Biofouling is a significant problem in various industries, including marine, medical, and water treatment. It occurs when microorganisms, such as bacteria, fungi, and algae, attach to surfaces and form biofilms, which can lead to material degradation, decreased performance, and increased maintenance costs. Traditional approaches to mitigate biofouling include the use of biocides, physical cleaning, and chemical treatments. However, these methods have limitations, such as environmental concerns, short-term effectiveness, and development of resistant organisms. One alternative approach is the use of lichen-associated symbionts, which have been found to produce compounds that inhibit biofilm formation and growth. These compounds could be used to develop eco-friendly and sustainable antifouling coatings. Another promising approach is the use of nanotechnology to develop novel coatings that prevent biofouling. Nanomaterials can be engineered to have hydrophobic structures, which deter microorganisms from attaching to surfaces. They can also be designed to contain nano biocides, which can kill organisms that come into contact with the surfaces. Overall, the use of lichen-associated symbionts and nanotechnology holds great potential for developing effective and sustainable solutions to mitigate biofouling. However, further research is needed to optimize these approaches and ensure their safety and efficacy in various applications. This review offers a brief overview on the mechanisms of biofouling and evaluate the potential of using lichen-associated symbionts and nanotechnology to prevent or reduce biofouling.
Biofouling, Marine Environment, Anti-microbials, Nanomaterials, Lichen
The process of biofouling involves the build-up of microorganisms, planktons, algae, or animals on wet surfaces, followed by their growth on solid surfaces submerged in water.1 In the maritime environment, marine bacteria, protozoa, and microalgae as well as invertebrates and macroalgae swiftly colonize both natural and manmade surfaces.2 In the maintenance of Mari culture facilities, shipping facilities, vessels, and seawater pipelines, both microfouling and macro fouling in the seas result in enormous material and monetary losses.3 The costs associated with bioleaching may arise due to increased fuel consumption, maintenance and repair of the affected surface and reduced efficiency. More than 5.7 billion US dollars are spent yearly by nations worldwide to prevent and manage marine biofouling. Numerous underwater constructions are covered in antifouling coatings to reduce the effects of fouler. However, these coatings are known to be very hazardous and generic in nature.4
The economy and the environment are both negatively impacted by biofouling. Following are the negative impacts of Biofouling: 1. Environmental Concerns: Biofouling is linked to environmental problems. This is just one of the harmful effects of biofouling. Ships utilize a lot of fuel, which releases greenhouse gases into the atmosphere and harms the ecosystem. 2. Water pollution: The chemicals used to clean water cause biofouling, and the development of biofilm degrades the water quality of rivers, lakes, and other bodies of water. 3. Ocean aquaculture: Fish species are disturbed by biofouling creation. Production of seafood is also decreasing. 4. Operating Costs: Biofouling speeds up the degradation of surfaces or structures where it develops. Spending on operations stems from this. 5. Mobility Issues: Loss of manoeuvrability is the most frequent result of biofouling.
Organisms and Adhesion In Marine Biofouling
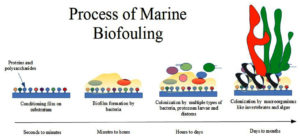
Marine biofouling is a serious problem because aquatic creatures quickly colonise ship hulls. The main problem is a complicated colonization process in which aquatic organisms, microbial slimes, and macrophytes quickly colonise the ship hulls. Bioaccumulation causes the surface to become more uneven, which increases hydrodynamic wear and drag. The cost of biofouling restoration is impacted by higher fuel consumption, the need to remove paint and repaint the surface, and other related environmental compliance requirements.5 Ocean biofouling has negative economic and ecological effects.6 The term “biofouling” describes marine organisms that have two distinct stages in their complex life cycles, such as a planktonic larval stage and an adult stage that develops after the larva metamorphoses and becomes benthonic and attaches to a natural surface like a rock or an artificial surface like a ship hull. The fouling community, which includes algae, barnacles, molluscs, bryozoans, polychaetes, tunicates, and hydrozoans, is what causes marine biofouling (Figure 1). Numerous different microorganisms can thrive when organic materials are present on the surface of the substratum. Typically, bacteria, protozoa, and diatoms that produce biofilms initiate the first colonisation.7 Diverse microbial species that are attached to the surface (and to one another) and contained in a matrix of extracellular polymeric molecules make up biofilms (EPS). Marine biofilms contain a variety of heterotrophic bacteria, particularly proteobacteria, cyanobacteria, archaea, and unicellular eukaryotes like diatoms. Microorganisms may control their adhesion to surfaces, film growth, swarming, and toxin release through a process known as quorum sensing (QS). During this method, a class of low molecular weight chemicals known as “autoinducers” is produced and released into the environment. These chemicals stimulate the expression of target genes, which alters bacterial activity, when their concentrations reach a specific point where diffusion is restricted.8
Strategies to Control Biofouling
Plants, insects, corals, and fish use a variety of physical and chemical antifouling measures.9 Fouling-resistant, fouling-release, and fouling-degradation strategies are used in antifouling coatings. By forming a super hydrated surface, foulants such as bacteria, algal spores, and protein are prevented from adhering fouling-resistant coatings.
