ISSN: 0973-7510
E-ISSN: 2581-690X
Xylooligosaccharides (XOS) act as prebiotics because they are not digested in the upper gastrointestinal tract and increase the population of beneficial gut microflora like Lactobacilli and Biofidobacteria. XOS with these importance’s are emerging as important oligosaccharide with rise in demand worldwide. For use of xylanase in the production of XOs, xylanolytic fungi were isolated from degraded corn cobs, screened for enhanced xylanase production with cultural and nutritional conditions optimized using one factor at a time approach. It was found that among the 11fungal isolates, isolate SKF-4 which was identified as Aspergillus fumigatus produced highest level of xylanase and lowest level of ²-xylosidase i.e. 143.0 IU/ml and 0.01 IU/ml respectively. The selected fungus A. fumigatus SKF-4 produced enhanced level of xylanase with wheat bran and proteose peptone as carbon and nitrogen source respectively at pH 5.0 and temperature 30°C. The partially purified enzyme from A. fumigatus SKF-4 showed highest xylanase activity at pH 5.0 and temperature 45°C. Partially purified xylanase of A. fumigatus SKF-4 could produce 1.44±0.4 mg/ml of xylobiose and low amount of xylose 0.25±0.1 mg/ml from beechwood xylan after 24hr. Xylanase from A. fumigatus SKF-4 found to be promising for xylooligosaccharides production.
Xylooligosaccharides, prebiotics, xylanase.
Xylooligosaccharides (XOs) are sugar oligomers containing 2-10 xylose molecules linked by b-1, 4 glycosidic bonds and may be substituted by D-glucouronic acid, arabinofuranose, ferulic acid and acetyl groups in different proportion depending upon the source and method of production. XOs with lower degree of polymerization (DP) i.e. 2 to 5 exert prebiotic effects when taken as a part of diet as they are neither hydrolyzed nor absorbed in the upper part of the gastrointestinal tract and selectively stimulate growth or activity of limited number of bacteria like Bifidobacteria, Lactobacilli and thus improve health of host (Moure et al., 2006; Vazquez et al., 2000).
XOs have health benefits like promoting growth of probiotics such as Bifidobacterium spp. (Gobinath et al., 2010 and Manisseri and Gudipati, 2010), Lactobacillus spp., L. brevis (Moura et al., 2008), L. fermentum and L. acidophilus (Chapla et al., 2012). They also aid in enhancing the biological availability of calcium by improving its absorption (Mussatto and Mancilha, 2007), improvement in bowel function and reducing the risk of colon cancer (Swennen et al., 2006), having cytotoxic effects on human leukemia cells (Ando et al., 2004), positive effects on the type II diabetes mellitus (Sheu et al., 2008) and acting as antioxidants (Chen et al., 2009).
XOs could be produced from various lignocellulosic biomasses like corn cobs, orange peels, tobacco stalk, cotton stalks, sugarcane bagasses etc. by employing chemical, autohydrolysis, enzymatic and chemo-enzymatic methods. Among the different methods that are used for XOS production, chemo-enzymatic methods are most widely used which consists of two steps i) extraction of xylan from lignocellulosic biomasses using suitable alkali ii) hydrolysis of xylan to xylooligosaccharides using xylanases.
India being an agrarian based country where there is abundant availability of agri- based lignocellulosic biomasses like paddy straw, wheat straw, corn strover, tobacoo stalk, cotton stalk etc and agro-industrial based product like rice bran, wheat bran etc which are major sources of cellulose, hemicelluloses and lignin. These biomasses which are either discarded or burned leads to environmental pollution can be easily converted to value added products like feed, biofuel and other neutracuetical products such as xylitol, xylooligosaccharides etc using microorganisms thus adding income generation for the farmers and ecofreindly disposal of these wastes.
For chemo-enzymatic production of XOs, the xylanase enzyme complex should have high ratio of xylanase to exo-xylanase or β-xylosidase activity because enzyme with high exo-xylanase or β-xylosidase activity produces high amount of xylose which causes inhibition effects for XOs production (Akpinar, 2009).
Xylanases are produced by various microorganisms like bacteria, yeast, fungi and actinomycetes (Fang et al., 2007) but filamentous and spore forming fungi are important for industrial production in two ways, firstly they secrete these enzymes directly into the medium (extracellular) thus reducing the downstream processing for obtaining the enzyme and secondly and their xylanase activities are much higher than those found in yeast and bacteria (Bakri et al., 2010).
For industrial processes the cost of enzyme contributes a significant proportion to the cost of production of any product like XOS. Hence there is urgent need for bioprospecting fungi producing xylanases which can rapidly utlilize cheap and easily available lignocellulosic biomass. Also, to exploit fungi for high titre enzyme production, all the cultural and nutritional conditions need to be optimized.
With above background, for the purpose of using the xylanase for xylooligosaccharides productions, eleven fungi were isolated from degraded corn cobs and screened for their efficiency in xylanase production. Different cultural and nutritional conditions affecting the xylanase production were optimized by one factor at a time approach. Xylanase was produced under optimized conditions and crude enzyme was partially purified, characterized for optimum pH and temperature. Further, crude enzyme was evaluated for xylooligosaccharides production using beechwood xylan.
