ISSN: 0973-7510
E-ISSN: 2581-690X
Bimetallic nanocomposites have evolved into a significant smart material in the recent past. Owing to the growing interest, we herein report the biosynthesis of bimetallic silver doped copper (Cu-Ag) nanocomposites using green methods by utilizing aqueous extract of Carica papaya leaves. The optical property and the surface morphology of the nanoparticles were determined by using various analytical techniques like Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Analysis (EDAX) and Transmission Electron Microscopy (TEM). The redox behaviour of the bimetallic nanocomposites was studied using Cyclic Voltammetry (CV) with platinum electrode in 0.1M KCl solution at different scan rates and concentrations. The FTIR revealed the presence of active components of the leaf extract which played the roles of surfactants, stabilizing, capping, and reducing agents. Similarly, SEM with EDAX exhibited the presence of spherically agglomerated Cu-Ag nanocomposites and TEM images revealed a particle size of 20 nm. The gradual increase in peak current was observed in CV with increase in the scan rates and concentrations apparently. The bimetallic nanocomposites showed potential anti-bacterial, anti-cancerous activity and the reports are provided in detail.
Green synthesis, cyclic voltammetry, Electron microscopy, bimetallic nanoparticles, antibacterial, anti-cancerous
The field of nanotechnology has emerged into great area of interest within the present scenario of research in material science. The synthesis of mono and bimetallic nanocomposites is important as they provide many pathways and applications such as biomedical drug delivery1,2 water treatment3 agriculture4 sensors5 and textiles.6 The efficacy in terms of number of particles that can be prepared and the high surface area to volume ratio are the significant reasons for nanoparticles to be effective in small amounts.7 The literature revealed the use of Physio-Chemical for the synthesis of mono and bimetallic nanocomposites. The preparation of Ag nanoparticles is generally done by the chemical reduction of silver salt solution in water or an organic solvent to produce an organic suspension and for synthesizing Cu nanoparticles, the most common approach is by creating microemulsions.8 It is also worthy to note that the microemulsion method is expensive since it requires a large amount of surfactant and organic solvents9,10 and other physical methods using laser ablation,11 radiolysis or aerosol techniques,12 although remarkably effective, requires costly instruments and large amount of energy.13 The chemicals employed are both toxic and cause environmental pollution. Therefore, more emphasis is shown on techniques and studies that use chemical free green approach for the synthesis of nanocomposites. A much-defined structure, morphology, size of nanoparticles is obtained through green methodologies than through physical and chemical methods.14 Green synthesis and stabilization of metal nanoparticles are found to be more efficient for the synthesis of nanocomposites.15,16 The main aim of this study is to reduce the usage of toxic chemicals for the synthesis by utilizing the plant extract as reducing and stabilizing agent. Metal ions are reduced to nanoparticles by plant extract which acts as reducing agent in a single step.17
Bimetallic nanocomposites are a result of alloying of two metals. Besides their individual properties, the two combined metals show more efficacious nature due to their synergistic effect, thus proving to be more advantageous over the individual monometallic nanoparticles. A review on literature reveals many methods of synthesis of bimetallic nanoparticles but a few using plant extracts. Therefore, plant extracts which contains alkaloids, flavonoids, terpenoids and polyphenols reduce the metal and stabilize the synthesized nanocomposites.18-20 There are reports on efficient biobased green methods to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract.21 The aqueous leaf extract contains polyphenols and the hydroxyl groups in polyphenols act as reducer and stabilizer in the synthesis of Cu-Ag bimetallic nanocomposites.22 Among all the nanoparticles, silver and copper are very important due to their physical, chemical, and biological properties and their applications in wide areas. This Ag-Cu bimetallic nanocomposites has significant catalytic property and can be a potent biosensor. Their high conductivity is another attractive feature that increase their usability. Their anticancer and antibacterial behaviour is of great medical scope as well.