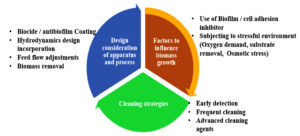
Combination of actions to control biofouling is preferred over a single action. Figure 2 represents the current approaches for biofouling control (ABC), which is symbolised by an umbrella shielding against biofouling.10 Surface coating is the most used technique to mitigate biofouling because of its effectiveness and low cost. It enables the removal of foulants from the substratum’s surface using simple mechanical or physical cleaning methods such as brushing or using water jets.11 These measures include reduced drag, adhesion, wettability, changes in microscale textures, continuous grooming behaviour, and antifouling compound secretion.9
Figure 2. The Biofouling Control Approach (BCA) encompasses three fundamental elements: the design and operation of equipment, the optimization of biomass growth conditions, and the utilization of effective cleaning agents10
Methods of Biofouling Control
Biofouling control strategies are roughly classed as non-acoustic and acoustic methods. The former refers to procedures that use high toxicity antifouling chemicals, whilst the latter uses non-toxic antifouling agents. A broad-spectrum, high-toxicity antifouling coating technique is referred to as non-acoustic means for biofouling management.4 Due to their low polymer-water interfacial energies and high degree of hydration, amphiphilic polymer nanocomposites are the most common fouling-resistant polymer coatings.12
As a result, Tributyltin was discovered to be more effective and to provide higher protection against fouling. Tributyltin has been used as a powerful antifoulant for decades. However, all of these techniques offer a significant environmental risk and serve as a biocide on both non-target and other beneficial organisms.13 Silicone based fouling release coatings have been created as an alternative to biocides, where they work by weakening the adherence of attached organisms. Rising environmental and safety standards have prompted intensive research into the development of new environmentally acceptable antifoulants.14 Marine AF coatings have been created using hydrophilic polymers as PEG, hydrogel, zwitterion, and hyper branched polymers.12 This became an extremely effective antifouling method, with an estimated 70% of the world fleet covered at one time.15 Unfortunately, TBT systems have a negative impact on the environment. With the addition of booster biocides, copper has surpassed TBTs as the primary biocide ingredient in antifouling coatings (Figure 3).16, 17 Copper-based antifouling continues to raise environmental issues.15 Cu2O is a p-type semiconductor with a narrow bandgap that, in contrast to semiconductors with wide bandgaps, has the unique ability to utilise sunshine.18
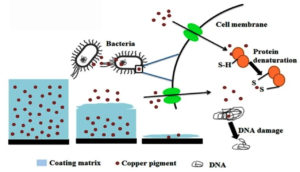
Figure 3. Anti-fouling action of copper against fouling bacteria.19 High concentrations of copper are detrimental to microorganisms since it has the ability to displace or substitute essential metals from their original binding sites. This occurs because copper exhibits a stronger attraction towards thiol-containing groups and oxygen sites, thus leading to toxicity for all microorganisms. Also, copper can cause confirmational changes in biomolecules such as DNA and protein.
As a result, there is a growing interest in producing environmentally friendly alternatives. Antifouling chemicals that occur naturally in the environment can be isolated and integrated into a paint matrix. Large quantities of these substances would have to be collected or synthesised at an economically reasonable price.4 Fouling release coatings lessen the fouling’s attachment strength, making it easier to remove with water flow or mechanical cleaning. According to reports, these coatings are highly pricey, have poor adherence to the substrate, and are readily destroyed.4, 20, 21
Mariners have employed metal salts as biocides and fouling control agents since ancient times. Copper has traditionally been used as a biocide. Antifouling paints containing copper salts have been created.22 Toxic anti-fouling paints being used today include dissolving matrix paints, ablative paints, and self-polishing systems. The majority of antifouling paints contain copper, usually in the form of cuprous oxide (Cu2O). Self-polishing copolymer (SPC) paints have a polishing effect because the polymer dissolves and then releases tributyltin (TBT) during normal conditions. Photocatalysis (PC) and photoelectrocatalysis (PEC) are AOPs that use in situ generation of hydroxyl radicals to oxidize organics, which are environmentally friendly, reusable, and can be used to degrade toxic organic pollutants.18 TBT eliminates settling fouling organisms while also making the surface smoother.23
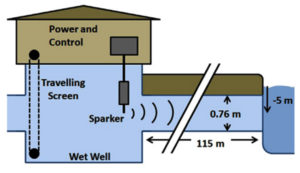
Acoustic antifouling control methods are non-toxic antifouling approaches that have included textured surface coatings, mechanical procedures, and electrical ways.4,24 For instance, Schaefer et al.25 have used acoustic sparkers for the control of biofouling and it is illustrated in Figure 4. Other procedures utilised for pipes include the use of chemicals, hot water, acids, and UV treatments.4, 17, 25 Acoustic antifouling methods may give a non-toxic alternative to non-acoustic approaches for biofouling prevention and are far more environmentally benign than non-acoustic methods.
Bioleaching is a process in which microorganisms are used to extract metals from ores and other materials. In the context of marine fouling, bioleaching can be used to remove unwanted deposits on surfaces. This is accomplished by introducing microorganisms that are capable of breaking down the organic material that makes up the fouling. These microorganisms can be naturally occurring or artificially introduced, and they work by producing enzymes that break down the fouling into smaller, more easily removable particles.27 However, bioleaching is not without its challenges. One issue is that the microorganisms used for bioleaching must be carefully selected and controlled to prevent unintended damage to the surface being cleaned. Additionally, the process may take longer than other cleaning methods, as it relies on the natural growth and metabolism of the microorganisms. Despite these challenges, bioleaching has shown promise as a viable alternative to traditional marine fouling removal methods.