Isolation, identification and maintenance of microorganisms
Degraded corn cob samples were collected from three different locations (i) dumping pits at Eterna village, Sonepat, Haryana; (ii) fields of Indian Institute of Maize Research, and (iii) Division of Agronomy, Indian Agricultural Research Institute, New Delhi and used for isolation of xylanolytic fungi.. Standard serial dilution method was used for isolation of xylanolytic fungi on Reese’s Minimal Medium (RMM) with 1% beechwood xylan SRL Pvt. Ltd., India). Pure fungal isolate were maintained on PDA (Potato Dextrose Agar) ( HI Media, Pvt. Ltd., India) slants at 4°C until further use with regular subculturing.
Identification of the promising fungal isolate was carried out based on both colony characteristics on PDA plate and microscopic observation of shape, color and arrangement of conidia, conidiophore, and vesicles (Raper and Fennell, 1965) at Indian Type Culture Collection (ITCC), IARI, New Delhi. Molecular characterization was based on Internal Transcribed Spacer (ITS) sequence analysis. For molecular identification, fungal genomic DNA was isolated by using HiPurA™ Fungal DNA Purification Kit (Hi Media Pvt. Ltd., India) by following the standard protocols provided. The ITS of fungal rDNA which includes ITS 1, 5.8S and ITS 2 regions was amplified using universal ITS region primers (ITS 1, 52 TCC GTA GGT GAA CCTGCG G3, and ITS 2, 52 TCC TCC GCT TAT TGA TAT-3). The amplified DNA was sequenced by Sanger sequencing method (Sci Genom Lab, Kochi, India) and the sequence obtained was compared with consensus fungal ITS sequences available in the GenBank database (NCBI) using BLAST programme.
Qualitative and quantitative screening of isolated fungi for xylanolytic enzymes
Qualitative screening for xylanolytic ability was ascertained by point inoculation of fungal isolates on xylan agar plates and incubating at 30°C for 3-4 days. Colonies developed were assayed for xylanase producing ability by Congo red (Hi Media Pvt., Ltd., India) staining (Teather and Wood, 1982).
Quantitative screening for xylanolytic ability was carried out by xylanase production under submerged fermentation in 100ml erlenmeyer flask containing 25 ml Reese’s minimal medium (Mandels & Reese, 1957) with 1% beechwood xylan.
Enzyme and protein assay
Endo-b-1, 4 – xylanase (xylanase) in crude extract was estimated by method described by Ghose and Bisaria (1987) using beechwood as substrate. Reducing sugars released were measured by the DNSA (SRL Pvt. Ltd., India) method (Miller 1959). β-xylosidase activity was assayed by the method described by Chang et al. ( 2005) using p-nitrophenyl- b-xylopyranoside (Sigma–Aldrich Corp.,St. Louis, Mo., U.S.A) as the substrate. One enzyme unit of xylanase or β-xylosidase was expressed as 1µmol xylose or p-nitrophenol (Hi Media Pvt. Ltd., India) formed per milliliter of culture filtrate per minute during hydrolysis under standard assay condition. The protein content in the crude enzyme was estimated by the method of Lowry et al. (1951).
Selection of the most efficient lignocellulosic biomass as carbon and nitrogen sources for maximum xylanase production using One factor at a time (OFAT) approach
Effect of different agri-based crude carbon sources on xylanase production by Aspergillus fumigatus SKF-4 was studied by supplementing one of the crude carbon substrates i.e. wheat bran, wheat straw, corn cob, rice bran, rice straw, and sugarcane bagasse (procured from local market) @1% (w/v) in 100ml erlenmeyer flask containing 25 ml Reese’s minimal medium. Similarly, to study their effect on xylanase production, different nitrogen sources (both organic and inorganic) like yeast extract, urea, soybean meal, proteose peptone, (NH4)2SO4, KNO3, NaNO3 (Hi Media Pvt. Ltd., India) @ 0.5% (w/v) were used in Reese’s minimal medium containing 1% wheat bran as carbon source. One ml of spore suspension containing 107 spores was used as inoculum and the flasks were incubated at 30°C under static condition for 6 days. After 6 days, fermentation broth was filtered and filtrate was used as crude enzyme for the estimation of extracellular enzyme (xylanase) and protein.
Optimization of pH and temperature for xylanase production using Aspergillus fumigatus SKF-4
To determine the optimum pH for maximum xylanase production, the final pH of the Reese’s minimal medium containing wheat bran @1% (w/v) as carbon and proteose peptone @ 0.5%(w/v) as nitrogen source was adjusted in the range 3-8 with 1N HCl or 1N NaOH (SRL Pvt. Ltd., India). After setting pH 5.0, temperature for xylanase production was determined by incubating the above medium at 20°C – 40°C.
Partial purification of crude xylanase from Aspergillus fumigatus SKF-4
Xylanase production was carried out in two litre optimized medium. After 6 days, fermentation broth was filtered and 8 litre chilled acetone (Ranken & Co Pvt. Ltd., India) was added and kept at -20°C for overnight. It was centrifuged at 10,000 rpm for 10min and the supernatant were discarded while pellet was concentrated and later dissolved in 25ml of citrate buffer pH 4.8.