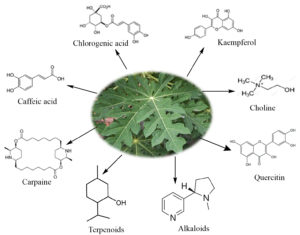
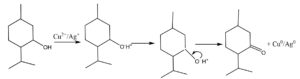
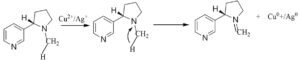
Carica papaya which belongs to the family Caricaceae is well known for its various nutritional and health benefits. The fruit and leaf extract exhibit antioxidant and antimicrobial properties. Their extracts are used to treat diabetes, indigestion and even for curing open wounds. The fruit is proven to have effective anticancer property too. Some biomolecules present in Carica papaya are caffeic acid, chlorogenic acid, kaempferol, quercitin, carpaine, choline, etc. These biomolecules (Fig. 1) are responsible for their reducing, capping and stabilizing properties, making it possible for the formation of nanocomposites. Schemes 1 and 2 show the mechanism of the reduction of copper and silver ions to their nanocomposites with the help of terpenoids and alkaloids respectively. In this study, we have synthesised Cu-Ag nanocomposites through simultaneous reduction of Ag and Cu ions using Carica papaya aqueous leaf extract and evaluated their redox, antibacterial and anticancerous efficacy.
Materials
The chemicals for the synthesis were chosen to be of Analytical grade (AR) and were used as such without any purification. Double distilled water was used for preparing the plant extract. The leaves of Carica papaya were collected from Chennai, India.
Preparation
Fresh Carica papaya leaves were collected from the city of Chennai, Tamilnadu, India. It was cleaned with distilled water to remove impurities which can potentially hamper the process and then it was dried completely without any moisture. The dried leaves were finely powdered using mechanical grinder and then stored. 5g of plant leaves with 100 mL of double distilled water is taken in a 500 mL beaker and warmed at 70°C for about 1h and then it was cooled to room temperature. This solution was filtered to get clear aqueous extract and was refrigerated for further use.
Biosynthesis of Ag-Cu bimetallic nanocomposites
In this green eco-friendly method, the stock solution (1mM) was prepared using silver nitrate [AgNO3], Cupric nitrate [Cu(NO3)2] and aqueous extract of Carica papaya leaves. Standard solutions of AgNO3 and [Cu(NO3)2] were prepared and a known volume of the solution was added to 1% solution of the extract to prepare the requisite Cu-Ag nanocomposites. To achieve the standardisation, several trials were carried out at different concentrations of standard solutions and plant extract and the normalization was achieved by adding 10 mL of aqueous plant extract to 10mL of each 1mM AgNO3 and 1mM [Cu (NO3)2] which was made to undergo constant stirring at room temperature (RT). The addition of plant extract was done slowly and carefully monitored to prevent the agglomeration of the particles and after complete addition of plant extract, the mixture is stirred for about 3h at 80°C at constant temperature and pH.
Electrochemical Measurements
To evaluate the electrochemical activity of Cu-Ag bimetallic nanocomposites, Cyclic Voltameter (CHI 604E) was employed. The three-electrode cell consists of Ag/AgCl in 1M KCl as reference electrode, Pt wire as counter electrode and platinum electrode as working electrode. The electrochemical analysis was carried out with 0.1M KCl as supporting electrolyte. The electrochemical response of Cu-Ag nanocomposites was plotted.
Antibacterial studies
Test bacterial Strain
The synthesized nanocomposites were tested for antibacterial activity using Bacterial strain Staphylococcus aureus MTCC 96 and the selected strain was obtained from MTCC (IMTECH, Chandigarh, India).
In vitro antibacterial activity
Well diffusion method23 was employed to determine the antibacterial activity. The sample plates were prepared by adding 25 mL of molten Mueller Hinton agar into a sterile Petri plate (Himedia, Mumbai, India). The plates were allowed to solidify, after 18h growth (OD adjusted to 0.6) 100 µl of test pathogenic bacteria cultures were transferred into the plate and the culture lawn was made by using sterile cotton swabs. The pathogenic bacteria could dry and settle and after five minutes a sterile cork borer was used to make 5 mm well on the agar. The test samples were dissolved in sterile water and loaded into wells with various concentrations such as 25µg/well, 50µg/well, 75µg/well and 100µg/well. The azithromycin (30µg/well) added well served as positive control. The plates were incubated at 37°C in an exceedingly bacteriological incubator for 24h.24 The antibacterial activity of the nanocomposites was determined by measuring the diameter of zone of inhibition around the sample well and using antibiotic zone inhibition as standard (Himedia, Mumbai, India).
