Dental caries is a polymicrobial infection affecting the dental hard tissues. Excessive carbohydrate intake leads to the accumulation of acid-producing and acid-resistant microorganisms in the oral region. It is a biofilm-dependent oral infection with cariogenic pathogens and the most prevalent disease globally. The prevention and control of caries play a vital role in global health management. Periodontal diseases and subgingival plaque etiology are due to the combined action of bacterial invasion and immune reaction, resulting in the devastation of periodontal tissues, culminating in tooth loss. The compact micro colony inhabiting the dental surfaces attaches with secreted polymer, forming a biofilm. Bacterial biofilm impervious to various drugs and chemicals poses a significant challenge in therapeutic scenarios of medical and odonatological infections. The quorum-sensing signaling mechanism in bacteria controls the metabolic and physiologic properties involved in bacterial existence, pathogenesis, and virulence. Hence, studies monitoring the molecular mechanism of quorum sensing and their restricted social interactions will be highly beneficial in the treatment regimen of the modern era. Natural bioactive compounds can be exploited for their medicinal value in combating oro-dental infections. Phytochemicals are promising candidates that could provide novel strategies for fighting infections. The current review highlights the mechanism of quorum sensing, plant products’ effect in controlling quorum sensing, and biofilm-induced dental infections like Periodontitis.
Phytochemicals, Flavanoids, Biofilm, Quorum Sensing and Quorum Quenching
The mouth is an ideal microbial incubator that harbors versatile microorganisms and is the primary entry point before reaching the gastrointestinal tract. More than 700 different species of bacteria have been identified so far. Some of them are commensals, and some are opportunistic pathogens. The oral cavity contains both resident microbial flora and transient microbial flora. Carious lesions and dental infections like gingivitis and inflammation of periodontal tissues may be triggered by bacterial biofilm. Biofilm is a dense microcommunity where microorganisms co-exist in mutual harmony. The very existence of microbes in biofilm accounts for their ability to communicate with each other by small molecules called autoinducers. The process by which microbes communicate with each other inside a biofilm is termed quorum sensing (QS).
The QS mechanism in bacteria is complicated but well organized. Several studies around the globe highlight the biochemical and molecular mechanisms of quorum sensing. Popova et al., state that the bacterial biofilm, upon growing into a particular cell density, communicates with each other with signaling molecules called autoinducers and triggers the gene activation for biofilm stability, pathogenesis, antibiotic resistance, etc.1 The current studies associated with the biofilm is the bacterial inter-communication with a shared circulatory system that permits the interchange of bacterial products in a nutrient-lacking medium. The clinical pathology of various oro dental infections is attributed to quorum sensing. By minimizing quorum sensing, bacterial invasion can be reduced drastically. Different compounds are studied for their ability to inhibit quorum sensing. Quorum-sensing inhibitors are molecules or substances that can hinder or minimize the quorum-sensing mechanism. Treating biofilms is a global challenge. Phytochemicals are promising candidates that could provide novel therapeutic approaches for combating infections. The current review concerns bacterial quorum sensing inhibition by phytochemicals to reduce cariogenic biofilm.
This review highlights the ability of plant-based quorum-sensing inhibitors to be used in the pharmaceutical industry and combinative drug therapy. Since ancient times, plant-based products have been used for their excellent medicinal value. An extensive literature review revealed plant-based products’ antibacterial, anti-inflammatory, anti-cariogenic, and anti-diabetic properties.2 Active components responsible for such activity include flavonoids, phenolic compounds, coumarins, tannins, quinones, alkaloids, etc.3 Various phytochemicals and their action potential are discussed in this review.