Physico-chemical characterization of partially purified xylanase from Aspergillus fumigatus SKF-4
To determine optimum temperature, assay of the xylanase activity of partially purified enzyme was carried out at a broad range of temperatures (30–70°C) at pH 5.0 for 30min. Similarly, optimum pH was determined by measuring the activity at pH 3-10 at 45°C for 30 min using following buffers: 50 mM citrate buffer (pH 3-6), sodium phosphate buffer (pH 7-8) and glycine–NaOH buffer (pH 9-10)
Evaluation of commercial and partially purified xylanase from Aspergillus fumigatus SKF-4 for xylooligosaccharide production
Beech wood xylan (1%) was used for production of xylooligosaccharides using 600U of partially purified xylanase from Aspergillus fumigatus SKF-4 and commercial xylanase (SRL Pvt. Ltd., India) per gm of dry substrate. A blank was also maintained by keeping heat inactivated enzyme and substrate. The concentration of xylooligosaccharides (Xylobiose) (Megazyme, Ireland) in the enzymatic hydrolysate was determined by high performance liquid chromatography (HPLC, Waters pump 515 model) equipped with Waters 2414 refractive index (RI) detector. The Aminex HPX-87H column was operated with 5 mM H2SO4 (Sigma–Aldrich Corp., St. Louis, Mo., U.S.A) as the mobile phase at a flow rate of 0.5 ml/min and the oven temperature was kept at 40°C.
Isolation, screening and identification of xylanolytic fungi
A total of 11 fungi were isolated from degraded corn cobs collected from three different locations. On the basis of qualitative screening for xylanase production using congo-red test, all the 11 fungi were found to produce zone of hydrolysis. Further these isolates were evaluated for their extracellular xylanolytic enzyme (xylanase and β-xylosidase) production under submerged fermentation (Table 1) and it was found that the fungal isolate SKF-4 produced maximum amount of xylanase (143.0 IU/ml) and lowest amount of β-xylosidase (0.01 IU/ml) as compared to other ten fungal isolates. Hence this fungus was selected for further studies (Plate 1 and 2).
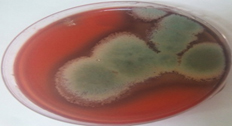
Plate 1. Zone of hydrolysis shown by Aspergillus fumigatus SKF-4 on xylan agar plate
Plate 2. Aspergillus fumigatus SKF-4 on PDA plate
Table (1):
Extracellular xylanolytic enzymes production by different fungal isolates under submerged fermentation.
Fungal isolate |
Xylanase (IU/ml) |
β- xylosidase (IU/ml) |
Protein (mg/ml) |
|---|---|---|---|
SKF-1 |
80.03 |
0.01 |
1.00 |
SKF-2 |
68.47 |
0.01 |
0.93 |
SKF-3 |
70.17 |
0.01 |
1.07 |
SKF-4 |
143.03 |
0.01 |
0.93 |
SKF-5 |
70.00 |
0.01 |
0.60 |
IKF-6 |
84.00 |
0.09 |
0.90 |
IKF-7 |
134.23 |
0.29 |
1.10 |
IKF-8 |
69.53 |
0.02 |
1.03 |
AKF-9 |
78.10 |
0.01 |
0.87 |
AKF-10 |
81.47 |
0.02 |
0.93 |
AKF-11 |
67.90 |
0.01 |
0.90 |
SE(m) |
3.04 |
0.01 |
0.03 |
CD at 5% |
8.99 |
0.01 |
0.09 |
For the identification of selected fungal isolate SKF-4, both morphological and molecular studies were carried out. Based on the colony characterization on PDA plates, the isolates appeared as white at early stage (1-2 days) and turned to castor -grey color due to sporulation in 5 days and the base of the colony turned pale yellow in color. Microscopic observation after staining with Lacto-phenol cotton blue it was found that conidial head was erect, compact and columnar, vesicle flask shaped, uniseriate, conidia were globose, echinulate and sclerotia or clestothecia were absent. Based on above characteristics, the fungal isolate SKF-4 was identified to be Aspergillus fumigatus. Further confirmation on identification of isolate was based on sequence analysis of ITS (Internal Transcribed Spacer) region. ITS sequence was compared with available database of Gene Bank using Mega BLAST. Results showed that ITS sequence of SKF-4 showed 97% identity with Aspergillus fumigatus (KF577886.1) of Gene Bank database. Sequence was deposited in Gene Bank and Accession no. KX463451 was obtained. Finally based on both morphological and molecular identification isolate SKF-4 was designated as Aspergillus fumigatus SKF-4
One factor at a time (OFAT) approach for selection of carbon and nitrogen sources for xylanase production
It is well known fact that cost of enzyme is an important concern for its industrial application. Large scale production of xylanase using beechwood xylan is economically not feasible as it is highly expensive. Hence cheap carbon source like lignocellulosic agri-residues such as wheat straw, sugarcane bagasse, paddy straw, corn cob and few processed by- products like wheat bran, rice bran were screened as carbon supplement for the production of xylanase using Aspergillus fumigatus SKF-4. On evaluation of carbon sources (Table 2), wheat bran produced highest level of xylanase (41.03 IU/ml) followed by corn cobs (25.47 IU/ml), wheat straw (33.27 IU/ml), paddy straw (20.63 IU/ml).