Anti-Cancerous studies
The anticancerous activity of the bimetallic nanocomposites was evaluated by trypsinization procedure25 on HeLa cells Dulbecco’s Modified Eagle’s Medium (DMEM), which was obtained after removing the growth medium. The procedure involves the addition of 10% fetal calf serum (FCS) into the disaggregated cells of DMEM in 25 mL flask. Further, the passage of cells is suspended, and the cells are homogenized with the help of a pipette.24 well culture plate is filled with 1 mL of homogenized cell suspension with various concentration of test sample (0 to 200ug/mL) and incubated at 37°C for 48hrs after which the cells were examined. Cytotoxicity assay was carried out using (3-(4, 5-dimethyl thiazol-2yl)-2, 5- diphenyltetrazolium bromide (MTT). The experimental procedure involves reduction of MTT and is found to be one of the most frequently used methods for measuring cell proliferation and cytotoxicity.
FTIR spectroscopy
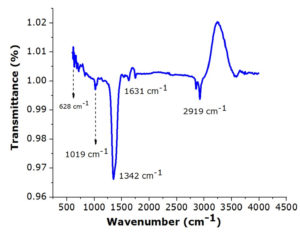
Silver doped copper (Ag-Cu) bimetallic nanocomposites were synthesized through a green chemistry route with aqueous extract of Carica papaya leaves by reduction of metal ions. The FTIR spectra indicated the O-H stretching of phenols and N-H stretching of amides, C-H bond of the aldehydic functional group, C=O group, C=C and C-C aromatic stretching, N-H, C-H and amino group which indicated the presence of phenols, polyphenols, amino acids and primary amines. The band 3588 cm-1 can be attributed to the overtone of the amide N-H band and the stretching vibration of the O-H group, is attributed to the OH groups in phenols, carbohydrates and/or proteins present in the sample as shown in the Fig. 2. The peaks at 2919 cm-1 and 2853 cm-1 are attributed to the C-H stretching and the band at 1631 cm-1 are due to the stretching vibration of carbonyl groups. The absorption band at 1342 cm-1 26 correspond to the C-N stretching vibration band of aliphatic amines. To summarise, the absorption bands observed at 3588 cm-1, 2919 cm-1, 1631 cm-1,1628 cm-1 and 1342 cm-1, are correlated to the amine and phenolic groups present in the aqueous plant extract27 and are involved in the reduction and stabilization of the Cu-Ag nanocomposites during the bio mediated synthesis.28
Electron microscopy analysis
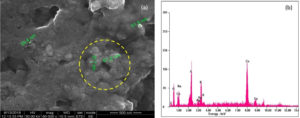
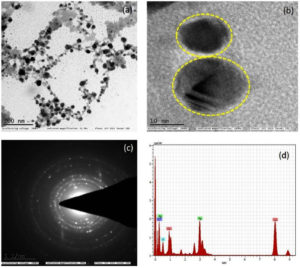
The SEM studies were carried out with an accelerating voltage of 30 KV at 60000x magnification, to determine the surface morphology of the particles. The diameters of the Cu-Ag bimetallic nanocomposites were found to be around 20 nm to 50 nm. EDAX is an analytical technique used for the elemental analysis of a sample which confirmed the presence of Ag and Cu nanoparticles as shown in Fig. 3(a-b). Presence of K and S might be originated from the Carica papaya leaves extract that are present on the surface of the nanocomposites due to adsorption.29 Therefore, the finding from EDAX is in accordance with TEM in which the biomolecules from the Carica papaya leaves was responsible as the stabilizing and reducing agents as shown in Fig. 4 a-d. From EDAX graph which is also observed that the intensity of Cu nanoparticles is greater than Ag nanoparticles. The Cu-Ag nanocomposites are mostly spherical in shape.30,31 The concentric circles in SAED images indicated the polycrystalline nature of the synthesized bimetallic nanocomposites (Fig. 4(c).
Fig. 3 a-b. SEM image and EDAX of the bimetallic nanocomposites of Cu-Ag formed using Carica papaya leaf extract.
Fig. 4 a-d. TEM micrograph of the Cu-Ag nanocomposites which revealed that nanoparticles were predominantly spherical and monodispersed.