Fouling Control Using Anti-Microbial Compounds
Currently, antimicrobial-based antifouling coatings are gaining momentum. Antimicrobials are compounds that are produced by various living organisms such as bacteria, fungi, plant etc., against microorganisms. Depending on the target organisms, antimicrobials are classified as antibacterials (Compounds that act against bacteria), antifungals (Compounds that act against fungi), antivirals (Compounds that act against virus) and antiparasitics (Compounds that act against parasites). Since, bacteria are the primarily associated with the marine biofouling, antibacterial agents are used in the manufacturing of antimicrobial-based antifouling coatings (Figure 5). Bacteria are prokaryotes, which means they lack a nucleus and are thus very simple in nature. A silicone/graphene oxide sheet-alumina nanorod ternary composite has been proposed as a potential superhydrophobic antifouling coating. The coating consists of a layer of silicone, a layer of graphene oxide sheet, and a layer of alumina nanorods. The silicone layer provides the coating with flexibility and durability, while the graphene oxide sheet layer provides a hydrophobic surface that repels water. The alumina nanorods, which are vertically aligned on the surface, create a rough surface with a high aspect ratio, further enhancing the hydrophobicity of the coating.28 As they are very distantly related to humans, several of their proteins can be targeted to produce novel antibacterial agents.29 It is very important that the antimicrobials used in the coating should not harm other organisms, especially humans. The combination of these three materials results in a coating with a high water contact angle and low sliding angle, making it superhydrophobic. This property makes the coating less prone to fouling, as it repels water and prevents the attachment of fouling organisms.28 The cell wall of bacteria is a crucial target since it has a structure that human cells lack and is formed of a material called peptidoglycan, which human cells also lack; hence, it is a significant target. Bacterial ribosome is another target not because they are absent in humans, but because their sizes differ, and most medications that attach to subunit of the bacterial ribosome do not interact with human counterparts. Similarly, a portion of the enzymes involved in bacterial nucleic acid replication differ from their human counterparts. Folic acid production is another interesting target. Folic acid is usually synthesized by the bacteria while humans have to obtain it from their food.30
According to Zhang et al.32, natural antifouling agents can be derived from organisms of both marine and terrestrial origin. Additionally, a wide range of protein resistant coatings are available, which aid in inhibiting bacterial attachment and subsequently the development of biofilms. The traditional methods, on the other hand, involve creating biocidal coatings for biological materials’ surfaces that may comprise antibiotics, quaternary ammonium salts, and silver.33 A silicone/ZnO nanorod composite coating has been proposed as a potential antifouling surface for marine applications. The coating is made up of a layer of silicone and a layer of vertically aligned zinc oxide (ZnO) nanorods. The silicone layer provides the coating with flexibility and durability, while the ZnO nanorods create a rough surface with a high aspect ratio that inhibits the attachment of fouling organisms. Additionally, ZnO has antimicrobial properties that may further prevent the growth of fouling organisms.34 The use of organic products to combat fouling has produced an effective and useful solution to the current environmental issue and economic difficulties brought on by a surge in marine biofouling. The effectiveness of the silicone/ZnO nanorod composite coating as an antifouling surface has been demonstrated in laboratory tests, showing a reduction in fouling compared to uncoated surfaces. However, further research is needed to determine the long-term effectiveness and durability of this coating in real-world conditions, as well as its potential environmental impacts.34 The development of the poly (methyl methacrylate-co-ethyl acrylate-co-hexafluorobutyl methacrylate-co-isobornyl methacrylate) copolymer (PBAF), which demonstrated an exceptional anti-biofouling activity of 98.2 % resistance to the E. coli strain, is one of the most significant methods for the antifouling activity. The integration of the fluorine into the copolymer was primarily responsible for this effective antifouling and bactericidal action. This results in a reduced surface energy and wettability of the synthetic antifouling agent. The major benefit of the PBAF-mediated coating is that it is environmentally friendly and it is extremely wear resistant.35 The antifouling qualities of PDMS, or Poly (dimethyl siloxane), another anti-fouling agent, are fairly constant, although their mechanical behaviour and precise release of antifoulants are lacking. Additionally, according to the studies, these antifouling coatings were required to have a notable degree of transparency, bactericidal and anti-algal capabilities, and a nine month anti–biofouling performance in field.36
Effect of Chemical Anti-Fouling Agents on Marine Ecosystem
As the primary component of antifouling paints, copper salts, typically cuprous oxide (Cu2O), were manufactured.22 Polishing paints are made of self-polishing copolymer (SPC), which dissolves and releases tributyltin (TBT) under normal working conditions. Tritylin was discovered to be more effective and to provide greater protection against fouling. The newest antifouling paint alternatives are based on copper compounds such as the copper thiocyanate (CuSCN) and cuprous oxide (Cu2O).
Novel Technologies
Finding a dynamic antifoulant is the most important task for the present and the future, and this is the main direction of aquatic industry research.37 The development of novel active biomolecules that might thwart the fouling process is greatly aided by increased molecular understanding of how bioadhesives function.
Inhibitors used and the Recent Innovations in Antibiofouling
TBT and copper based paints were mostly used organometallic compounds incorporated in paint to prevent biofouling. One of the most promising alternatives to heavy metal based paints is offered by the development of antifouling coatings in which the active ingredients are compounds naturally occurring in organisms and operating as natural anti-settlement agents.38 A study looked into how commercially available enzymes affected the bryozoan Bugula neritina’s larval attachment. At doses of 0.03 to 1 ul, proteases of six deep-sea Pseudoalteromonas species dramatically reduced larval attachment. In field tests, the protease and trypsin were each independently integrated into a water-soluble paint matrix that resisted biofouling. Their research showed how proteolytic enzymes could be used to protect against fouling. Since biofouling is one of the major threats to the developing marine industries finding for an immediate long lasting resilient solution is a great need. Marine water is being used in various studies for the isolation of antagonistic organisms that can prevent fouling.
Antagonistic marine fungus isolated from coastal waters of Tamil Nadu was selected from initial screening for in vitro analysis of antifouling effect. Another study by Bernbom et al.37 studied the antifouling activity of marine bacteria against Pseudoalteromonas species and zoospores of the green alga Ulva australis, which showed the antifouling reducing ability of these organisms. Bavya et al.39 showed the ability of marine actinomycetes as a potential source for the development of antifouling compounds. Mahadevan et al.40 reported that green seaweed (Ulva reticulata) and 2 out of 10 bacterial strains showed wide spectrum antibiotic activity against biofilm forming bacteria.
In vitro Studies In Antifouling
Salta et al.24 made simultaneous studies on marine and terrestrial sources for detaining biofilm formation against two marine bacteria namely Cobetia marina and Marinobacter hydro carbonoclasticus. The toluene soluble part of C. crispus (CCT) was found to inhibit bacterial attachment at higher concentrations for both the bacteria. At higher concentration hexane extract of Curcuma longa can be used as a natural biocide. The study also showed that the obtained extract does not have any toxic effect on the marine life. Acevedo et al.41 investigated the antifouling activity of organic extracts from some epibiont-free Colombian Caribbean Sea sponges and a sea-cucumber. After 45 and 90 days, significant differences in fouling cover percentages between painted panels and controls were found (p < 0.05). Red Sea puffer fish mucus powder extracts were incorporated into simple paint formulations and showed significant results in the first six weeks. The study successfully identified one of the potential new sources of natural antifouling compounds.
