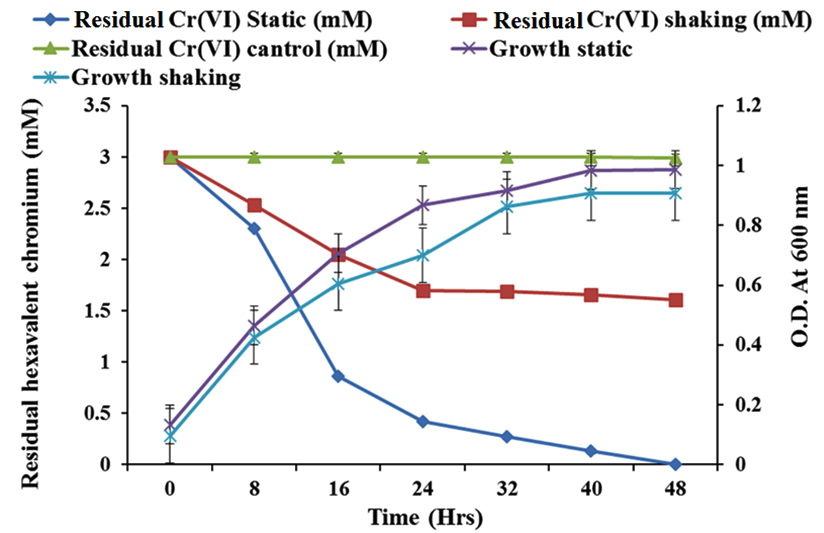
Fig. 2. Reduction of Cr(VI) and cell growth under static and shaking condition by SN6
Effect of metal ions on growth of SN6 and Cr (VI) reduction
Hexavalent chromium reduction in the presence of different metal ions at 1mM concentration was checked on bacterial consortium SN6. Reduction of hexavalent chromium by SN6 as slightly influenced in the presence of Ca2+, Cu2+, Zn2+, Ni2+, Co2+ and Cd2+. However Hg2+ and Ag+ drastically decrease the growth of SN6, subsequently it slowed reduced rate of Cr(VI) reduction (data not shown).
Effect of temperature and pH on Cr(VI) reduction
Cr(VI) reduction by SN6 under temperature range 33-41°C at 3mM initial Cr(VI) concentration at pH 7 was investigated (Fig. 3A). Bacterial consortium SN6 completely reduce 3mM Cr(VI) at 35°C within 40 h. Temperature above 40°C drastically affects the growth and consequently Cr(VI) reduction. Growth of Acinetobacter AB1 was maximum at 30°C whereas reduction of Cr(VI) was optimum in the range of 30-37°C (Essahale et al., 2012). Dhal et al., (2010) observed 35°C optimum temperature for Cr(VI) reduction by Bacillus sp. isolated from chromite contaminated soil. Temperature >40°C affects growth of bacteria and reduction of chromium negatively. This could be due to the effect of raised temperature on metabolic activity of cell. According to Narayani and Shetty (2013), at a temperature higher than optimum gradually decreases chromium reduction this could be due to alteration in membrane structure or inactivation of protein synthesizing due to alteration in ribosome.
