ISSN: 0973-7510
E-ISSN: 2581-690X
Contamination of soil / water resources by petroleum products poses severe threats to underground water and soil quality. In the present study biosurfactant producing bacterial cultures were used to degrade petrol engine oil under in situ conditions in the plant rhizosphere system. Two bacterial isolates used in this study were recovered from Haldia oil refinery site & petrol station of Pantnagar and identified as Pseudomonas aeruginosa (JX100389) and P. plecoglossicida (JX149549). Application of consortium C2, (Pseudomonas aeruginosa and P. plecoglossicida) degraded 56.14% petrol engine oil @ 2% in the soil planted with wheat (Triticum aestivum var. 2565) crop after 120 days. GC-MS of biodegraded fuel showed the presence of new product like octanoic acid-2-ethylhexyl ester and 1, 2-benzenedicarboxylic acid.
Biophytoremediation; biosurfactant; consortium; bioavailability.
Petroleum contaminated soil causes severe pollution of ground water, threatens the safety of potable water, limits the use of ground water, and causes enormous economic losses and ecological disaster1. Pollution of soil by petroleum hydrocarbons causes major changes in soil water, soil air, exchangeable iron and available phosphorous and sulphate. These changes affect plant growth adversely. However, certain microorganisms acclimatized to grow and thrive in oil polluted environment and play an important role in the treatment of pollutant by degradation process. Plant microbe and soil interactions are known to convert certain toxic pollutant to non toxic forms2-4. One of the limiting factors in this process is the bioavailability (i.e the extent to which hydrophobic contaminant available to microorganisms) of some fractions of the oil. Hydrocarbon degrading microorganisms produce biosurfactants of diverse chemical nature and molecular size. Biosurfactant are environment friendly, biodegradable, less toxic and non-hazardous hence preferred over chemical surfactants5. Biosurfactant increases the surface area of hydrophobic water-insoluble substrates and improve their bioavailability, thereby enhancing the growth of bacteria and the rate of bioremediation through emulsification6. They have better foaming properties and higher selectivity. They are active at extreme temperatures, pH and salinity7. Biosurfactants can be produced from industrial wastes and their by-products. This feature favours their cheap production and also allows utilizing waste substrates and reducing their polluting effect at the same time8-11. Hydrophobic pollutants present in petroleum hydrocarbons, soil/ water environment require solubilisation before being degraded by microbial cells. Present study is based on the use of microbial consortia and hyper accumulator crop (bio-phytoremediation). Crops like mustard and wheat facilitates the growth of microorganism in the rhizospheric zone by excreting root exudates and the microorganism support plant growt by producing IAA, siderophore, P solubilisation and indirectly by producing HCN and antibiotics which inhibits the growth of phytopathogens12. Characterizations of bacterial isolates recovered from the oil contaminated sites were characterized on the basis of their emulsification potential for different oil.
Most important species of oil degrading bacteria belong to genera Pseudomonas. These microorganisms excrete a variety of biosurfactants which are biodegradable and consequently environmentally safe and help in solubilisation of xenobiotic compounds. Different groups of microorganisms plant growth promotory properties are reported to produce biosurfactants.
Strains and culture conditions
All the bacterial strains (H1A= Pseudomonas aeruginosa and P1A= P. plecoglossicida) used in this study were isolated from oil contaminated sites (Source: Haldia & Pantnagar (220 3’ 37.65’’N latitude, 880 06’ 35.09’’ E longitude and 290 01’ 20.00N latitude and 790 29’ 15.00E longitude. These two strains were selected and characterized on the basis of 16SrDNA sequencing. These two bacterial strains (H1A and P1A) which were previously used for biodegradation of mobil oil hydrocarbon by13.
PGPR Properties
The plant growth promotory properties viz. phosphate solubilization, siderophore production, indole acetic acid production, ammonia production, hydrogen cyanide (HCN) production were tests performed for biosurfactant production and their characterization included turbidity test, foaming, cell biomass and emulsification index (mobil oil)13.
Consortia development
Based on PGPR properties bacterial strains were selected to develop consortium. Bacterial strains showing compatibility on kings B agar medium were selected for consortium. Compatibility among the strains was also checked in MSM broth medium in the presence and absence of hydrocarbons (petrol engine oil 2%). The absorbance was taken from 0 to 120h at an interval of 24h using a spectrophotometer (Lamda35, Perkin Elmer).