Because of their symbiotic characteristics and because they make up a significant portion of the biota in India (over 2,900 species, or 14.8% of all known species), lichens are the subject of the current study (19,500 spp).42 Notably, India’s lichen-geographical hotspots include the Western Ghats and the Eastern-ern Himalayan highlands.43 Its varied assortment of microorganisms can colonise a wide range of surfaces; as a result, they are most frequently discovered on tree bark, rock, and as a component of the soil crust. Additionally, lichens’ interactions with microbes can be influenced by both abiotic and biotic factors.44 Figure 6 represents Lichen generated biological molecules can be used to make inexpensive, eco-friendly nanoparticles, which opens the door to new inventiveness and creativity. A technical wonder, nanomaterials provide a variety of ways to improve the effectiveness of the microorganism in which they are added.45
Because of their wide spectrum of fungicidal and bactericidal effects and their capacity to interact with different macromolecules and ligands in microbial cells, silver nanoparticles are the best material of choice to create.47 Moreover, due to its anti-inflammatory qualities, Ag has been successfully employed to treat wound healing and tissue regeneration, as well as to limit bacterial metabolism.48 Table represents Ag nanoparticles differ from their macroscale counterparts in terms of their physiochemical characteristics.49 This is mostly caused by the materials’ tiny size and vast surface area, to use an analogy. As a result of advancements in the synthesis, production, and stabilisation of metal nanoparticles, a new generation of goods as well as academic studies in the field of nanoscience have been produced.49-53 Due to its unique qualities and numerous applications, such as the development of less harmful antibacterial treatments, nanoparticle production has drawn more interest. It can be synthesized environmentally friendly as well as inexpensively.54
Table:
Microorganisms involved in Biofouling.
S.No |
Microorganism |
Type of Biofouling |
Reference |
|---|---|---|---|
1 |
Pseudomonas aeruginosa |
Bacterial biofilm formation |
Yebra et al.4 |
2 |
Vibrio spp. |
Microfouling |
Salta et al.24 |
3 |
Candida albicans |
Fungal biofilm formation |
Vrouwenvelder et al.57 |
4 |
Ulva spp. |
Macrofouling |
Choi et al.58 |
5 |
Ectocarpus spp. |
Macrofouling |
Rolin et al.59 |
A growing number of commercial items contain metal nanoparticles, especially Ag, although it is unclear how these materials may affect people’s health and the environment.55 Ag Nps are efficient and environmentally beneficial solutions for fouling control, hence the research focuses on biofouling.56 Marine biofouling is brought on by the adherence of macrophytes and bacterial slimes.57 Bioaccumulation increases surface irregularity when the boat base passes through the waves and oceans, which may increase hydrodynamic drag and wear. Increased fuel consumption, the need to remove paint and repaint surfaces, and other associated environmental compliance requirements all result in higher costs for biofouling remediation.5
Nanotechnology in Fouling Control
Nanotechnology is widely believed to be to blame for a number of ecological issues, including waste pollution and the release of hazardous chemicals. In order to handle toxic substances, precautionary measures must be developed, and many chemicals have side effects in addition to their intended uses.60
Antifouling is the process of cleaning and conditioning films created by the adsorption of oily molecules onto the sea surface. Sol-gel coating, silicone coating, and photocatalytic coating are receiving increased attention as part of the most recent eco-friendly anti-fouling technology. This is due to the fact that the release rate of zinc ions from nano ZNO was comparable to that of non-nano formulations. In long-term use, there is no discernible difference in the antifouling efficiency of nano and non-treating agents.61
Hydrophobic coatings and nano roughness could be used as non-toxic antifouling substitutes for SHCs. Future research is required to evaluate the fouling capacities of SHCs as well as the investigation of fouling resistance in long-term trials.62 New techniques including employing low surface energy non-toxic marine antifouling agents are made with acrylic resin and other pigments. Hydrophobic coatings and nano roughness have been suggested as potential non-toxic alternatives to traditional self-polishing hull coatings (SHCs) for antifouling purposes. The idea behind this is to create a surface that is less hospitable to the settlement and growth of fouling organisms such as barnacles and algae. Hydrophobic coatings can make the hull surface water-repellent, making it more difficult for fouling organisms to attach themselves to the hull. On the other hand, nano roughness involves creating microscopic bumps and ridges on the surface of the hull, which can also make it harder for fouling organisms to attach. Both of these methods have the potential to reduce the need for SHCs, which can contain toxic chemicals that can harm marine life. However, further research is needed to determine the effectiveness and long-term durability of these alternatives, as well as their potential environmental impacts. Antifouling paints were created by immobilising marina leaves with various nanoparticles and paint compositions. They have varying and increasing concentrations of oxidising (anionic) and non-oxidizing (cationic) substances. These substances are effective at preventing the growth of biofilms on marine surfaces.63
Antifouling agents containing nano-coatings made of silicone, phosphorous, and sulphur with the epoxy resin Diglycidyl ether of bisphenol (DGEBA) as the base material had an impact on corrosion and fouling behavior. These materials were cured using Aradur 140, a polyamidoimidazoline and XY 54 curative. A new technique for preventing fouling in marine environments makes use of a nanocomposite covering made of adhesive tannic acid (TA), polyethylene glycol, silica hybrid nanoparticles, and poly sulfobetaine methacrylate-vinyltrimethoxysilane.64 Antifouling agents’ functional coating was given hydrophilicity, high roughness, and exceptional abrasion resistance. This was made using an easy deposition technique and was successful in preventing the adherence of proteins and consequent biofouling deposition on marine surfaces.65
Nanotechnological devices have been shown to be excellent replacements for frequently used aquaculture equipment, such as fishing nets, cages, and enclosures.66 It comprises of a self-refreshing interface that successfully stopped the development of biofilms, fundamentally inhibiting marine biofouling. Antifouling techniques are centred on ionic polymers or polymeric micro- and nano-molecular structures. These products have been found to have long-lasting anti-fouling performance. Metal alloys, on the other hand, are heavy and expensive and have a shorter lifespan.67
Application of Lichens In Biofouling Control
Using lichen derived enzymes to hydrolyze fouling organism adhesives is one way to prevent biofouling.68 Organometallic antifoulants are outperformed by enzymes in terms of effectiveness, biodegradability, and environmental friendliness. Enzymes not only hydrolyze but also produce biocides, which have an impact on how marine fouling organisms attach. Over the past 20 years, there has been a lot of study done on enzyme-based fouling reduction techniques. Fouling will not immediately show up on the submerged material. There are three processes at play: microbial invasion, bioadhesive deposition, and subsequent growth. Therefore, it is essential and expanding quickly to produce ecologically friendly coatings (Figure 7).69,70