Cu-Ag nanocomposites electrochemical response
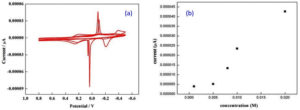
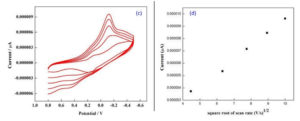
The redox behaviour of the bimetallic nanocomposites is determined by using Cyclic Voltammetry.32,4 The bimetallic nanoparticle with the colloidal media (Carica papaya leaf extract) of different concentrations (0.001M, 0.005M, 0.008M, 0.01M and 0.02M) were studied using CV technique at various scan rates from 20 mV/s to 100 mV/s. It is assumed that the interaction of the bimetallic nano system with the colloidal surrounding can generate oxides33 which may contribute to the electrochemical response observed as shown in Fig. 5 a-b. The CV curve shows strong anodic and cathodic peaks34 and the anodic peak was attributed to the oxidation of Ag0 into Ag+ whereas cathodic peak indicates the reduction of the two components, Cu2+ into Cu+ and Ag+ into Ag0 respectively. Since the electrochemical reaction is a multi-electron and multi-step in nature it is observed that the bimetallic system had undergone quasi-reversible electron transfer process as shown by the CV curves in Fig. 5 c-d.
Fig. 5 a-b. Randles sevcik plot for bimetallic nanoparticle of colloidal media with different concentration 0.001M, 0.005M, 0.008M, 0.01M, 0.02M at scan rate of 60 mV/s.
Fig. 5 c-d. Randles sevcik plot for bimetallic nanoparticle of colloidal media with different scan rate 20 mV/s to 100 mV/s at concentration of 0.008M.
Antibacterial Activity
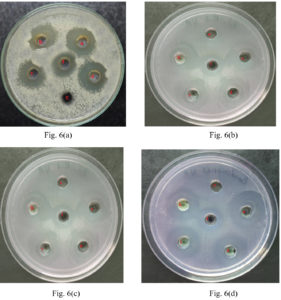
The synthesized Cu-Ag bimetallic nanocomposites were tested for its antibacterial activity against gram positive bacteria Staphlyococcus aureus [Fig. 6 a], Bacillus subtilis [Fig. 6 b] and gram negative bacteria Escherichia coli [Fig. 6 c], Pseudomonas aeruginosa [Fig. 6 d]. The zone of inhibition (ZOI) test is a qualitative way of determining the antibacterial efficacy of the bimetallic nano composites and the study revealed that the ZOI was 24 mm for the concentration 100µg/well as shown in Table 1. There is tremendous increase in the antibacterial activity when Cu-Ag bimetallic nanocomposites were used. This can be widely used in antimicrobial coating in medical instruments and in textiles. Test samples were more active than the standard antibiotic which was clearly seen in Fig. 6.
Table (1):
Antibacterial activity of Cu-Ag bimetallic nanoparticles synthesized from Carica papaya leaf extract.
Concentrations (mg/well) |
ZOI (mm) Staphylococcus aureus |
ZOI (mm) Baccillus Subtilis |
ZOI (mm) Pseudomonous aeruginosa |
ZOI (mm) Escherichia coli |
|---|---|---|---|---|
25 |
17 |
10 |
7 |
8 |
50 |
19 |
12 |
8 |
10 |
75 |
21 |
15 |
9 |
12 |
100 |
24 |
17 |
10 |
14 |
Fig. 6 a, b, c, d. are the antibacterial activity of synthesized Cu-Ag nanocomposites against Staphylococcus aureus(+), Bacillus subtilis(+), Escherichia coli(-) and Pseudomonous aeruginosa(-) respectively.
Anti-cancerous Activity
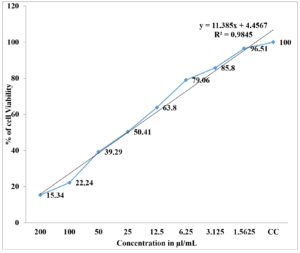
The studies of the in-vitro cytotoxicity activity revealed that the HeLa cell lines (25) are prone to be cytotoxic when tested with Cu-Ag bimetallic nanocomposites against standard doxorubicin. The tested sample concentrations are treated for about 48 hrs in which the cytotoxicity effect is observed in HeLa cell line35 and it is also indicative that there is an increase in cytotoxicity of HeLa cell lines with increased concentration of showed drug. The IC50 concentration against HeLa cell lines to the tested Cu-Ag bimetallic nanoparticles was found to be 48.19(µl/mL). The maximum percentage of HeLa cell lines viability was observed at a concentration of 3.1 µl/mL and this indicates that the bimetallic nanocomposites has a potential cytotoxic activity shown in Table 2.