Table (2):
Selection of carbon source for xylanase production by Aspergillus fumigatus SKF-4.
Carbon sources |
Xylanase activity (IU/ml) |
|---|---|
Wheat bran |
41.03 |
Wheat straw |
33.27 |
Sugarcane bagasse |
11.93 |
Paddy straw |
20.63 |
Rice bran |
9.83 |
Corn cob |
25.47 |
SE(m) |
1.09 |
C.D. @5% |
3.40 |
These results indicated that wheat bran can be selected as an appropriate carbon source for higher xylanase production using the fungi SKF-4. Similar results were found by other researchers where among the crude carbon sources screened for xylanase production using Penicillium sp. SS1, highest xylanase production was observed on wheat bran (21.8 IU/ml) followed by rice bran (20.6 IU/ml) and sawdust (10.7 IU/ml) (Bajaj et al., 2011). Wheat bran was found to be good substrate for xylanase production using different fungal isolates like Aspergillus niger DFR6 (Pal and Khanum, 2011), A. niger (Okafor et al., 2007), Penicillum citrinum xym2 (Saha and Ghosh, 2014), Rhizopus var. microsporus 595 (Shuvaeva and Sysoeva, 2010). Other carbon sources like sugarcane bagasse and rice bran showed lower xylanase production i.e. 11.93 IU/ml and 9.83 IU/ml respectively in comparison to wheat bran, corn cob, paddy straw and wheat straw. This result coincides with findings of Anthony et al. (2003) where on screening of various lignocellulosic biomasses for xylanase production, sugarcane bagasse produced lowest level of xylanase. Use of agri-residue as carbon source for mass production of enzyme not only helps in reducing the cost of enzyme but also eco-friendly disposal of these wastes.
Nitrogen sources provide necessary ingredients for protein synthesis required for growth of microorganism and enzyme production. Different nitrogen supplements like i.e. Soybean meal, Yeast extract, Proteose peptone, Urea, Potassium nitrate, Ammonium sulfate were evaluated for xylanase production using Aspergillus fumigatus SKF-4. All the nitrogen supplements were added in Reese minimal medium @ 0.5 % (w/v). On evaluation, proteose peptone was found to be the most suitable organic source followed by yeast extract (27.5 IU/ml), soybean meal (19.70 IU/ml), urea (14.97 IU/ml) and hence selected for further work (Table 3).
Table (3):
Selection of nitrogen supplement for production of xylanase by Aspergillus fumigatus SKF-4.
Nitrogen source |
Xylanase activity (IU/ml) |
|---|---|
Control |
21.90 |
Soyabean meal |
19.70 |
Yeast extract |
27.50 |
Proteose peptone |
48.50 |
Urea |
14.97 |
Potassium nitrate |
17.63 |
Ammonium sulfate |
23.27 |
SE(m) |
0.89 |
C.D. @5% |
2.70 |
Similar results were observed by Shah and Madamwar (2005) where maximum xylanase activity was obtained from Aspergillus foetidus when proteose peptone was used as the nitrogen source. Liao et al. (2012) also found that among the organic nitrogen and inorganic nitrogen sources screened, peptone was the best nitrogen source for xylanase production by Penicillium oxalicum GZ-2 followed by yeast extract and Murthy and Naidu, 2010 found that Penicillium sp CFR 303 produced maximum xylanase production with peptone as nitrogen source. Among all the organic sources tested, peptone was found to be the best inducer for xylanase enzyme production by Streptomyces cyaneus SN32 (Ninawe and Kuhad, 2005).
Ammonium sulfate was found to be most suitable inorganic nitrogen source (27.50 IU/ml) followed by, potassium nitrate (17.63IU/ml). Among the inorganic nitrogen sources ammonium sulfate produced highest level of xylanases by Pleurotus ostreatus (Qinnghe et al., 2004). Comparing both organic and inorganic sources, organic sources were most suitable than inorganic source where average production of xylanse was 24.6 IU/ml and 19.4 IU/ml respectively. Organic nitrogen sources are complex and supply nutritional constituents besides nitrogen. Urea, soybean meal and potassium nitrate could induce less xylanase compared to control. Similar results were also observed by Bajaj et al. (2011), where these nitrogen sources reduced the xylanase production by Penicillium sp. SS1 when both inorganic and organic nitrogen sources were screened for their effect on xylanase production. Urea has been reported as recalcitrant source of nitrogen for xylanase production as it leads to repression in xylanase biosynthesis (Bakri et al., 2003). Bajaj and Abbass (2011) also reported that Urea and potassium nitrate led to reduction of xylanase production by Aspergillus fumigatus MA28.