Lichens are major components of earthbound and marine environments.72 Since lichen-associated bacteria make up a crucial part of lichen thalli, the conventional understanding of lichens should be widened to take bacteria into account. Since they are usually numerous and diverse, lichen-associated bacteria (LAB) may contribute to lichen symbiosis in several ways. The majority of microorganisms found in lichens are not photosynthetic.73 Recent studies on lichen-associated bacteria suggest that lichen-associated microbes are a crucial part of lichen thalli.74 Each of the four lichen species from which bacteria were recovered had its own unique bacterial community, with Alphaproteobacteria predominating in all of the communities.75 The extent of this symbiotic interaction is still mostly unknown. Future research and testing are necessary to determine any potential benefits that these lichen-associated bacteria may have. For a wide range of biotechnological applications, industrial enzymes are essential. In enzyme-based antifouling coatings, proteases are the most crucial antifouling active components. Some of the enzymes utilised as antifouling active ingredients include lipase, protease, cellulase, amylase, and wood polyenzyme.76
E-waste can be recycled using various techniques, including chemical, mechanical, fire, and physical. Chemical treatment for recycling is currently in use, but because inorganic chemicals are used, it causes greater environmental problems. Because it is an environmentally beneficial way, this paper synthesised the nanoparticle from e-waste using the biological process. In order to minimise the copper and ferrous nanoparticle biologically, lichen-associated bacteria like Parmotrema tintorum and P. recticulatum were used to extract the particles from the e-waste (Figure 8). Utilizing PSA, the generated particles were almost 10 and 100 nanometers. At the 2.5% composition, the formation of ferrous nanoparticles was verified by the peak value procured at 430 nm and 540 nm for copper nanoparticles. Their antifouling qualities were examined by causing synthesized nanoparticles to interact with the paint and be applied to an iron surface. Recent studies have shown that the use of nanomaterials can minimise fouling activity, prevent adverse impacts on other marine species, and overcome some microbes’ resistance to anti-foulants. This study uses copper and ferrous nanoparticles that were created from e-waste materials with the aid of bacterial reduction to help reduce environmental pollutants.77
Figure 8. a) Lichen samples from various regions of Tamil Nadu, India, b) Lichen-associated bacterial (LAB) colonies isolated and streaked on nutrient agar, c) gram straining of LAB and d) Anti-microbial activity of LAB extract against common fouling bacteria77
A straightforward approach was used to create lichen-like nickel oxide nanostructure, which was then analysed. The electrocatalytic oxidation of acetylcholine (ACh) on the modified electrode was then studied. The nanostructure was then used to modify a carbon paste electrode and for the construction of a sensor. When nickel micro- and nanoparticles’ electrocatalytic efficiency was compared to that of nickel oxide nanostructures, the lichen-like nickel oxide nanostructure had the highest efficiency. Chronoamperometry, steady-state polarisation curve analysis, and cyclic voltammetry were used to study the mechanism and kinetics of the electro-oxidation process. It was reported on the catalytic rate constant, charge transfer coefficient, and diffusion coefficient of ACh during the electro-oxidation of ACh by active nickel species. For the estimation of ACh, a quick and accurate hydrodynamic amperometry approach was created. The sensor’s benefits included a straightforward fabrication process that didn’t require the use of any reagents or enzymes, an immobilisation step, high electrocatalytic activity, extremely high sensitivity, long-term stability, and an antifouling exterior property toward ACh and its oxidation product.78
The model states that when a droplet of liquid comes into contact with a surface, it spreads out to maximize the contact area. If the surface is rough, the liquid will conform to the surface roughness, increasing the contact area and adhesion. However, if the surface is hydrophobic, the liquid will bead up and not wet the surface, reducing the contact area and adhesion. The Cassie-Baxter model, on the other hand, takes into account the roughness of the surface and the air pockets trapped within it. In this model, the liquid droplet sits on top of the air pockets, resulting in a reduced contact area and adhesion. This is known as the Cassie state. Both the Wenzel and Cassie-Baxter models can be used to explain the hydrophobicity and fouling-repellency of surfaces. A surface with a high contact angle and low sliding angle is more likely to repel liquid droplets and fouling organisms, making it easier to keep clean. The use of hydrophobic coatings and surface texturing based on these models has been proposed as a potential solution to reduce fouling in various marine and industrial applications, such as ship hulls and heat exchangers. However, further research is needed to fully understand the effectiveness and durability of these approaches, as well as their potential environmental impacts.
ZnO Recently, Zn, Si, and Ti nanoparticles as well as polymers have been employed to stop biofouling, and numerous production methods have previously been documented79-81. However, it is vast and dangerous to produce nanoparticles using conventional chemical processes. In the process of microbial synthesis, bacteria, algae, and fungus are used to remove metal ions from the precursor and create metal oxide nanoparticles.82,83,84 The presence of the two organisms that is beneficial to one or both symbionts is known as symbiosis. Lichen is a common example of a symbiotic relationship between a photobiont and a mycobiont. Because of their excellent symbiosis, lichens can survive in almost any habitat. In harsh environments, the context of bioactive molecules produced by the mycobiont, other metabolites are more likely to develop. Because lichens include a large number of phenolic compounds including hydroxyl groups and an aromatic phenol ring, antibacterial activity has been seen in lichens. Due to their ability to produce less reactive phenoxyl radicals, phenols serve as free radical scavengers and hydrogen donors. Because of their symbiotic characteristics and because they make up a significant portion of the biota in India (approximately 2,900 species, or 14.8% of all known species), lichens are the subject of the current study (19,500 spp).42
In conclusion, biofouling is a complex and significant issue with detrimental economic and environmental impacts. The process involves the accumulation and growth of various microorganisms, plankton, algae, and animals on wet surfaces, leading to the colonization of both natural and manmade structures in the maritime environment. The consequences of biofouling, such as material and monetary losses, increased fuel consumption, maintenance, and repair costs, and reduced operational efficiency, pose significant challenges for industries and infrastructure maintenance. Nations worldwide invest billions of dollars annually to combat and manage marine biofouling. Addressing the challenges posed by biofouling requires the development of sustainable and efficient solutions. It is essential to explore alternatives to hazardous antifouling coatings and invest in innovative approaches that minimize the impact on marine ecosystems while ensuring the economic viability of maritime industries. Nanoparticles derived from the microorganisms, Lichens, and their metabolites represent a promising avenue in the quest for environmentally friendly solutions to combat biofouling. Their natural antifouling properties and potential for use in coatings and treatments offer an alternative approach that can contribute to the preservation of marine ecosystems, the reduction of economic losses, and the promotion of sustainable practices in maritime.