Table (2):
In vitro cytotoxic effect of Cu-Ag bimetallic nanocomposites Vs HeLa cell lines.
Sample concentration(ml/mL) |
HeLa Cell viability(%) |
|---|---|
0 |
100 |
1.5 |
94.02 |
3.1 |
76.61 |
6.2 |
74.49 |
12.2 |
61.62 |
25.0 |
51.32 |
50.0 |
41.03 |
100.0 |
18.77 |
Table (3):
A comparative study of bimetallic Ag-Cu nanocomposites found in literature.
No. |
Name and part of plant |
Size and morphology |
Studies for applications |
Ref. |
|---|---|---|---|---|
1. |
Curcuma longa rhizome |
45 nm |
Photocatalytic, antimicrobial |
37 |
2. |
Vitex negundo leaves |
60 nm (spherical) |
Antibacterial |
38 |
3. |
Achras sapota fruit |
20-40 nm (spherical) |
Cytotoxicity |
39 |
4. |
Annona muricata leaves |
– |
Bactericidal, antidiabetic, antioxidative, anticancer, catalytic |
40 |
5. |
Rheum emodi roots |
40-50 nm (spherical) |
Anticancer, antibacterial |
41 |
6. |
Ricinus communis leaves |
18 nm (spherical) |
Antibacterial, antifungal |
42 |
7. |
Moringa oliefiera leaves |
78 nm (globular) |
Antibacterial |
43 |
8. |
Palm leaves |
13 nm (spherical) |
– |
44 |
9. |
Muntingia calabura flowers |
40-78 nm (irregular) |
Antibacterial |
45 |
10. |
Amaranthus tricolour stem |
10-15 nm (spherical) |
Dimethoate sensing |
46 |
11. |
Artemisia roxburghiana root |
8-26 nm (spherical) |
Antimicrobial, antioxidant |
47 |
12. |
Toddy Borassus flabellifer |
80 nm |
Antitumour, antioxidant, antibacterial |
48 |
13. |
Carica papaya leaves |
90-150 nm (star-like structure) |
Catalytic degradation of toxic chlorpyrifosis |
49 |
14. |
Opuntia ficus indica leaves |
10-20 nm (ellipsoidal) |
– |
50 |
15. |
Justicia spicigera leaves |
53.3 nm |
– |
51 |
The Cu-Ag bimetallic nanocomposites have been synthesized with Carica papaya aqueous leaf extract in an exceedingly pure eco-friendly manner which contained the bioactive components that acted as reducing and stabilizing agents. The FTIR spectra showed the presence of bioactive components of the aqueous plant extract which had helped in reducing and stabilizing Cu-Ag bimetallic nanocomposite thus making the entire process greener and eco-friendly. The SEM/EDX analysis confirms the presence of metallic Cu and Silver with a visualization to surface morphology and substantiated with TEM images. The TEM revealed the average particle size of the bimetallic nanocomposite to be 20 nm which was in accordance with SEM /EDAX studies. The CV studies showed the redox behaviour of the bimetallic nanocomposites and therefore the peak current gradually increases with increase in scan rates and concentrations. Linear plots of Peak Current (ip ) vs. ν1/2 (Scan rate ) provide evidence for a chemically reversible redox process and also the presence of Ag0/Cu0 couple. Antibacterial activity confirms that Cu-Ag bimetallic nanocomposites are of high antibacterial efficacy and hence it can be employed in the potential treatment against bacteria. The investigation on the synthesized bimetallic nanocomposites on cancer cells may provide a pathway for further research in treatment of cancer using bimetallic nanocomposites in future. Table 3 gives the review of Ag-Cu nanoparticles in literature.
ACKNOWLEDGMENTS
The authors would like to thank the Principal and Secretary, HOD Department of Chemistry, Dwaraka Doss Goverdhan Doss Vaishnav College, Chennai, India. We sincerely thank sophisticated analytical instrument facility, Indian Institute of Technology (IIT) Madras, Vellore Institute of Technology (VIT) and Apex biotechnology training and research institute for their support in studies.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
All datasets generated or analyzed during this study are included in the manuscript.