Selection of pH and temperature for enhanced xylanase production
pH of the medium plays a major role in the growth, development of the microorganism and enzyme production (Bajaj and Abass, 2011). Hence, after selecting the carbon and nitrogen sources for the production of higher amount of xylanase using Aspergillus fumigatus SKF-4, the pH selection was carried out. The optimized medium with wheat bran (1%w/v) and proteose peptone (0.5%w/v) was evaluated within pH range 3-8 for xylanase production. The data showed that (Fig 1) although the enzyme was produced at pH 3.0 and 4.0 but the maximum enzyme was at pH 5.0, indicating acidophilic nature of the fungi Aspergillus fumigatus SKF-4.
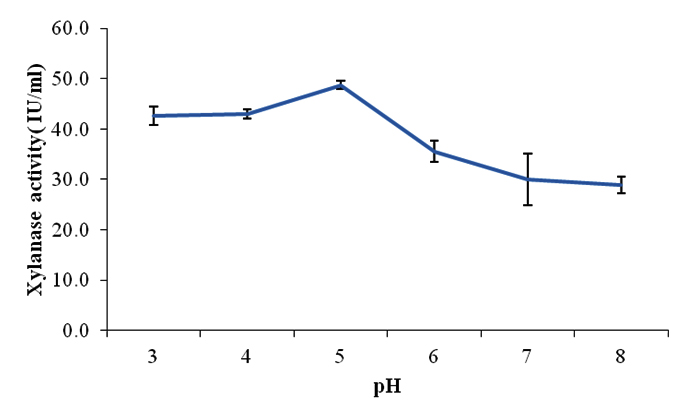
Fig. 1. Effect of pH on xylanase production by Aspergillus fumigatus SKF-4
Gupta et al., 2009 and Murthy and Naidu, 2010 reported that acidic pH (5–6.5) as the most appropriate for maximum enzyme production from fungi. Majority of fungal species have been reported to produce maximum xylanase at acidic pH (Subramanian and Prema, 2000). As the acidity decreased and the medium reached towards neutral, there was decline in the enzyme production which may be due to pH mediated changes in microbial metabolism (Srinivasan and Radhakrishnan, 2010). The decline was much lower than the amount produced at 3 and 4. The enzyme produced at 3.0, 4.0 and 5.0 were 42.7 IU/ml, 43.0 IU/ml and 48.7 IU/ml respectively while pH 6.0, 7.0 and 8.0 showed 35.6 IU/ml, 30.0 IU/ml and 28.9 IU/ml.
Incubation temperature is also a critical factor in the growth and metabolism of organism. To determine the optimum temperature for maximum production of xylanase, the optimized medium consisting wheat bran (1% w/v) as carbon source, proteose peptone (0.5%) as nitrogen source and optimum pH (5.0) was used for further studies. The temperature range was selected from 20°C – 40°C and all the flasks were incubated at respective temperatures. The xylanase activity was measured after six days of incubation. Fig. 2 shows the xylanase production at different temperature where it has been found that the enzyme production increased with increase in temperature from 20-30°C and after that the production started declining.
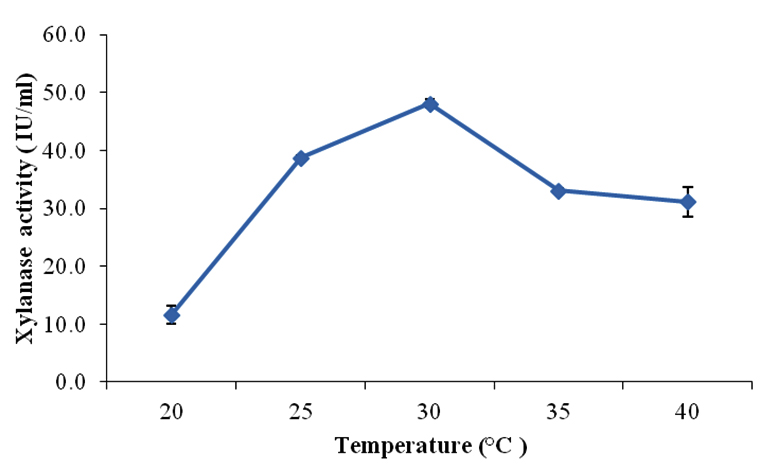
Fig. 2. Effect of temperature on xylanase production using Aspergillus fumigatus SKF-4
At 20°C xylanase production was 11.7 IU/ml at 25°C, 38.7 IU/ml and maximum was at 30°C (48.1 IU/ml) indicating mesophilic nature of the organism under study. Irfan et al. (2014) reported maxiumum production of xylanase enzyme at 30°C using Trichoderma viridae -IR05. Similar activities were reported for xylanase production with Aspergillus niger with optimum incubation temperature around 30°C (Wejse et al., 2005 and Laxmi et al., 2008). After 30°C, enzyme unit declined to 33.1IU/ml at 35°C and further decreased at 40°C (31.1 IU/ml). Reduced level of production of xylanase at higher temperature may be due to the fact that microorganism may synthesize reduced no. of protein essential for growth and other physiological processes including enzyme production (Gawande and Kamat, 1999).