ACKNOWLEDGMENTS
The authors would like to thank to the Management, CEO, Principal and Department of Biotechnology, K.S.Rangasamy College of Technology, Tiruchengode, for providing necessary facilities and also constant support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Zhang X, Wang L, Levänen E. Superhydrophobic surfaces for the reduction of bacterial adhesion. Rsc Advances. 2013;3(30):12003-20.
Crossref - Alvarez, PJJ, Chan CK, Elimelech M, Halas NJ and Villagrán D. Emerging opportunities for nanotechnology to enhance water security, Nat. Nanotechnol., 2018;13(8): 634–641.
Crossref - Bixler GD, Bhushan B. Biofouling: lessons from nature. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2012;370(1967):2381-417.
Crossref - Yebra DM, Kiil S, Dam-Johansen K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progress in organic coatings. 2004;50(2):75-104.
Crossref - Dobretsov S, Teplitski M, Bayer M, Gunasekera S, Proksch P, Paul VJ. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling. 2011;27(8):893-905.
Crossref - Qiu H, Feng K, Gapeeva A, Meurisch K, Kaps S, Li X, Yu L, Mishra YK, Adelung R, Baum M. Functional polymer materials for modern marine biofouling control. Progress in Polymer Science. 2022:101516.
Crossref - Dobretsov S, Dahms HU, Qian PY. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling. 2006 Jan 1;22(1):43-54.
Crossref - Golberg K, Eltzov E, Shnit-Orland M, Marks RS, Kushmaro A. Characterization of quorum sensing signals in coral-associated bacteria. Microbial ecology. 2011;61:783-92.
Crossref - Sullivan T, O’Callaghan I. Recent developments in biomimetic antifouling materials: A review. Biomimetics. 2020;5(4):58.
Crossref - Vrouwenvelder J, Kruithof J. Biofouling of spiral wound membrane systems. Iwa Publishing 2011.
Crossref - Maan AM, Hofman AH, de Vos WM, Kamperman M. Recent developments and practical feasibility of polymer-based antifouling coatings. Advanced functional materials. 2020;30(32):2000936.
Crossref - Selim MS, Shenashen MA, El-Safty, SA, et al. Recent progress in marine foul-release polymeric nanocomposite coatings. Progress in Materials Science, 2017;32.
Crossref - Farr H, Ruttenberg B, Walter RK, Wang YH, White C. Potential environmental effects of deepwater floating offshore wind energy facilities. Ocean & Coastal Management. 2021;207:105611.
Crossref - Wynne KJ, Swain GW, Fox RB, Bullock S, Uilk J. Two silicone nontoxic fouling release coatings: hydrosilation cured PDMS and CaCO3 filled, ethoxysiloxane cured RTV11. Biofouling. 2000;16(2-4):277-88.
Crossref - Thomas KV, Brooks S. The environmental fate and effects of antifouling paint biocides. Biofouling. 2010;26(1):73-88.
Crossref - Brooks S, Waldock M. The use of copper as a biocide in marine antifouling paints. In Advances in marine antifouling coatings and technologies 2009: 492-521. Woodhead Publishing.
Crossref - Guo ZY, Yuan XS, Geng HZ, Wang LD, Jing LC, Gu ZZ. High conductive PPy–CNT surface-modified PES membrane with anti-fouling property. Applied Nanoscience. 2018 Aug;8:1597-606.
Crossref - Koiki BA, Arotiba OA. Cu 2 O as an emerging semiconductor in photocatalytic and photoelectrocatalytic treatment of water contaminated with organic substances: A review. RSC advances, 2020;10(60): 36514-36525.
Crossref - Sathe P, Laxman K, Myint MT Z, Dobretsov S, Richter J, Dutta J. Bioinspired nanocoatings for biofouling prevention by photocatalytic redox reactions. Scientific Reports, 2017;7(1), 3624.
Crossref - Brady Jr RF. A fracture mechanical analysis of fouling release from nontoxic antifouling coatings. Progress in organic coatings. 2001;43(1-3):188-92.
Crossref - Schultz MP, Kavanagh CJ, Swain GW. Hydrodynamic forces on barnacles: Implications on detachment from fouling-release surfaces. Biofouling. 1999;13(4):323-35.
Crossref - Carteau D, Vallée-Réhel K, Linossier I, et al. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Progress in Organic Coatings. 2014;77(2):485-93.
Crossref - Gopikrishnan V, Radhakrishnan M, Pazhanimurugan R, Shanmugasundaram T, Balagurunathan R. Natural products: potential and less explored source for antifouling compounds. J Chem Pharm Res. 2015;7(7):1144-53.
- Salta M, Wharton JA, Dennington SP, Stoodley P, Stokes KR. Anti-biofilm performance of three natural products against initial bacterial attachment. International Journal of Molecular Sciences. 2013;14(11):21757-80.
Crossref - Omae I. Organotin antifouling paints and their alternatives. Applied organometallic chemistry. 2003;17(2):81-105.
Crossref - Schaefer R, Claudi R, Grapperhaus M. Control of zebramussels using sparker pressure pulses. Journal-American Water Works Association, 2010;102(4), 113-122.
Crossref - Legg M, Yücel MK, De Carellan IG, Kappatos V, Selcuk C, Gan TH. Acoustic methods for biofouling control: A review. Ocean Engineering, 2015;103:237-247.
Crossref - Sand W, Gehrke T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria. Research in Microbiology. 2006; 157(1): 49-56.
Crossref - Wang H, Zhou Q. Evaluation and failure analysis of linseed oil encapsulated self-healing anticorrosive coating. Prog. Org. Coat., 2018;118: 108–115.
Crossref - Purssell E. Antimicrobials. Understanding pharmacology in nursing practice. 2020:147-65.
Crossref - Pan Y, Wang Z, Ma J, et al. Folic Acid-Modified fluorescent-magnetic nanoparticles for efficient isolation and identification of circulating tumor cells in ovarian cancer. Biosensors. 2022;12(3):184.
Crossref - Drexelius MG, Neundorf I. Application of antimicrobial peptides on biomedical implants: Three ways to pursue peptide coatings. International Journal of Molecular Sciences, 2021; 22(24): 13212.
Crossref - Zhang L, Wang L, Zhang Y, Wang D, Guo J, Zhang M, Li Y. The performance of electrode ultrafiltration membrane bioreactor in treating cosmetics wastewater and its anti-fouling properties. Environmental research. 2022;206:112629.