Pot Trial
The experimental soil was mixed, allowed to dry to get water content less than 1%, sieved with 2 mm sieve and autoclaved at 121°C for 1hr for three consecutive days. Autoclaved soil (1.5 kg) was transferred to each pot and mixed with 30g (2%) of petrol engine oil hydrocarbons. Microbial consortium were also added to the experimental pots (50 ml i.e. ~ 8 × 10 5 cells ml-1). Suitable sets without hydrocarbons and/or bacterial consortium were kept as control. Surface sterilized seeds of wheat (Triticum aestivum var. 2565) were sown at a depth of 2.5 cm. Observation regarding germination (seedling germination percentage) and plant health parameters (i.e. seedling survival, average plant height, chlorophyll content using Spadmeter (optisciences 2000), plant biomass, average shoot height, average stem dry weight with fruit, average seed weight, average root weight and average yield per pot) (data not shown). This experiment was carried out for 120 days in poly house at room temperature.
GCMS analysis
Approximately 5g of samples from petrol engine oil contaminated soil was taken initially (0 days, control) and after cultivation of wheat at 120 days. To each of the soil samples, 15 ml of acetone was added followed by 1hr shaking. Obtained residue was filtered and washed twice with acetone, followed by filtration and concentration on a rotatory vaccum evaporator at 50°C and analyzed for the presence of residual hydrocarbons using FTIR. For Gas Chromatography Mass Spectrophotometry (GC-MS) analysis of the extracted residual hydrocarbons sample was dissolved in 1 ml hexane. The quantification of hydrocarbon compound was done by high resolution gas chromatography using QP-2010 gas chromatograph (Shimadzu) equipped with a HP-DB 5MS column (60 m × 0.25 mm, 0.25 ¼m film thickness) coupled with mass spectrometer detector (HP 5972) at AIRF, JNU, New Delhi (India).
These bacterial strains were found rod shaped and Gram– and showed homology with Pseudomonas (homology 97-99%).
All the morphological and Plant growth promotory traits of the recovered test strains were shown in Table 1 &213. Application of these bacterial strains can help in solubilizing and sequester iron from soil and provide it to plant cells under stress. Once these Sid+ strains bind iron and make microbial iron siderophores availability of complex soluble Fe++ become carry to plants14, 15. Indole acetic acid (IAA) production were best shown by H1A> H1B which can be used to enhance various stages of plant roots and different availability13. Biosurfactant, produced by these organisms, could be rhamnolipid as evident by16. Similar kind of study has also been reported by17.
Consortia are better degraders of hydrocarbons as compared to single organism. So based on the emulsification index, compatibility studies and plant growth promotory properties bacterial consortium were developed in which both organisms were compatible with each other. Under in vitro conditions consortium (C2) utilized petrol engine oil as carbon source and showed enhanced exponential growth as compared to control in the absence of any carbon source.
Consortia are better degraders of hydrocarbon as compared to single organism18. Based on emulsification index, compatibility studies and plant growth promotory properties microbial consortia were developed showed compatible or better growth when combined together as compare to single organism (data not shown).
In order to investigate the optimum condition for the biodegradation of petroleum hydrocarbons soil was spiked with 2% petrol engine oil and bacterized with efficient consortium. In situ experiment was performed to assess the combined impact of phyto (plant) & bioremediation (microorganism) in remediating soil from the toxic effect of petroleum hydrocarbon in wheat cropping system under the influence of C2 bacterial consortium.