Partial purification of xylanase from Aspergillus fumigatus SKF-4 and its physico-chemical characterization
On partial purification of crude xylanase from Aspergillus fumigatus SKF-4 by acetone precipitation method, xylanase activity increased two times than the initial. On physico-chemical characterization of partially purified xylanase produced by Aspergillus fumigatus SKF-4 with respect to pH it was found that there was sharp rise in enzyme activity as the acidity started decreasing from pH 3.0-4.0 and at pH 4.0 slight increase (20 IU/ml) was observed (Fig 3). Maximum xylanase activity (135.7 IU/ml) was observed at pH 5.0.
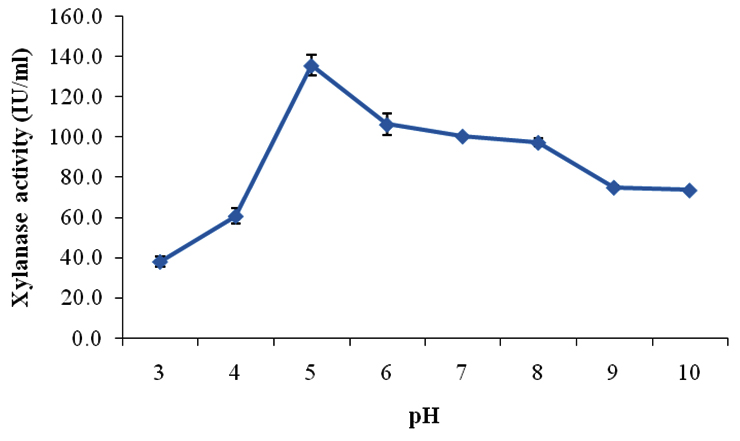
Fig. 3. Effect of pH on xylanase activity produced by Aspergillus fumigatus SKF-4
Xylanase from several Aspergillus fumigatus strains with acidic characteristics have been reported, like strain AR1 with optimum pH of 6.5 (Anthony et al., 2003), strain MKU1 with optimum pH of 5.0 (Thiagarajan et al., 2006), and strain RP04 with optimum of 5.0-5.5 (Peixoto-Nogueira et al., 2009). As the medium pH changed from acidic to neutral, activity started declining but still at pH 7.0 and 8.0 there was substantial activities i.e. 100.4 IU/ml and 97.3 IU/ml respectively. Results indicated that enzyme was much suitable in slightly acidic to slightly alkaline range corresponding to pH 5.0-8.0. After pH 8.0 there was sharp decline in the xylanase activity and it reached decline phase. The majority of xylanases reported to date are optimally active in the acidic, neutral or slight alkaline pH range (Subramanian and Prema 2000; Sudan and Bajaj 2007). The characteristics found in the crude xylanase enzyme under study will help in withstanding harsh conditions of the industrial processes.
Partial characterization of crude enzyme produced by Aspergillus fumigatus SKF-4 was carried out to find out the optimum temperature. The results showed ( Fig 4) that there was slight increase in activity as the temperature increased from 35°C (67.1 IU/ml) to 40°C (95.7 IU/ml) but as the temperature increased from 40°C to 45°C the enzyme activity increases sharply and maximum activity (136.3 IU/ml) was found at 45°C.
This result coincides with the fact that the optimum temperature for xylanase produced by most fungi is in the range of 40-60°C (Kulkarni et al., 1999). Bajaj and Abbass, (2011) also reported that optimum temperature for xylanase produced by Aspergillus fumigatus as 50°C. Several other fungal xylanases have been reported to show optimum activity at 50°C like Penicillium citrinum (Dutta et al., 2007), Aspergillus sydowii (Nair et al., 2008), Penicillium sp (Murthy and Naidu 2010). After 45°C though xylanase activity started declining still significant xylanase activity (114.9 IU/ml) was observed at 50°C but as the temperature Increased further there was sharp decline in enzyme activity and at 60°C xylanase activity of 33.3 IU/ml was observed. After 60°C, the enzyme activity reached a static condition.
Evaluation of commercial and partially purified xylanase from Aspergillus fumigatus SKF-4 for xylooligosaccharides production from beechwood xylan
Partially purified xylanase obtained from Aspergillus fumigatus SKF-4 and the commercial xylanase were evaluated for XOS production using beechwood xylan. Results obtained (Table 4) showed that enzyme obtained from fungal isolate Aspergillus fumigatus SKF-4 was superior than commercial enzymes in terms of xylobiose and xylose production.
It was found that Aspergillus fumigatus SKF-4 produced marginally higher amount xylobiose (1.44±0.4 mg/ml) as compared to commercial xylanase (1.04±0.02 mg/ ml) while xylose was much lower (0.25±0.1 mg/ml) i.e. 7.8 times less than that of commercial xylanase (1.94±0.1 mg/ml). The output received using the fungal isolate Aspergillus fumigatus SKF-4 is highly desirable for xylooligosaccharides production from lignocellulosic biomass like corn cob.
The present study established the potential of xylanases produced from Aspergillus fumigatus SKF- 4 isolated from corn cobs for XOS production. Aspergillus fumigatus SKF-4 was able to produce high titer of xylanase enzyme complex using cheap carbon sources like wheat bran with proteose peptone as nitrogen additive at pH 5.0 incubated at 30°C. This fungus was able to convert beech wood xylan into high amount of xylobiose and lesser xylose within 24 hrs. Hence Aspergillus fumigatus SKF-4 could be a potential organism for xylooligosaccharides production from lignocellulosic biomass.