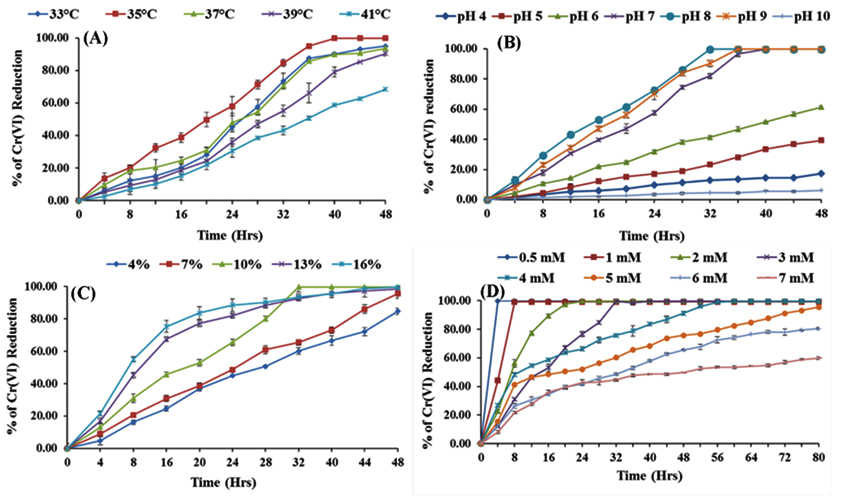
Fig. 3. Effect of experimental conditions on Cr(VI) reduction by SN6 (A) Temperature, (B) pH, (C) In-oculum size (D) Initial chromium concentration
Bacterial consortium SN6 grew well in the range of pH 7.0 to 9.0, 99.75% Cr(VI) reduction was achieved at pH 8.0. in 32 h at 35°C in LB medium (Fig. 3B). pH of the medium governs ionization of the proteins and also can deactivate or alters the structure of reactive site of an enzyme and hence its activity (Kathiravan et al., 2010). Yang et al., (2009) found pH 8.0 suitable for growth and Cr(VI) reduction by Intrasporangium sp. Q5-1. Liu et al., (2004) reported pH 7.0 for maximum Cr(VI) reduction by Pseudomonas aeruginosa. Wide pH range for Cr(VI) reduction is reported because of the dominance of chromate (CrO42-) ions in an aqueous medium in the range of pH 6.0 to 9.0 (McLean and Beveridge 2001)
Effect of inoculum size and initial Cr(VI) concentration
Reduction of hexavalent chromium by SN6 increased with the increase in inoculum size from 4-16% (Fig. 3C). Inoculum size above 10% i.e. 13% and 16% achieved more than 80% Cr(VI) transformation to Cr(III) in 24 h. Whereas with 10% inoculum size maximum Cr(VI) reduction take place in 32 h of incubation. Mc Lene et al., (2000) observed a similar trend that as an increase in the inoculum size Cr(VI) reduction increases up to a certain extent with Pseudomonas CRB5. Desai et al., (2008) also used 10% inoculum size for the maximum Cr(VI) reduction by Pseudomonas sp. G1DM21.
The effect of initial hexavalent chromium concentration on SN6 was studied over a range of 0.5-7 mM of Cr(VI) as shown in fig. 3D. Considerable amount of Cr(VI) reduction was achieved by SN6 over chromate concentration range studied. Bacterial consortium SN6 has ability to 5mM Cr(VI) was completely reduced to Cr(III) in 80 h while increase in the initial concentrations to 6.0 and 7.0 mM reduce the reduction to 80 and 60% after 80 h of incubation respectively. Yang et al., (2009) reported the similar type of results with Intrasporangium sp. Q5-1. 98 % of Cr(VI) was removed after 36, 48, and 84 h when initial chromium concentration was 2, 3 and 4 mM respectively. Several reports assist the present investigation that as chromate concentration increases rate of Cr(VI) reduction decreases with the correlation of bacteria growth. This could be due to the toxic and mutagenic effect of Cr(VI) on bacterial cells.
Characterization of reduced products
Chromium after reduction in trivalent form can be in cell pellets and in solution which depends largely on complexing agents in the media along with culture media and bacterial cell wall composition. To develop a sustainable process for industrial Cr(VI) waste removal its essential to know the state and form of reduced Cr(III) species, which could be in the form of hydroxide, adsorption on cell or form a nutrient chromium complex (Li et al., 2008). Cheng et al., (2010) also reported that presence of organic components like amino acids and organic acid from LB medium make Cr(III) unavailable for bacterial cell as an effect Cr(III) remains in soluble phase rather than immobilized in bacterial cell.