Crossref - Banerjee I, Mondal D, Martin J, Kane RS. Photoactivated antimicrobial activity of carbon nanotube− porphyrin conjugates. Langmuir. 2010;26(22):17369-74.
Crossref - Mardosaitė, R, Jurkevičiu̅tė, and S. Račkauskas, “Superhydrophobic ZnO nanowires: Wettability mechanisms and functional applications. Cryst. Growth Des., 2021;21(8): 4765–4779.
Crossref - Song F, Zhang L, Chen R, et al. Bioinspired durable antibacterial and antifouling coatings based on borneol fluorinated polymers: Demonstrating direct evidence of antiadhesion. ACS Applied Materials & Interfaces. 2021;13(28):33417-26.
Crossref - Tong Z, Guo H, Di Z, et al. Squid inspired elastomer marine coating with efficient antifouling strategies: Hydrophilized defensive surface and lower modulus. Colloids and Surfaces B: Biointerfaces. 2022;213:112392.
Crossref - Bernbom N, Ng YY, Kjelleberg S, Harder T, Gram L. Marine bacteria from Danish coastal waters show antifouling activity against the marine fouling bacterium Pseudoalteromonas sp. strain S91 and zoospores of the green alga Ulva australis independent of bacteriocidal activity. Applied and Environmental Microbiology. 2011;77(24):8557-67.
Crossref - Bazes A, Silkina A, Douzenel P, et al. Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. Journal of Applied Phycology. 2009;21:395-403.
Crossref - Bavya M, Mohanapriya P, Pazhanimurugan R, Balagurunathan R. Potential bioactive compound from marine actinomycetes against biofouling bacteria. 2011.
- Mahadevan G, Murugan A, Mahendran S, Gautam K, Ravi V. Antifouling activity of the green seaweed Ulva reticulata and its epiphytic bacterial strains against marine biofilm bacteria. International Journal of Advanced Life Sciences. 2013;6(5):417-24.
- Acevedo MS, Puentes C, Carreño K, et al. Antifouling paints based on marine natural products from Colombian Caribbean. International Biodeterioration & Biodegradation. 2013;83:97-104.
Crossref - Subba Rao T, Murthy PS, Veeramani P, et al. Assessment of biogrowth assemblages with depth in a seawater intake system of a coastal power station. Biofouling. 2021;37(5):506-20.
Crossref - Keshari N, Adhikary SP. Characterization of cyanobacteria isolated from biofilms on stone monuments at Santiniketan, India. Biofouling. 2013;29(5):525-36.
Crossref - Cappitelli F, Salvadori O, Albanese D, Villa F, Sorlini C. Cyanobacteria cause black staining of the National Museum of the American Indian Building, Washington, DC, USA. Biofouling. 2012;28(3):257-66.
Crossref - Truong VK, Webb HK, Fadeeva E, et al. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling. 2012;28(6):539-50.
Crossref - Hamida RS, Ali MA, Abdelmeguid NE, Al-Zaban MI, Baz L, Bin-Meferij MM. Lichens—A potential source for nanoparticles fabrication: A review on nanoparticles biosynthesis and their prospective applications. Journal of Fungi, 2021; 7(4):291.
Crossref - Nwabor OF, Singh S, Wunnoo S, Lerwittayanon K, Voravuthikunchai SP. Facile deposition of biogenic silver nanoparticles on porous alumina discs, an efficient antimicrobial, antibiofilm, and antifouling strategy for functional contact surfaces. Biofouling. 2021;37(5):538-54.
Crossref - Loo CY, Young PM, Lee WH, Cavaliere R, Whitchurch CB, Rohanizadeh R. Non-cytotoxic silver nanoparticle-polyvinyl alcohol hydrogels with anti-biofilm activity: designed as coatings for endotracheal tube materials. Biofouling. 2014;30(7):773-88.
Crossref - Huang CF, Chiang HJ, Lan WC, Chou HH, Ou KL, Yu CH. Development of silver-containing austenite antibacterial stainless steels for biomedical applications Part I: microstructure characteristics, mechanical properties and antibacterial mechanisms. Biofouling. 2011;27(5):449-57.
Crossref - Ramasubramanian B, Reddy MV, Zaghib K, Armand M, Ramakrishna S. Growth mechanism of micro/nano metal dendrites and cumulative strategies for countering its impacts in metal ion batteries: A review. Nanomaterials. 2021;11(10):2476.
Crossref - Brindha R, Ajith SR, Nandhini M, et al. Evaluation of anticorrosive behaviour of ZnO nanotetra-pods on a AZ91-grade Mg alloy. Bulletin of Materials Science. 2019;42:1-2.
Crossref - Pushparaj K, Liu WC, Meyyazhagan A, et al. Nano-from nature to nurture: A comprehensive review on facets, trends, perspectives and sustainability of nanotechnology in the food sector. Energy. 2022;240:122732.
Crossref - Manikandan V, Balasubramanian B, Bharti B, et al. Development of ZnO/MOGAC nanocomposites for enhanced photocatalytic removal of PO 4 3− and NO 3-ions from wastewater under various light irradiations. Biomass Conversion and Biorefinery. 2022:1-8.
Crossref - Kalaimurugan D, Balamuralikrishnan B, Govindarajan RK, et al. Production and characterization of a novel biosurfactant molecule from Bacillus safensis YKS2 and assessment of its efficiencies in wastewater treatment by a directed metagenomic approach. Sustainability. 2022;14(4):2142.
Crossref - Yaqoob AA, Umar K, Ibrahim MN. Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Applied Nanoscience. 2020;10:1369-78.
Crossref - Liu C, Geng L, Yu Y, Zhang Y, Zhao B, Zhao Q. Mechanisms of the enhanced antibacterial effect of Ag-TiO2 coatings. Biofouling. 2018;34(2):190-9.
Crossref - Xu H, Zhang J, Lv X, Niu T, Zeng Y, Duan J, Hou B. The effective photocatalysis and antibacterial properties of AgBr/Ag2MoO4@ ZnO composites under visible light irradiation. Biofouling. 2019;35(7):719-31.
Crossref - Yang WJ, Neoh KG, Kang ET, Lee SS, Teo SL, Rittschof D. Functional polymer brushes via surface-initiated atom transfer radical graft polymerization for combating marine biofouling. Biofouling. 2012;28(9):895-912.