ACKNOWLEDGMENTS
The authors are thankful to Director, Indian Agricultural Research Institute, New Delhi for providing the necessary facilities and financial support to undertake this study.
- Moure, A., Gullón, P., Domínguez, H., Parajó, J.C. Advances in the manufacture, purification and applications of xylooligosachharides as food additives and nutraceuticals. Process Biochem., 2006; 41: 1913.
- Vazquez M.J., Alonso, J.L., Domínguez, H., Parajó, J.C. Xylooligosaccharides: manufacture and applications. Trends Food Sci. Technol., 2000. 11: 387.
- Gobinath D., Madhu A.N., Prashant, G., Srinivasan, K., Prapulla, S.G. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Braz. J. Nutr. 2010; 104: 40–47.
- Manisseri, C., Gudipati, M. Bioactive xylo-oligosaccharides from wheat bran soluble polysaccharides. LWT – Food Sci. Technol., 2010; 43: 421-430.
- Moura, P., Carvalheiro, F., Esteves M.P., Gírio F.M. Prebiotics xylooligosaccharides as a high-value co-products on an integrated biorefinery approach from lignocellulosic feedstock: International Conference and Exhibition on Bioenergy Bioenergy. 2008
- Chapla, D., Pandit, P. Shah, A. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour. Technol., 2012; 115: 215–221.
- Mussatto, S.I. Mancilha, I.M. Non-digestible oligo saccharides: A review. Carbohydr. Polym., 2007; 68: 587.
- Swennen, K., Courtin, C.M. Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr., 2006; 46:459.
- Ando, H., Ohba, H., Sakaki, T., Takamine, K., Kamino, Y., Moriwaki, S., Bakalova, R., Uemura, Y., Hatate, Y. Hot compressed-water decomposed products from bamboo manifest a selective cytotoxicity against acute lymphoblastic leukemia cells. Toxicol. In Vitro., 2004; 18: 765.
- Sheu, W.H., Lee, I.T., Chen, W., Chan, Y.C. Effects of xylooligosaccharides in type 2 diabetes mellitus. J. Nutr. Sci. Vitaminol. (Tokyo)., 2008; 54: 396.
- Chen, L.L., Zhang, M., Zhang, D.H., Chen, X.L., Sun, C.Y., Zhou, B.C., Zhang, Y.Z. Purification and enzymatic characterization of two b-endoxylanases from Trichoderma sp. K9301 and their actions in xylooligosaccharides production. Bioresour. Technol., 2009; 100: 5230.
- Akpinar, O., Erdogan, K., Bostanci S. Production of xylo oligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr. Res., 2009; 344: 660.
- Fang, H.Y., Chang, S.M., Hsieh, M.C., Fang, T.J. Production, optimization growth conditions and properties of the xylanase from Aspergillus carneus M34. J. Mol. Catal. B: Enzym., 2007; 49 (16): 36-42.
- Bakri, Y., Masson, M., Thonart., P. Isolation and Identification of Two New Fungal Strains for Xylanase Production. Appl. Biochem. Biotechnol., 2010; 162:1626–1634.
- Teather, R. M., Wood, P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol., 1982; 43(4): 777-780.
- Ghose, T.K., Bisaria, V.S. Measurement of hemicellulase activities part 1: xylanases. Pure Appl. Chem., 1987; 59: 1739-1752.
- Miller, G.L. Use of dinitrosalicylic acid reagent for determinationof reducing sugar. Anal. Chem., 1959; 31(3):426–428
- Chang, S.C., Chou, H.C., Cheng, M.K., Wei, D. L. Purification and characterization of ²-xylosidase from an isolated Xylaria regalis 76072314. Fung. Sci., 2005; 20: 3-4.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. Protein measurement with the Folin-Phenol reagents. J. Biol. Chem., 1951; 193: 265-275.
- Bajaj, B. K., Sharma, M., Sharma, S. Alkalistable endo-b-1, 4-xylanase production from a newly isolated alkalitolerant Penicillium sp. SS1 using agro-residues. 3 Biotech., 2011; 1: 83–90.
- Pal, A., Khanum, F. Production and extraction optimization of xylanase from Aspergillus niger DFR5 through solidstatefermentation. Bioresour. Technol., 2010; 101(19): 7563–7569.
- Okafor, U. A., Emezue,T. N., Okochi, V. I., Onyegeme-Okerenta, B. N., Nwodo-Chinedu, S. Xylanase production by Penicillium chrysogenum (PCL501) fermented on cellulosic wastes. Afr. J. Biochem. Res., 2007; 1: 048- 053.
- Saha., S.P. Ghosh, S. Optimization of xylanase production by Penicillium citrinum xym2 and application in saccharification of agro-residues. Biocatal. Agric. Biotechnol., 2014; 3:188–196.
- Shuvaeva, G.P., Sysoeva, M.G. Xylanase of the micromycete Rhizopus var. microsporus 595: preparation, structural and functional characteristics and application. Appl. Biochem. Microbiol., 2010; 46: 641–64.