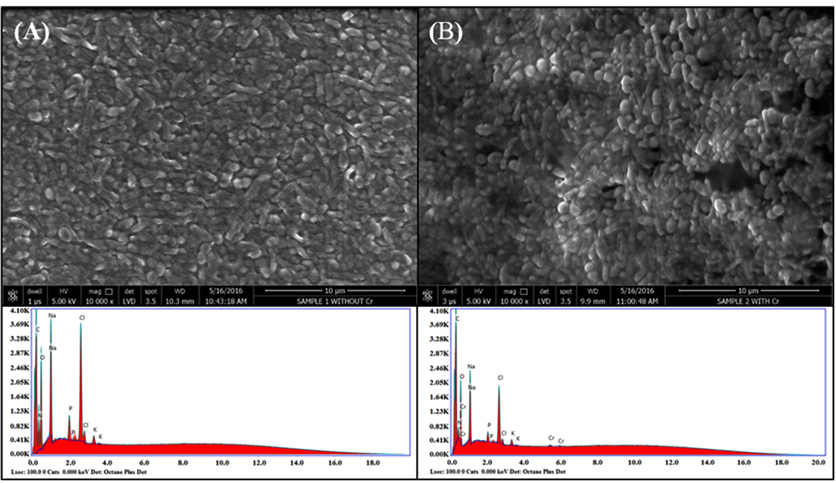
Fig. 4. (A) SEM-EDAX images of SN6 without Cr(VI); (B) SEM-EDAX images of Cr(VI) treated SN6
SEM-EDAX of SN6 incubated for 32 h in the presence and absence of Cr(VI) in LB broth was carried out and micrographs were presented in Fig. 4. The micrograph of SN6 grown with/without Cr(VI) showed different cell morphologies. In the presence of Cr(VI), the cell size increases as evident by the SEM micrograph (Fig 4B). Yang et al., (2009) reported an increase in the cell length of Intrasporangium sp.Q5-1 when incubated with K2CrO4 for 20 h. The EDAX of SN6 grown in the presence of Cr(VI) showed very small Cr peaks (Fig 4B) (bound or precipitated on the cell surface) while characteristic Cr peak was absent in the EDAX micrograph of the cells grown without Cr(VI) (Fig 4A). In addition, the peaks of another existing elements like C, O, N, Na, Cl, and K, were observed in EDAX bacterial cells grown in the presence and absence of Cr(VI).
To know the physical state of reduced Cr(III) species (crystalline or amorphous) and to know the bonding pattern of reduced chromium, powder XRD pattern and FT-IR spectra of SN6 grown in LB medium with or without Cr(VI) was performed (data not shown). The powder XRD pattern of SN6 grown with or without Cr(VI) is very similar. Result in the present investigation was similar with Dhal et al., (2010). The peaks formed in XRD analysis at 2q values 31.6, 45.4, 56.4 and 66.2 do not match with either Cr(OH)3 or any other form of Cr(III). This indicates the formation of a minute amount of amorphous Cr(OH)3. The FT-IR analysis of SN6 grown in the presence and absence of Cr(VI) in identical reduction experiment failed to reveal noteworthy difference in the size, shape and place of different characteristic peaks for the chromium oxides.
Characterization of cell pellets was further confirmed by total chromium analysis of supernatant by Inductive Coupled Plasma Spectroscopy (ICP-AES). The results indicated that more than 99.45% of total chromium remains in the supernatant fraction. A similar type of results was obtained by Megharaj et al., (2003) with Anthrobacter sp. and Bacillus sp.
Phytotoxicity study
The findings of phytotoxicity revealed that the presence of Cr(VI) in soil highly affect the growth of V. radiata L. The germination time prolonged and percentage of germination decreased to 40% (Table: 3). After germination, growth of V. radiata L. retarded due to the presence of Cr(VI), whereas in the presence of Cr(III), the growth pattern and germination percentage were in agreement with a blank. Several researchers had reported the similar type of results. Samantary (2002) reported that in the presence of Cr(VI), chlorophyll and protein content of V. radiata L. decreased, resulting in retarded growth of seedling. Wyszkowski et al., (2013) conducted an experiment of Cr(VI) and Cr(III) phytotoxicity on oats, concluded similar types of results.
The present investigation provided information on effective Cr(VI) biotransformation i.e. reduction of Cr(VI) by newly isolated native bacterial consortium-SN6, a potential Cr(VI) reducer and can be used for bioremediation in industrial effluents containing Cr(VI) to exploit its ability to reduce Cr(VI) and to tolerate heavy metals. Consortium-SN6 was enriched from the chromate contaminated soil of Lambha, Ahmedabad, India. The ability of SN6 to reduce Cr(VI) depends on pH, temperature, initial Cr(VI) concentration and inoculum size. Under favourable condition i.e. pH 8.0, temperature 35°C, 10% inoculum size and Cr(VI) concentration 3mM, complete reduction of Cr(VI) to Cr(III) was achieved in 32 h. Furthermore, SN6 has the ability to reduce Cr(VI) effectively in the presence of many metal ions. Characterization of reduced product revealed that Cr(III) remain majorly in the supernatant. Phytotoxicity on V. radiata L. conforms the detoxification of toxic, and mutagenic Cr(VI).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Ackerley, D. F., Barak, Y., Lynch, S. V., Curtin, J. & Martin, A. Effect of chromate stress on Escherichia coli K-12. Journal of Bacteriology. 2006; 188: 3371-3381.