AVAILABILITY OF DATA
This article does not contain any studies with human participants or animals performed by any of the authors.
- Chaloupka K, Malam Y and Seifalian AM. Nanosilver as a new generation of nano product in biomedical applications. Trends Biotechnol. 2010;28(11):580-588.
Crossref - Camelio S, Babonneau D, Declemy A, Fonda E, Traverse A, Girardeau T. Relations between the optical properties of Cu-Ag:Si3N4 nanocermets and the microstructure of the metallic nanoclusters. Journal of Non-Crystalline Solids. 2006;352(23-25):2501-2505.
Crossref - Renuka R, Renuka Devi K, Sivakami M, Thilagavathi T, Uthrakumar R, Kaviyarasu K. Biosynthesis of silver nanoparticles using phyllanthus emblica fruit extract for antimicrobial application. Biocatalysis and Agricultural Biotechnology. 2020;24:101567.
Crossref - Zhang D, Liu X. Synthesis of polymer stabilized monometallic Cu and bimetallic Cu/Ag nanoparticles and their surface enhanced Raman scattering properties. J Mol Struct. 2013;1035:471-475.
Crossref - Heidari A. Applications of single-walled carbon nanotubes(SWCNT) in the production of Glucose Biosensors and improving their performance using Gold colloidal nanoparticles and usage of polyaniline nanostucture-Based Biosensors for dectecting Glucose and cholestrol. Malays J Chem. 2020;22(2):121-162.
Crossref - Yusop HM, Wan Ismail WN. A review on synthesis of plant mediated metal nanoparticles for fabric coating. Malays J Chem. 2021;23(2):40-54.
Crossref - Tseng W J, Mou Kao S, Hsieh JH. Photocatalytic and bactericidal activity of mesoporous TiO2-Ag nanocomposite particles. Ceramics International. 2015;41(9):10494-10500.
Crossref - Karthikeyan B, Loganathan B. Strategic green synthesis and characterization of Au/Pt/Ag trimetallic nanocomposites. Materials Letters. 2012;85:53-56.
Crossref - Zou X, Silva R, Huang X, Al-Sharab JF, Asefa T. A self cleaning porous TiO2-Ag core shell nanocomposite material for surface-enhanced Raman scattering. Chem Commun. 2013;49(4):382-384.
Crossref - Ji Y, Yang S, Guo S, Song X, Ding B, Yang Z. Bimetallic Ag/Au Nanoparticles:A low Temperature Ripening Strategy in Aqueous Solution. Colloids Surf A: Physicochem Eng Aspects. 2010;372(1-3):204-209.
Crossref - Yang D, Yang N, Ge J. Controlled deposition of ultra small Ag particles of TiO2 nanorods:oxide/metal hetero-nanostructures with improved catalytic activity. Cryst Eng Comm. 2013;15(36):7230-7235.
Crossref - Li H, Yan Guo C, Ling Xu C. A highly sensitive non-enzymatic glucose sensor based on bimetallic Cu-Ag superstructures. Biosensors and Bioelectronics. 2015;63:339-346.
Crossref - Xie H, Yea X, Duan K, et al . CuAu-ZnO-graphene based bimetallic alloy-semiconductor catalyst with its enhanced photocatalytic degradation performance. Journal of Alloys and Compounds. 2015;636:40-47.
Crossref - Mohseni MS, Khalilzadeh MA, Mohseni M, et al. Green synthesis of Ag nanoparticles from pomegranate seeds extract and synthesis of Ag-starch nanocomposite and characterization of mechanical properties of the films. Biocatalysis and Agricultural Biotechnology. 2020;25:101569.
Crossref - Kokilavani S, Syed A, Thomas AM, et al. Development of multifunctional Cu sensitized Ag-dextran nanocomposite for selective and sensitive detection of mercury from environmental sample and evaluation of its photocatalytic and anti-microbial applications. Journal of Molecular Liquids. 2021;321:114742.
Crossref - Rizwan M, Amin S, Malikovna BK, et al. Green synthesis and antimicrobial potential of silver nanoparticles with Boerhavia Procumbens extract. J Pure Appl Microbiol. 2020;14(2):1437-1451.