Crossref - Choi, H. R., Park, S. H., Kim, D. H., Kim, J. Y., & Heo, M. S. (2016). Phylogenetic diversity and community analysis of marine bacteria associated with Ulva pertusa. Journal of Life Science, 26(7): 819-825.
Crossref - Rolin C, Inkster R, Laing J, McEvoy L. Regrowth and biofouling in two species of cultivated kelp in the Shetland Islands, UK. Journal of Applied Phycology, 2017;29: 2351-2361.
Crossref - Szewczyk P. The role of nanotechnology in improving marine antifouling coatings. Zeszyty Naukowe Akademii Morskiej w Szczecinie. 2010(24 (96):118-23.
- Miller DJ, Araújo PA, Correia PB, Ramsey MM, Kruithof JC, van Loosdrecht MC, Freeman BD, Paul DR, Whiteley M, Vrouwenvelder JS. Short-term adhesion and long-term biofouling testing of polydopamine and poly (ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water research. 2012;46(12):3737-53.
Crossref - Fu W, Pei T, Mao Y, Li G, Zhao Y, Chen L. Highly hydrophilic poly (vinylidene fluoride) ultrafiltration membranes modified by poly (N-acryloyl glycinamide) hydrogel based on multi-hydrogen bond self-assembly for reducing protein fouling. Journal of Membrane Science. 2019;572:453-63.
Crossref - Abdelsalam KM, Shaltout NA, Ibrahim HA, Tadros HR, Aly-Eldeen MA, Beltagy EA. A comparative study of biosynthesized marine natural-product nanoparticles as antifouling biocides. Oceanologia. 2022;64(1):35-49.
Crossref - Kumar SA, Sasikumar A. Studies on novel silicone/phosphorus/sulphur containing nano-hybrid epoxy anticorrosive and antifouling coatings. Progress in Organic Coatings. 2010;68(3):189-200.
Crossref - Sun PF, Kim TS, Kim HS, Ham SY, Jang Y, Park YG, Tang CY, Park HD. Improved anti-biofouling performance of pressure retarded osmosis (PRO) by dosing with chlorhexidine gluconate. Desalination. 2020;481:114376.
Crossref - Ashbridge Z, Fielden SD, Leigh DA, Pirvu L, Schaufelberger F, Zhang L. Knotting matters: orderly molecular entanglements. Chemical Society Reviews. 2022.
Crossref - Wang W, Kang S, Vikesland PJ. Surface-enhanced Raman spectroscopy of bacterial metabolites for bacterial growth monitoring and diagnosis of viral infection. Environmental Science & Technology. 2021; 55(13):9119-28.
Crossref - Nagaraj V, Skillman L, Li D, Xie Z, Ho G. Control of biofouling by xanthine oxidase on seawater reverse osmosis membranes from a desalination plant: enzyme production and screening of bacterial isolates from the full-scale plant. Letters in applied microbiology. 2017;65(1):73-81.
Crossref - Rubavathi S, Ayyappadasan G, Saranya D. In Vitro enzymatic screening and assessment of the Lichen Associated Bacteria and its evaluation of antifouling studies. 2022;91(3):188-200.
Crossref - Rubavathi S, Ayyappadasan G, Brindha R. Self-potent anti-microbial and anti-fouling action of silver nanoparticles derived from lichen-associated bacteria. Applied Nanoscience. 2022;12(8):2397-408.
Crossref - Vilas-Boas C, Carvalhal F, Pereira B, et al. One step forward towards the development of eco-friendly antifouling coatings: Immobilization of a sulfated marine-inspired compound. Marine drugs, 2020; 18(10):489.
Crossref - Subbaiyan R, Ganesan A, Dhanuskodi S. Ecolichenology of Eastern Ghats diversity against climatic fluctuations in Kolli Hills, India. Biodiversitas, 2023 24: 625-635.
Crossref - Biosca EG, Flores R, Santander RD, Díez-Gil JL, Barreno E. Innovative approaches using lichen enriched media to improve isolation and culturability of lichen associated bacteria. PLoS One. 2016;11(8):e0160328.
Crossref - Bates ST, Cropsey GW, Caporaso JG, Knight R, Fierer N. Bacterial communities associated with the lichen symbiosis. Applied and environmental microbiology. 2011;77(4):1309-14.
Crossref - Grube M, Cardinale M, de Castro JV, Müller H, Berg G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. The ISME journal. 2009;3(9):1105-15.
Crossref - Kavitha G, Inbakandan D, Nalini S, Riyaz SU. Antifouling activity of alkaline protease from halotolerant Bacillus sp. isolated from marine source. Indian Journal of Geo Marine Sciences, 2019 ; 48 (8):1274-1279. http://nopr.niscpr.res.in/handle/123456789/49701
- Rubavathi S, Ayyappadasan G, Bhurniammal S, Srilekha R, and Brindha R. Synthesis and Characterization of Ferrous and Copper Nanoparticles from E Waste Using Biological Reduction by Lichen Associated Bacteria and Their Application in Antifouling Activity, Applied Biochemistry and Biotechnology, 2022.
Crossref - Sattarahmady N, Heli H, Vais RD. An electrochemical acetylcholine sensor based on lichen-like nickel oxide nanostructure. Biosensors and Bioelectronics. 2013;48:197-202.
Crossref - Kolangare IM, Isloor AM, Karim ZA, Kulal A, Ismail AF, Asiri AM. Antibiofouling hollow-fiber membranes for dye rejection by embedding chitosan and silver-loaded chitosan nanoparticles. Environmental Chemistry Letters. 2019;17:581-7.
Crossref - Nasar A, Rajender B, Khan A, Asiri AM, Ashraf GM. Optimization of glucose powered biofuel cell anode developed by polyaniline-silver as electron transfer enhancer and ferritin as biocompatible redox mediator. Scientific Reports. 2017;7(1):1-9.
Crossref - Ibrahim GP, Isloor AM, Asiri AM, Farnood R. Tuning the surface properties of Fe 3 O 4 by zwitterionic sulfobetaine: application to antifouling and dye removal membrane. International Journal of Environmental Science and Technology. 2020;17:4047-60.
Crossref - Kanchi S. One-pot biosynthesis of silver nanoparticle using Colocasia esculenta extract: Colorimetric detection of melamine in biological samples. Journal of Photochemistry and Photobiology A: Chemistry. 2020;391:112310.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.