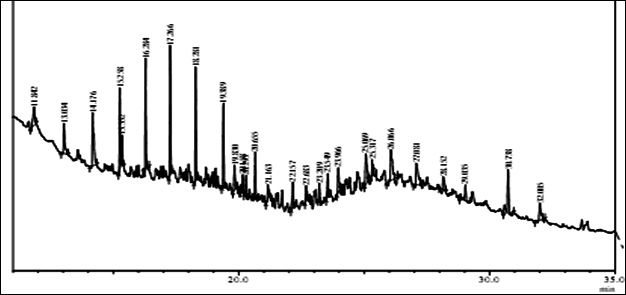
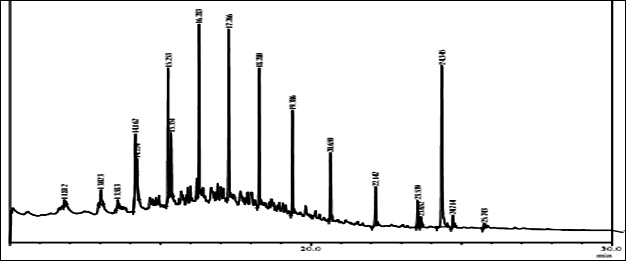
Fig. 1. GC/MS spectrum of petrol engine oil contaminated soil at 0 DAS and 120 DAS
The GC-MS analysis showed that the compounds appearing in the control sample at 0 DAS in different retention time were absent from the samples after 120 days of treatment (Fig. 2). It indicates that compounds were degraded by the developed bacterial consortium and utilized as carbon and energy sources. The metabolic capability and cell growth were also observed at different time intervals from 0 to 120 days after sowing (DAS). However, during the course of 120 DAS consortium C2 & wheat crop have managed to reduce or mitigate the concentration of petrol engine oil hydrocarbon in polluted soil, as indicated by the increased the biodegradation potential cell growth during the incubation period at different time (0, 20, 40, 60, 80 and 120 DAS) intervals (Table 1). Further, the peaks were characterized for the type of metabolite produced during the biodegradation of petrol engine oil hydrocarbons in wheat rhizospheric soil. In wheat with consortium C2, new peak was observed at retention time (RT) 23.652 & 24.345 and identified as octanoic acid-2-ethylhexyl ester and 1, 2-benzenedicarboxylic acid. (Fig 2). In addition to this plant play significant effect on pollution removal as the wheat crop has significant contribution for mobil oil biodegradation13. The mass fragment and structure of new intermediate products were shown at Fig 3. The average% degradation of petrol engine oil (2%) contaminated soil in C2PE2W (C2= consortia, PE2= 2% petrol engine oil, W= wheat plant) was 56.14 % after 120 DAS (Table 1). Similar type of study, at 50 days, methyl ester dodecanoic acid and methyl tetradecanoate etc. were detected from petroleum products hydrocarbon19.
Table (1):
Metabolic capability and growth (cell count) of microbial consortium.
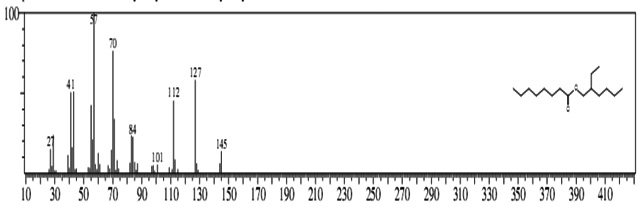 1, 2-benzenedicarboxylic acid (RT = 24.345)
1, 2-benzenedicarboxylic acid (RT = 24.345)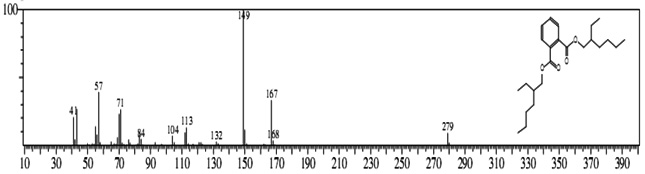 Fig.2. New peak observation in C2PE2W treatments and its mass spectrum
Fig.2. New peak observation in C2PE2W treatments and its mass spectrumAfter the development of consortium for petrol engine oil (C2= Pseudomonas aeruginosa & P. plecoglossicida). Consortium was capable of degradation of 2% petrol engine oil hydrocarbons in wheat (Triticum aestivum var. 2565) rhizosphere. Consortium C2 degraded PEO hydrocarbons in wheat rhizosphere with a potential of 56.14% in 120 DAS. Various plants can be used for remediation but owing to the importance of plants and especially wheat (Triticum aestivum var. 2565) the plant was selected for studying the interaction with biosurfactant producing plant growth promoting rhizobacteria (PGPR) in petroleum products contaminated soil anticipating that this study will be helpful in use of oil contaminated sites for farming and better understanding of remediation mechanism of such sites.