Crossref - Bawazeer S, Rauf A, Ur Rahman K, et al. Green synthesis and antimicrobial potential of silver/gold nanoparticles functionalized with Debregeasia Salicifolia D.Don. J Pure Appl Microbiol. 2020;14(4):2513-2523.
Crossref - Thomas B, Vithiya B, Prasad T, et al. Antioxidant and photocatalytic activity of aqueous leaf extract mediated green synthesis of silver nanoparticles using passiflora edulis f. flavicarpa. J Nanosci Nanotechnol. 2019;19(5):2640-2648.
Crossref - Badineni V, Maseed H, Arla SK, Yerramala S, Naidu BVK, Kaviyarasu K. Effect of PVA/PVP protective agent on the formation of silver nanoparticles and its photocatalytic and antimicrobial activity. Materials Today: Proceedings. 2021;36(2):121-125.
Crossref - Hassan SWM, Hala H Abd El-latif. Characterization and application of the biosynthesized silver nanoparticles by Marine Pseudomonous Sp.H64. J Pure Appl Microbiol. 2018;12(3):1289-1299.
Crossref - Atarod M, Nasrollahzadeh M, Mohammad Sajadi S. Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J Colloid Interface Sci. 2016;462:272-279.
Crossref - Raveendran P, Fu J, Wallen SL. Completely green synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940-13941.
Crossref - Holder IA, Boyce ST. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentration non-toxic for human cells in culture. Burns. 1994;20(5):426-429.
Crossref - Vijayalakshmi RV, Kuppan R, Kumar PP. Investigation on the impact of different stabilizing agents on structural, optical properties of Ag@SnO2 core – shell nanoparticles and its biological applications. Journal of Molecular Liquids. 2020;307:112951.
Crossref - Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays . J Immunol Methods. 1983;65:55-63.
Crossref - Kumar KM, Sinha M, Mandal BK, Ghosh AR, Kumar KS. Biobased green method to synthesis paladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta A: Molecular and Biomolecular Spectroscopy. 2012;91:228-233.
Crossref - Valodkhar M, Modi S, Pal A, Thakore S. Synthesis and anti bacterial activity of Cu, Ag and Cu -Ag alloy nanoparticles.A green approach. Materials Research Bulletin. 2011;46(3):384-389.
Crossref - Perugu S, Nagati V, Bhanoori M. Green synthesis of silver nanoparticles using leaf extract of medically potent plant Saraca indica: a novel study. Appl Nanosci. 2016;6(5):747-53.
Crossref - Adyani SH, Soleimani E. Green synthesis of Ag/Fe3O4/RGO nanocomposites by Punica Granatum peel extract: Catalytic activity for reduction of organic pollutants. Int J Hydrog Energ. 2019;44(5):2711-2730.
Crossref - Metz KM, Sanders SE, Pender JP, et al. Green synthesis of metal nanoparticles via natural extracts: the biogenic nanoparticle corona and its effects on reactivity. ACS Sustainable Chem Eng. 2015;3(7):1610-1617.
Crossref - Tiwari AD, Mishra AK, Mishra SB, Kuvarega AT, Mamba BB. Stabilisation ofsilver and copper nanoparticles in a chemically modified chitosin matrix. Carbohydr Polym. 2013;92(2):1402-1407.
Crossref - Elgrishi N, Routree K, Mccarthy BD, Routree E, Eisenhart TT, Dempsey JL. A practical beginner’s guide to cyclic voltammetry. J Chem Educ. 2018;95(2):197-206.
Crossref - Abondanza TS, Oliveria CR, Barbosa CMV, et al. Bcl-2 expression and apoptosis induction in human HL60 leukamemic cells treated with a novel organotellurium(IV)compound RT-04. Food Toxicol. 2008;46(7):2540-2545.
Crossref - Amanulla AM, Magdalane CM, Saranya S, Sundaram R, Kaviyarasu K. Selectivity, stability and reproducilitity effect of CeM – CeO2 modified PIGE electrode for photochemical behaviour of energy applications. Surfaces and Interfaces. 2021;22:100835.
Crossref - Artun FT, Karagoz A, Ozcan G, et al. In vitro anticancer and cytotoxic activities of some plants extracts on HeLa and Vero cell lines. Proceedings. 2017;1(10):1019.
