ISSN: 0973-7510
E-ISSN: 2581-690X
Silver nanoparticles (AgNPs) have become indispensable due to their wide range of applications across various industries, particularly in enhancing human health and environmental sustainability. Recently, the use of biological sources for AgNP synthesis has gained significant attention, as it minimizes adverse effects on animals, plants, humans, and the environment. This study focuses on the biosynthesis of AgNPs using the culture supernatant of Bacillus sp. EPS003, a soil isolate. The UV-visible spectrum of the biosynthesized nanoparticles revealed a surface plasmon resonance peak at 420 nm, confirming the formation of AgNPs. Functional groups were identified through Fourier transform infrared (FTIR) spectroscopy analysis. The synthesized AgNPs had an estimated size of 71.34 nm, and their stability was confirmed using zeta potential measurements. Furthermore, the biosynthesized AgNPs exhibited excellent dye degradation potential. They demonstrated maximum catalytic activity within a few minutes of incubation against eosin yellow and methyl orange dyes, achieving decolorization efficiencies of up to 62.3% and 73.4%, respectively. These biosynthesized AgNPs may be useful in bioremediation applications, helping to mitigate environmental stress caused by the release of toxic dye-rich industrial effluents.
Silver Nanoparticles, Photodegradation, Catalytic Activity, Environment, Biosynthesis
Nanotechnology is an emerging field that integrates biology, physics, chemistry, material science, and medicine. This technology enables the development of new functional materials at the nanoscale, possessing exceptional physical, chemical, optical, mechanical, electrical, magnetic, and biological properties.1 In recent years, metal nanoparticles-including copper, iron, gold, silver, titanium, zinc, cerium, and thallium-have been widely synthesized and applied in various industrial and environmental sectors.2,3 Among these, silver is the most commonly used due to its superior and unique functional characteristics. Silver has long been employed as an antimicrobial agent and antibiotic substitute. More recently, Silver nanoparticles (AgNPs), with significantly improved structural and functional properties, have replaced conventional silver and silver-based compounds.4,5 In environmental bioremediation, AgNPs demonstrate remarkable efficiency compared with other compounds. AgNPs can be synthesized using physical, chemical, and biological methods. However, physical methods are relatively slow and inefficient, while chemical synthesis often involves harsh reagents that pose environmental risks.6 Consequently, biological methods have gained prominence due to their eco-friendliness and the superior structural and functional properties of biosynthesized AgNPs compared with those synthesized using physical and chemical approaches.7 AgNPs have been extensively studied for applications in therapy, diagnostics, biosensors, composite fibers, cryogenic superconducting materials, cosmetics, and electronic components.8 Their applicability depends on factors such as size, shape, composition, structure, and the reducing agents used in synthesis.9,10 The biological agents employed in AgNP synthesis play a crucial role in determining their potential and application. Common biological sources include bacterial or fungal culture supernatants and plant extracts.11 Biosynthesis involves oxidation and reduction reactions facilitated by natural capping agents, converting bulk silver or silver-based compounds into nanoparticles.12 This method overcomes several limitations of conventional nanoparticle synthesis techniques. Industrialization has led to significant contamination of water resources with toxic pollutants, particularly industrial effluents rich in synthetic dyes. These pollutants originate from textile, paper, plastic, leather, food, cosmetic, and pharmaceutical industries. When released into water bodies, synthetic dyes cause eutrophication, reduce oxygen levels, and obstruct sunlight penetration, severely impacting aquatic ecosystems and communities that are dependent on these water sources. Thus, it is crucial to treat dye-laden effluents before discharge into water bodies or land. Conventional treatment methods include adsorption, ultrafiltration, and physical, chemical, and electrochemical approaches.13,14 Unfortunately, majority of these methods are less useful in addressing the contamination problem caused by the release of huge concentrations of dyes in the industrial effluents and are less efficient, cost intensive, time consuming, and often result in other secondary problems. Photocatalytic dye degradation has emerged as a promising alternative, utilizing reducing agents in the presence of sunlight.15 Metal nanoparticles, particularly AgNPs, have demonstrated effective photocatalytic properties, leading to rapid dye decolorization.16 However, the widespread application of AgNPs is restricted due to the hazardous chemicals used in their synthesis. In this study, we aimed to achieve the green synthesis of AgNPs using the culture supernatant of a novel soil isolate, Bacillus sp. EPS003, and to evaluate its photocatalytic degradation potential against eosin yellow and methyl orange dyes.
All chemicals used in this study were procured from HiMedia Laboratories Private Limited, Mumbai.