Table (2):
All plant health parameters of control and treated samples.
| Treatment | Germination (%) DAS | Average plant Height (DAS) | RW | SPW | SW | Chlorophyll index (45 DAS) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 15 | 30 | 30 | 45 | 60 | 90 | |||||
| Control (COPEOW) | 80 | 80 | 80 | 15.45±.05 | 23.92±0.02 | 33.71±0.08 | 51.66±0.04 | 0.68±0.10 | 0.714±0.12 | 1.81±0.09 | 2.74±0.02 |
| C2PE2W | 10 | 10 | 100 | 11.20±.03 | 27.60±0.06 | 31.98±0.02 | 52.66±0.01 | 1.08±0.03 | 0.73±0.11 | 2.08±0.04 | 3.60±0.0 |
DAS: Days after sowing; RW: Root weight; SPW: Shoot+ pods weight; SW: Seed weight
ACKNOWLEDGMENTS
This work was financially supported by UGC.
- Xu S.Y., et al., Enhanced dissipation of phenathrene and pyrene in spiked soils by combined plants cultivation. Science Of Total Environment, 2006, 363; 206-215.
- Cunningham S.D and W.R. Berti, Remediation of contaminated soils with green plants: an overview, In Vitro Cell Development Biology, 1993, 29: 207-212
- Cunningham S.D., et al., Phytoremediation of contaminated soils, Trends Biotechnology, 1995, 13; 393-397.
- Singh P. O., D.J. Parmar and A.V. Pandya: Selective screening of potential crude oil degrading microbes from crude oil contaminated site. International Research Journal of Environmental Sciences, 2015; 4(1): 78-79.
- Singh SP., et al., Optimization of nutrient requirements and culture conditions for the production of rhamnolipid from Pseudomonas aeruginosa (MTCC 7815) using mesua ferrea seed oil. Indian Journal of Microbiology, 2013; 53, 467-476
- Kelkar D.S., et al., Hydrocarbon emulsification and enhanced crude oil degradation by lauroyl glucose ester. Bioresource Technology, 2007; 98; 1505-1508.
- Kebria D.Y., et al., Isolation and characterization of a novel native Bacillus strain capable of degrading diesel fuel. International Journal of Environment Sciences, 2009; 6: 435- 442.
- Kosaric N., Biosurfactants and their application for soil bioremediation. Food Technology Biotechnology, 2001; 39: 295–304.
- Rahman K.S.M., et al., Enhanced bioremediation of n-alkane petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresource Technology, 2003; 90: 159–168.
- Das K and A.K. Mukherjee., Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source: some industrial applications of biosurfactants. Process Biochemistry, 2007, 42: 1191–1199.
- Das P., S. et al., Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere, 2008; 72: 1229–1234.
- Ahemad M and M. Kibret., Mechanisms and applications of plant growth promotingrhizobacteria: Current perspective, Journal of Kin Sau Uni Science, 2014; 26: 1–20.
- Kumar R., et al., Enhanced biodegradation of mobil oil hydrocarbons by biosurfactant producing bacterial consortium in wheat and mustard rhizosphere. Petroleum & Environment Biotechnology, 2013. ISSN: 2157-7463.
- Meyer J.M., Pyoverdins: Pigments siderohores and potential taxonomic markers of fluorescent pseudomonas species. Archives Microbiology, 2000; 174: 135-142.
- Meyer J.M., et al., Siderohore- mediated iron uptake in fluorescent psudeomonas: characterization of the pyoverdine- receptor binding site of three cross- reacting pyoverdines. Archieves of Biochemistry and Biophysics, 2002; 397(2): 179-183.
- Sadoudi-Ali ahmed D., et al., Treatment of an oil polluted soil by injecting Pseudomonas aeruginosa and produced rhamnolipid. International Journal of Environment Engineering Science and Technology Research, 2014; 2: 1-9.
- Chandankere R., et al., Enhanced production and characterization of biosurfactant produced by a newly isolated Bacillus amyloliquefaciens USTBb using response surface methodology. International Journal Current Microbiology Applied Sciences, 2014; 3: 66-80.
- Sunday A.A., et al., Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World Journal of Microbiology & Biotechnology, 2007; 23: 1149-1159.
- Rosado E.D and J. Pitchel., Phytoremediation of soil contaminated with used motor oil: II. Greenhouse studies. Environment Engineering Sciences, 2004; 21: 169-180.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