- Ali, N. A., Ater, M., Sunahara, G. I. & Robidoux, P. Y. Phytotoxicity and bioaccumulation of copper and chromium using barly (Hordeum vulgare L.) in spiked artificial and natural forest soils. Ecotoxicology and Environmental Safety. 2004; 57: 363-374.
- Arias, Y. M. & Tebo, B. M. Cr(VI) Reduction by Sulfidogenic and Non sulfidogenic Microbial Consortia. Applied and Environmental Microbiology. 2003; 69(3): 1847–1853.
- Bachate, S. P., Nandre, V. S., Ghatpande, N. S. & Kodam, K. M. Simultaneous reduction of Cr(VI) and oxidation od As(III) by Bacillus firmus TE7 isolated from tannery effluent. Chemosphere. 2013; 90: 2273-2278.
- Balapure, K. H., Jain, K., Chattaraj, S., Bhatt, N. S. & Madamwar, D. Co-metabolic degradation of diazo dye-Reactive blue 160 by enriched mixed cultures BDN. Journal of Hazardous Materials. 2014; 279: 85-95.
- Camargo, F. A., Bento, F. M. Okeke, B. C. & Frankenberger, W. T. Chromate reduction by chromium resistant bacteria isolated from soils contaminated with dichromate. Journal of Environmental Quality. 2003; 32: 1228-1233.
- Cheng, Y., Yan, F., Huang, F., Chu, W., Pan. D., Chen, Z., Zheng, J., Yu, M., Lin, Z. & Wu, Z. Bioremediation of Cr(VI) and immobilization as Cr(III) by Ocrobactrum anthropic. Environmental Science and Technology. 2010; 44: 6357-6363.
- Desai, C., Jain, K. & Madamwar, D. Hexavalent chromate reductase activity in cytosolic fraction of Pseudomonas sp. G1DM21 isolated from Cr(VI) contaminated industrial landfill. Process Biochemistry. 2008; 43: 713-721.
- Dey, S. & A. K. Paul. Occurrence and Evaluation of Chromium Reducing Bacteria in Seepage Water from Chromite Mine Quarries of Orissa, India. Journal of Water Research and Protection. 2010; 2: 380-388.
- Dey, S. & Paul, A. K. Evaluation of in vitro reduction of hexavalent chromium by cell-free extract of Arthrobacter sp. SUK 1201. British Microbiology Research Journal. 2013; 3(3), 325-338.
- Dhal, B., Thatoi, H., Das, N. & Pandey, B. D. Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. Journal of Chemical Technology and Biotechnology. 2010; 85: 1471-1479.
- Essahale, A., Malki, M., Marin, I. & Moumni, M. Hexavalent chromium reduction and accumulation by Acinetobacter AB1 isolated from fez tanneries in morocco. Indian Journal of Microbiology. 2012; 52(1): 48-53.
- Fude, L., B. Harris, M. M. Urrutia, & T. J. Beveridge. Reduction of Cr(VI) by a Consortium of Sulfate-Reducing Bacteria (SRB III). Applied and Environmental Microbiology. 1994; 60(5): 1525-1531.
- Gonzalez, C. F., Ackerley, D. F., Park C. H. and Matin, A. A soluble flavoprotein contributes to chromate reduction and tolerance by Pseudomonas putida. Acta Biotechnologica. 2003; 23: 233-239.
- He, M., Li, X., Liu, H., Miller, J. S., Wnag, G. & Rensing, C. Characterization and genomic analysis of a highly chromate resistant and reducing bacterial strain Lysinibacillus fusiformis ZC1. Journal of Hazardous Materials. 2011; 185: 682-688.
- Huang, H., Wu, K., Khan, A., Jiang, Y., Ling, Z., Liu, P., Chen, Y., Tao, X., & Li, X. A novel Pseudomonas gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresource Technology. 2016; 207: 370-378.
- Kang, C., Wu, P., Li, L., Yu, L., Ruan, B., Gong, B. & Zhu, N. Cr(VI) reduction and Cr(III) immobilization by resting cellsof Pseudomonas aeruginosa CCTCC AB93066: spectroscopic, microscopic, and mass balance analysis. Environmental Science and Pollution Research. 2017; 24: 5949-5963.
- Kathiravan, M. N., Karthick, R., Muthu, N., Muthukumar, K. & Velan, M. Sonoassisted microbial reduction of chromium. Applied Biochemistry and Biotechnology. 2010; 160: 2000-2013.