Collection of cell-free supernatant
A 1% inoculum of Bacillus sp. EPS003 was added to freshly prepared sterile nutrient agar supplemented with 10% sucrose and incubated overnight at 37 °C in a shaking incubator. The overnight culture was then centrifuged at 6000 rpm for 15 min and the cell-free supernatant was collected.17
Biosynthesis of AgNPs
A 1 mM silver nitrate (AgNO3) solution was prepared in water and used for AgNP synthesis. The reaction mixture consisted of a 1:1 ratio of cell-free supernatant and 1 mM AgNO3 as shown in Figure 1a. The reaction was incubated in the dark for 24 h, and the color change was observed (Figure 1b).18
Characterization of biosynthesized AgNPs
UV-visible spectroscopy and Fourier transform infrared spectroscopy analysis
The reduction of AgNO3 to AgNPs was confirmed using a UV-visible spectrophotometer (Dynamica Halo DB-20 Double Beam Spectrophotometer, Australia). A wavelength scan from 200 to 800 nm was performed, and the UV-Vis spectrum was plotted to identify the surface plasmon resonance peak of AgNPs. Further characterization was conducted using FTIR spectroscopy (IR Tracer 100, Shimadzu Corporation, Japan) in the range of 500-4000 cm-1 at a resolution of 4 cm-1.
Zeta size and potential analysis
Bioreduced AgNPs were analyzed for size and zeta potential using a Zetasizer (Zetasizer Nano ZS, ZEN3600, Malvern, UK). The mean particle size and surface charge were determined.
Evaluation of photocatalytic potential of AgNPs
A 10 mL solution of 1 mM eosin yellow and methyl orange dyes was prepared in water. One milliliter of biosynthesized AgNPs was added to the dye solution, mixed thoroughly, and placed under sunlight for up to 6 h. Color changes in the dye solution were visually observed, and the optical density (OD) was measured at the respective maximum absorption wavelengths: Eosin Yellow (517 nm) and Methyl Orange (465 nm). The percentage of dye degradation was calculated using Equation 1:
% dye degraded = 100 × (C0 – C / C0) …(1)
Where C0 is the initial OD at time
t = 0, and C is the OD after 6 h of photocatalytic degradation.19
The color change in the mixture containing 1 mM AgNO3 was observed visually, as shown in Figure 1. Over 24 h of incubation, the initially yellow mixture gradually turned brown to dark brown, confirming the formation of AgNPs. During biosynthesis, metabolites present in the cell-free supernatant act as reducing agents, converting silver ions (Ag+) into silver atoms (Ag0).20 These Ag serve as nucleation sites and gradually grow into AgNPs. Sunlight plays a crucial role in enhancing the unique properties of biosynthesized AgNPs. Thus, the color change from yellow to brown serves as an initial indication of AgNP formation. Further confirmation was obtained by analyzing the absorption peak in the UV-Vis spectrophotometer, corresponding to the expected wavelength range.
Figure 1. Biosynthesis of AgNPs: a) Yellow colour of 1 mM AgNO3 and cell free supernatant mixture; b) Colour change from yellow to brown after 24 h of incubation
Characterization of biosynthesized AgNPs
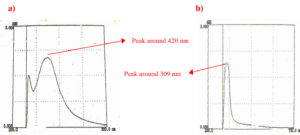
The spectral characteristics of the biosynthesized AgNPs were studied using UV-Vis and FTIR spectroscopy. As shown in Figure 2, the surface plasmon resonance (SPR) peak for biosynthesized AgNPs appeared at approximately 420 nm, whereas AgNO3 exhibited a peak at 309 nm. The structural and functional properties of biosynthesized AgNPs depend on their interaction with light, which occurs through SPR. The collective oscillation of free electrons in biosynthesized AgNPs, induced by incident light, typically falls within the 400-450 nm range, producing the characteristic brown color.21
Figure 2. a) UV-Vis spectrum: Surface Plasmon Resonance peak of biosynthesized AgNPs was observed at 420 nm; b) Surface Plasmon Resonance peak of AgNO3 was observed at 309 nm
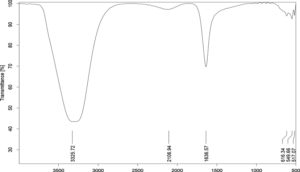
FTIR spectroscopic analysis revealed peaks corresponding to various functional groups, as shown in Figure 3. The most intense peak bands were observed at 3325.72, 2106.94, 1636.57, 516.34, 549.66, and 517.07 cm-1. The peak at 3325.72 cm-1 may be attributed to the stretching vibrations of hydroxyl groups present in water molecules, phenols, or alcohols in the biomolecules within the culture supernatant used for biosynthesis. The peak at 1636.57 cm-1 indicates the presence of amide groups, suggesting the involvement of proteins and peptides in biosynthesis. Peaks below 700 cm-1 correspond to the stretching vibrations of Ag-O bonds, further confirming the biological synthesis of AgNPs.22
Figure 3. FTIR spectrum of biosynthesized AgNPs: Characteristic peaks for AgNPs were observed at 3325.72, 2106.94, 1636.57, 516.34, 549.66, and 517.07 cm-1
Determination of size and stability of biosynthesized AgNPs
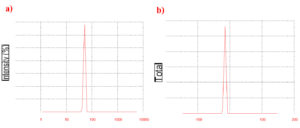
The average particle size of the biosynthesized AgNPs was determined using a zeta sizer, revealing a size of approximately 71.34 nm (Figure 4a). The overall negative charge (-15.8 mV), as indicated by the zeta potential analysis (Figure 4b), may be attributed to biomolecules such as proteins, polysaccharides, phenolic compounds, and flavonoids present in the cell-free extract, which act as reducing and stabilizing agents.23 These biomolecules predominantly contain negatively charged functional groups, such as carboxyl (-COO–), hydroxyl (-OH–), or phosphate groups. When adsorbed onto the nanoparticles, these groups impart a net negative charge, leading to high stability due to strong intermolecular interactions.