- Anthony, T., Raj, K. C., Rajendran, A., Gunasekaran, P. High molecular weight cellulase-free xylanase from alkali-tolerant Aspergillus fumigatus AR. Enzyme Microb. Technol., 2003; 32: 647–654
- Shah, A.R., Madamwar, D. Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem. 2005; 40:1763–1771.
- Liao, H., Xu, C., Tan, S., Wei, Z., Ling, N., Yu, G., Raza, W., Zhang, R., Shen, Q., Xu, Y. Production and characterization of acidophilic xylanolytic enzymes from Penicillium oxalicum GZ-2. Bioresour Technol., 2012; 123: 117–124.
- Murthy, P.S., Naidu, M.M. Production and application of xylanase from Penicillium sp. utilizing coffee byproducts. Food Bioprocess Technol., 2010; 5: 657.
- Ninawe, S., Kuhad, R.C. Use of xylanrich cost effective agroresidues in the production of xylanase by Streptomyces cyaneus SN32. J. Appl. Microbiol., 2005; 99 (5): 1141–1148.
- Qinnghe, C., Xiaoyu, Y., Tiangui, N., Cheng, J., Qiugang, M. The screening of culture condition and properties of xylanase by white-rot fungus P. ostreatus. Process Biochem., 2004; 39: 1561-1566.
- Bakri, Y., Jacques, P., Thonart, P. Xylanase production by Penicillium canescens10–10c in solid state fermentation. Appl Biochem Biotechnol., 2003; 108:737–748.
- Bajaj, B. K., Abbass, M. Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28. 3 Biotech., 2011; 1(3): 161-171.
- Gupta, V.K., Gaur, R., Gautam, N., Kumar, P., Yadav, I.J., Dharmwal, N.S. Optimization of xylanase production from Fusarium solani F7. Am. J. Food Technol., 2009; 4: 20–29.
- Subramanian, S., Prema, P. Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol. Lett., 2000; 183:1–7.
- Srinivasan, K., M. Radhakrishnan. Characterization of proton production and consumption associated with microbial metabolism. BMC Biotechnol., 2010; 10: 2–10.
- Irfan, M., Nadeem, M., Syed, Q. One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride IR05 in solid-state fermentation. J. Radiat. Res. Appl. Sci., 2014; 7(3): 317-326.
- Wejse, P.L., Ingvorsen, K., Mortensen, K. K. Salinity and temperature effects on accessibility of soluble and cross-linked insoluble xylans to endo-xylanases. IUBMB Life., 2005; 57(11): 761–763.
- Laxmi, G.S., T. Sathish, Ch. Subba Rao, P. Brahmaiah, M. Hymavathi, and R.S. Prakasham. Palm fiber as novel substrate for enhanced xylanase production by isolated Aspergillus sp. RSP 6. Curr. Trends Biotechnol. Pharm. 2008; 2(3): 447–455.
- Gawande, P.V., Kamat, M.Y. Production of Aspergillus xylanase by lignocellulosic waste fermentation and its application. J. Appl. Microbiol., 1999; 87: 511-519.
- Thiagarajan, S., Jeya, M., Gunasekaran, P. Purification and characterization of a high molecular weight endoxylanase from the solid state culture of an alkali tolerant Aspergillus fumigatus MKU1. World J. Microbiol. Biotechnol., 2006; 22: 487–492.
- Peixoto-Nogueira, S.C., Michelin, M., Betini, J.H.A., Jorge, J.A., Terenzi, H.F., Polizeli, M.L.T.M. Production of xylanase by Aspergilli using alternative carbon sources: application of the crude extract on cellulose pulp biobleaching. J. Ind. Microbiol. Biot., 2009; 36: 149-155
- Sudan, R., Bajaj, B.K. Production and biochemical characterization of xylanase from an alkalitolerant novel species Aspergillus niveus RS2. World J. Microbiol. Biotechnol., 2007; 23:491–500.
- Kulkarni, N., Shendye, A., Rao, M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol. Rev. 1999; 23: 411-456.
- Dutta, T., Sengupta, R., Sahoo, R., Sinha Ray, S., Bhattacharjee, A., Ghosh, S. A novel cellulasefree alkaliphilic xylanase from alkalitolerant Penicillium citrinum: production, purification and characterization. Lett. Appl. Microbiol., 2007; 44: 206–211.
- Nair, S.G., Sindhu, R., Shankar, S. Purification and biochemical characterization of two xylanases from Aspergillus sydowii SBS 45. Appl. Biochem. Biotechnol. 2008; 149: 229–243.
- Kulkarni, N., Shendye, A., Rao, M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol. Rev. 1999; 23: 411-456.
- Mandels, M., Reese, E.T. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J. Bacteriol., 1957; 73: 269-278.
- Raper, K.B., Fennell, D.I. The genus Aspergillus. 1965., Williams & Wilkins, Baltimore
- Sudan, R., Bajaj, B.K. Production and biochemical characterization of xylanase from an alkalitolerant novel species Aspergillus niveus RS2. World J. Microbiol. Biotechnol., 2007; 23:491–500.
© The Author(s) 2016. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