- Li, B., Pan, D., Zheng, J., Cheng, Y., Ma, X., Huang, F. & Lin, Z. Microscopic investigations of the Cr(VI) uptake mechanism of living Ochrobactrum anthropi. Langmuir. 2008; 24: 9630-9635.
- Liu, Y. G., Xu, W. H. & Zeng, G. M. Experimental study on reduction by Pseudomonas aeruginosa. Journal of Environmental Science. 2004; 16: 795-801.
- McLean J., Beveridge, T. J. & Phipps, D. Isolation and characterization of a chromium-reducing bacterium from a chromate copper arsenate-contaminated site. Environmental Microbiology. 2000; 2: 611-619.
- McLean, E. O., Soil pH and lime requirement. In: Method of soil analysis, Part 2, 2nd edn. Agron Monogr 9, ASA and SSSA, Madison, Wis 1982.
- McLean, J. & Beveridge, T.J. Chromate reduction by a pseudomonad isolated from a site contaminated with chromate copper arsenate. Applied Environmental Microbiology. 2001; 67: 1076-1084.
- Megharaj, M., Avudainayagam, S. & Naidu, R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Current microbiology. 2003; 47: 51-54.
- Mishra, R. R., Dhal, B., Dutta, S. K., Dangar, T. K., Das, N. N. & Thatoi, H. N. Optimization and characterization of chromium(VI) reduction in saline condition by moderately halophilic Vigribacillus sp. Isolated from mangrove soil of Bhitarkanika, India. Journal of Hazardous Materials. 2012; 227-228, 219-226.
- Narayani, M. & Shetty, K. V. Chromium resistant bacteria and their environmental condition for hexavalent chromium removal: a review. Critical Reviews on Environmental Science and Technology. 2013; 43: 955-1009.
- Ohtake, H., Cervantes, C. & Silver, S. Decreased chromate uptake in Pseudomonas fluorescence carrying a chromate resistance plasmid. Journal of Bacteriology. 1987; 169: 3853-3856.
- Piñon-Castillo, H. A., Brito, E. M. S., Goni-Urriza, M., Guyoneaud, R., Duran, R., Nevarez-moorillon, G. V., Gutierrez-Corona, J. F., Caretta, C. A. & Reyna-Lopez. G. E. Hexavalent chromium reduction by bacterial consortia and pure strains from an alkaline industrial effluent. Journal of Applied Microbiology. 2010 109(6): 2173-2182.
- Samantary, S. Biochemical response of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere. 2002; 47: 1065-1072.
- Sayel, H., Bahafid, W., Joutey, N. T., Derraz, K., Benbrahim, K. F., Koraichi, S. I. & Ghachtouli, N. E. Cr(VI) reduction by Enterococcus gallinarum isolated from tannery waste-contaminated soil. Annual microbiology. 2012; 62: 1269-1277.
- Srinath, T., Khare, S., & Ramteke, P. W. Isolation of hexavalent chromium-reducing Cr-tolerant facultative anaerobes from tannery effluents. Journal of General and Applied Microbiology. 2001; 47: 307-312.
- Standard Methods for the Examination of Water and Wastewater 1998 20th edn, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA.
- Thacker, U. & Madamwar, D. Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World Journal of Microbiology and Biotechnology. 2005; 21: 891-899.
- Thacker, U., Parikh, R., Shouche, Y. & Madamwar D. Reduction of chromate by cell-free extract of Brucella sp. Isolated from Cr(VI) contaminated sites. Bioresource Technology. 2007; 98: 1541-1547.
- Thacker, U., Parikh, R., Shouche, Y. & Madamwar, D. Hexavalent chromium reduction by Providencia sp. Process Biochemistry. 2006; 41: 1332-1337.
- Wyszkowski, M. & Radziemska, M. Assessment of Tri- and Hexavalent Chromium Phytotoxicity on Oats (Avena sativa L.) Biomass and Content of Nitrogen Compounds. Water, Air and Soil Pollution. 2013; 224: 1619-1633.
- Xu, W., Jian, H., Liu, Y. G., Zeng, G. M., Li, X. & Zhang, W. Bioreduction of chromate by an isolated Bacillus anthracis Cr-4 with soluble Cr(III) product. Water, Air & Soil Pollution. 2015; 226: 82-90.
- Yang, J., He, M. & Wang, G. Removal of toxic chromate using free and immobilized Cr(VI)-reducing bacterial cells of Intrasporangium sp.Q5-1. World Journal of Microbiology and Biotechnology 2009. DOI 10.1007/s11274-009-0047-x.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