Figure 4. Particle size and stability of biosynthesized AgNPs: a) Average particle size of biosynthesized AgNPs was obtained around 71.34 nm. b) Zeta potential analysis of biosynthesized AgNPs showed overall negative charge of -15.8 mV
Photocatalytic degradation of eosin yellow and methyl orange dyes
Initially, the color of the dye solutions was visually observed, and their OD was measured. Observations were recorded at regular intervals, and at the end of 6 h, the final color and OD values for both control and experimental samples were documented (Table). The experiment was conducted in triplicate, and the reported values represent the average results.
Table:
Estimation of % dye degradation
Eosin yellow |
OD517 at time, t = 0 h 2.265 |
OD517 at time, t = 6 h 0.854 |
% dye degradation 62.3 |
Methyl orange |
OD465 at time, t = 0 h 2.973 |
OD465 at time, t = 6 h 0.79 |
% dye degradation 73.4 |
The dye degradation potential of biosynthesized AgNPs was comparable to that of chemically synthesized AgNPs. As reported by Al-Zaban et al.,24 methylene blue dye degradation using AgNPs synthesized with honey reached approximately 60% within 6 h and required 72 h to achieve a degradation efficiency of 92%. In some cases, chemically synthesized AgNPs exhibited superior dye degradation efficiency. However, due to the hazardous effects of chemical reducing agents used in AgNP synthesis, biosynthesized AgNPs are the preferred choice, particularly for water treatment applications. Chemical reducing agents such as ethylene glycol and sodium citrate are known to cause serious health issues, including respiratory disorders, nausea, and weakness.25 As evident from Figure 5, the color of the dye solution in the control remained unchanged, whereas the test samples containing AgNPs exhibited a significant reduction in color intensity within 6 h. The loss of color intensity may be attributed to the generation of reactive oxygen species (ROS) by AgNPs under sunlight exposure. These ROS can degrade eosin yellow and methyl orange, transforming them into colorless or less intensely colored products.26 The change in the color of eosin yellow and methyl orange is illustrated in Figures 5a and 5b, respectively. The intensity of eosin yellow decreased drastically over 6 h of sunlight exposure, with similar results observed for methyl orange. At the end of the experiment, biosynthesized AgNPs achieved 62.3% and 73.4% degradation of eosin yellow and methyl orange dyes, respectively. The degradation process was effective within 6 h. While some chemically synthesized AgNPs exhibited superior dye degradation potential, the environmental and health hazards associated with chemical synthesis highlight the advantages of biosynthesized AgNPs.
The biosynthesis of AgNPs provides a sustainable and eco-friendly alternative to conventional chemical and physical synthesis methods. Utilizing microorganisms not only reduces environmental impact but also enhances the functional properties of AgNPs through natural capping agents. This green approach enables the production of nanoparticles with controlled size, shape, and stability, making them highly suitable for photocatalytic applications. The photocatalytic performance of biosynthesized AgNPs demonstrated significant potential in dye degradation under light irradiation, offering a promising solution for environmental remediation.
Future research should focus on optimizing synthesis parameters to enhance the photocatalytic efficiency and scalability of biosynthesized AgNPs. Additionally, exploring synergistic effects by combining biosynthesized nanoparticles with other photocatalysts can further drive innovation in sustainable nanotechnology. This study underscores the pivotal role of green chemistry in advancing nanomaterial development for cleaner and more sustainable technologies.
ACKNOWLEDGMENTS
The authors thank Mepco Schlenk Engineering College, Sivakasi, for providing the necessary infrastructure facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Abbasi R, Shineh G, Mobaraki M, Doughty S, Tayebi L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: a review. J Nanopart Res. 2023;25(3):43.