Crossref - Ismail M, Khan I, Khan SA, et al. Green synthesis of antibacterial bimetallic Ag-Cu nanoparticles for catalytic reduction of persistent organic pollutants. Journal of Materials Science: Materials in Electronics. 2018;29:20840-20855.
Crossref - Mamatha G, Sowmya P, Madhuri D, et al. Antimicrobial Cellulose Nanocomposite Films with In Situ Generations of Bimetallic (Ag and Cu) Nanoparticles Using Vitex negundo Leaves Extract. Journal of Inorganic and Organometallic Polymers and Materials.2021;31(15):802-815.
Crossref - Thakore SI, Nagar PS, Jadeja RN, Thounaojam M, Devkar RV, Rathore PS. Sapota fruit latex mediated synthesis of Ag, Cu mono and bimetallic nanoparticles and their in vitro toxicity studies. Arabian Journal of Chemistry. 2019;12(5):694-700.
Crossref - Velidandi A, Pabbathi NPP, Dahariya S, Baadhe RR. Green synthesis of novel Ag-Cu and Ag-Zn bimetallic nanoparticles and their in vitro biological, eco-toxicity and catalytic studies. Nano-Structures & Nano-Objects. 2021;26:100687.
Crossref - Sharma D, Ledwani L, Kumar N, Mehrotra T, Pervaiz N, Kumar R. An Investigation of Physicochemical and Biological Properties of Rheum emodi Mediated Bimetallic Ag-Cu Nanoparticles. Arabian Journal for Science and Engineering. 2021;46:275-285.
Crossref - Lopez-Ubaldo F, Sanchez-Mendieta V, Olea-Mejia OF, Maria S G-P, Luckie RAM. Biosynthesis of Ag/Cu bimetallic nanoparticles using Ricinus communis and their antibacterial and antifungical activity. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2020;11(2):025018.
Crossref - Seetha J, Mallavarapu UM, Akepogu P, et al. Biosynthesis and study of bimetallic copper and silver nanoparticles on cellulose cotton fabrics using Moringa oliefiera leaf extraction as reductant. Inorganic and Nano-Metal Chemistry.2020;50(9):828-835.
Crossref - Mohamad NAN, Arham NA, Junaidah J, Hadi A , Idris SA. Green Synthesis of Ag, Cu and AgCu Nanoparticles using Palm Leaves Extract as the Reducing and Stabilizing Agents. IOP Conf. Series: Materials Science and Engineering. 2018;358:012063.
Crossref - Patra N, Sahoo A, Behera A. Synthesis and Differential Antibacterial Activity of Bioconjugated Bimetallic Nanoparticles. Pharmaceutical Chemistry Journal. 2020;54(8):865-869.
Crossref - Ansari Z, Saha A, Singha SS, Sen K. Phytomediated Generation of Ag, CuO and Ag-Cu Nanoparticles for Dimethoate Sensing. Journal of Photochemistry and amp; Photobiology, A: Chemistry.2018;367:200-211.
Crossref - Tripathi PK, Sati C. Green synthesis of Ag/Cu bimetallic nanoparticles and determination of it’s antimicrobial and antioxidant activities. Journal of Emerging Technologies and Innovative Research. 2019;6:627-633. URL:https://www.jetir.org/view?paper=JETIR1904M94
- Merugu R, Gothalwal R, Deshpande KP, Mandal SD, Padala G, Chitturi KL. Synthesis of Ag/Cu and Cu/Zn bimetallic nanoparticles using toddy palm: Investigations of their antitumor, antioxidant and antibacterial activities. Materials Today: Proceedings. 2021;44(1):99-105.
Crossref - Rosbero TMS, Camacho DH. Green preparation and characterization of tentacle-like silver/copper nanoparticles for catalytic degradation of toxic chlorpyrifos in water. J Environ Chem Eng. 2017;5(3):2524-2532.
Crossref - Rocha-Rocha O, Cortez-Valadez M, Hernandez-Martinez AR, et al. Green Synthesis of Ag-Cu Nanoalloys Using Opuntia ficus-indica. Journal of Electronic Materials. 2017;46(2):802-807.
Crossref - Gonzalez-Mendoza D, Valdez-Salas B, Bernardo- Mazariegos E, et al. Influence of monometallic and bimetallic phytonanoparticles on physiological status of mesquite. Open Life Sci.2019;14:62-68.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.