Crossref - Nishan U, Ullah I, Muhammad N, et al. Investigation of Silver-Doped Iron Oxide Nanostructures Functionalized with Ionic Liquid for Colorimetric Sensing of Hydrogen Peroxide. Arab J Sci Eng. 2023;48(6):7703-7712.
Crossref - Asad M, Muhammad N, Khan N, et al. Colorimetric acetone sensor based on ionic liquid functionalized drug-mediated silver nanostructures. J Pharm Biomed Anal. 2022;221:115043.
Crossref - Ananda T, Modi A, Managuli V, Mukhopadhyay C. Antimicrobial Property of Silver Nanoparticles: Effects of Concentration and Temperature on Bacterial Isolates. J Pure Appl Microbiol. 2023;17(2):1118-1127.
Crossref - Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver Nanoparticles and Their Antibacterial Applications. Int J Mol Sci. 2021;22(13).
Crossref - Osman AI, Zhang Y, Farghali M, et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ Chem Lett. 2024;22(2):841-887.
Crossref - Arshad F, Naikoo GA, Hassan IU, et al. Bioinspired and Green Synthesis of Silver Nanoparticles for Medical Applications: A Green Perspective. Appl Biochem Biotechnol. 2024;196(6):3636-3669.
Crossref - Al-asbahi MGSS, Al-Ofiry BA, Saad FAA, Alnehia A, Al-Gunaid MQA. Silver nanoparticles biosynthesis using mixture of Lactobacillus sp. and Bacillus sp. growth and their antibacterial activity. Sci Rep. 2024;14(1):10224.
Crossref - Zhao X, Xu X, Ai C, Yan L, Jiang C, Shi J. Advantages of silver nanoparticles synthesized by microorganisms in antibacterial activity. In: Abd-Elsalam KA, ed. Green Synthesis of Silver Nanomaterials. 2022:571-586.
Crossref - Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996-9031.
Crossref - Bloch K, Pardesi K, Satriano C, Ghosh S. Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine. Front Chem. 2021;9:624344.
Crossref - Bamal D, Singh A, Chaudhary G, et al. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials. 2021;11(8):2086.
Crossref - Katheresan V, Kansedo J, Lau SY. Efficiency of various recent wastewater dye removal methods: A review. J Environ Chem Eng. 2018;6(4):4676-4697.
Crossref - Al-Tohamy R, Ali SS, Li F, et al. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf. 2022;231:113160.
Crossref - Khan S, Noor T, Iqbal N, Yaqoob L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega. 2024;9(20):21751-21767.
Crossref - Altammar KA. A review on nanoparticles: characteristics, synthesis, applications, and challenges. Front Microbiol. 2023;14:1155622.
Crossref - Samuel MS, Jose S, Selvarajan E, Mathimani T, Pugazhendhi A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J Photochem Photobiol B. 2020;202:111642.
Crossref - Yurtluk T, Akcay FA, Avc A. Biosynthesis of silver nanoparticles using novel Bacillus sp. SBT8. Prep Biochem Biotechnol. 2018;48(2):151-159.
Crossref - Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J Adv Res. 20167(1):17-28.
Crossref - Singh P, Kim YJ, Zhang D, Yang DC. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016;34(7):588-599.
Crossref - Elsayed MA, Othman AM, Hassan MM, Elshafei AM. Optimization of silver nanoparticles biosynthesis mediated by Aspergillus niger NRC1731 through application of statistical methods: enhancement and characterization. 3 Biotech. 2018;8(3):132.
Crossref - Alfryyan N, Kordy MGM, Abdel-Gabbar M, Soliman HA, Shaban M. Characterization of the biosynthesized intracellular and extracellular plasmonic silver nanoparticles using Bacillus cereus and their catalytic reduction of methylene blue. Sci Rep. 2022;12(1):12495.
Crossref - Raza S, Ansari A, Siddiqui NN, Ibrahim F, Abro MI, Aman A. Biosynthesis of silver nanoparticles for the fabrication of non cytotoxic and antibacterial metallic polymer based nanocomposite system. Sci Rep. 2021;11(1):10500.
Crossref - Al-Zaban MI, Mahmoud MA, AlHarbi MA. Catalytic degradation of methylene blue using silver nanoparticles synthesized by honey. Saudi J Biol Sci. 2021;28(3):2007-2013.
Crossref - Vishwanath R, Negi B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Current Research in Green and Sustainable Chemistry. 2021;4:100205.
Crossref - Krishna PG, Mishra PC, Naika MM, et al. Photocatalytic Activity Induced by Metal Nanoparticles Synthesized by Sustainable Approaches: A Comprehensive Review. Front Chem. 2022;10:917831.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.