Understanding of dental biofilm as a therapeutic target
A dental biofilm is a polymicrobial environment comprising hundreds of microbial species encapsulating themselves in a dense polymeric environment and causing infection.4,5 Biofilms contain microcolonies of bacteria distributed in a glycocalyx matrix. Most of the bacteria in a biofilm are anaerobic but also facilitate the growth of facultative anaerobes, microaerophilic, and capnophilic bacteria in a density-dependent manner in biofilm and periodontal pockets.6 The bacteria in the biofilm adhere to each other and form mushroom-shaped sessile microcolonies.7 Every microcolony has an independent existence in a compact, customized environment. Biofilm progresses rapidly with several visible layers of bacteria and secretes an enormous amount of extracellular polysaccharides, a striking feature of bacterial biofilm. Bacterial cells are embedded in a polymeric matrix with carbohydrates and mineral constituents and constitute a lower level of bacterial biofilm.8 The next is a typical loose irregular layer that covers the external medium. An outer fluid layer around the biofilm contains both a stationary and a movable fluid layer. Several micro-colonies are connected by water channels, which allow the inflow of nutrients and other molecules in and out of the biofilm. This trafficking mechanism is a type of primitive circulatory system.9 Every micro-colony is a diverse combination of microbial species surviving harmoniously as independent entities.
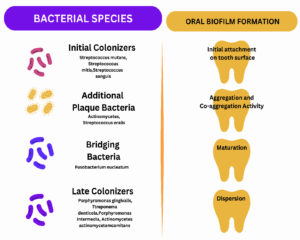
Interestingly, the biofilm colony’s center comprises obligate anaerobes, and the aerobic population colonizes around the fluid channel’s outer area. Accordingly, the biofilm structure provides an array of customized living conditions like altered pH, oxygen tension, nutrient availability, etc. Though oral biofilm is a polymicrobial community, Streptococcus mutans is the pioneer pathogen triggering carious lesions and progressing to different oral infections.10 Among the various virulence factors of Streptococcus mutans, sucrose-dependent colonization on the tooth surface and the action of glucosyl and fructosyl transferase play a significant role in initiating cariogenicity.11 Inter-bacterial communication among bacterial species is crucial, allowing a few species to progress and inhibit other bacterial populations.12 These communications may harm the host. Different types of bacterial interaction are shown in Table 1. Table 2 demonstrates the biofilm composition.1,5 The various oral bacteria involved in biofilm and the process of biofilm formation are depicted in Figure 1.
Table (1):
Relationship between bacteria and the extent of its pathogenic potential
Interaction |
Mechanism |
Examples of periodontal pathogens |
|---|---|---|
Mutualism |
Beneficial co-existence |
1. P. gingivalis & T. denticola; 2. T. forsythia & F. nucleatum |
Synergism |
The Sum of the Pathogenic potential of the two species greatly outweighs their pathogenic potentials. |
|
Commensalism |
One of the two species benefits |
|
Antagonism |
Interactions in a negative way |
1. S. mutans & A. actinomycetemcomitans; 2. S. sanguis & A. actinomycetemcomitans |
Competitive relations |
Competitive Interactions |
1. P. gingivalis & A. viscosus, A. naeslundii, S. mutans, S. mitis. |
Table (2):
Composition of biofilm and type of microbial interactions
Important pathogens associated with dental plaque |
Organic components |
Inorganic components |
Microbial interactions |
|---|---|---|---|
1. A. actinomycetemcomitans 2. P. gingivalis 3. P. intermedia 4. T. forsythia 5. F. nucleatum 6. P. micros 7. C. rectus |
Polysaccharides Proteins Glycoproteins Lipids |
Calcium Phosphorous Sodium Potassium Fluoride |
Synergism Mutualism Commensalism Competitive relations |
Figure 1. The mouth, a polymicrobial environment, harbors various microorganisms. The biofilm formation starts with the adhesion of pioneer bacteria known as initial colonizers. Initial colonizers are then followed by additional plaque bacteria, bridging bacteria, and late colonizers. The process of oral biofilm progression takes place in 4 steps via attachment, aggregation, and co-aggregation activity, maturation, and dispersal of bacteria, allowing the initiation of a new cycle
Formation of biofilm
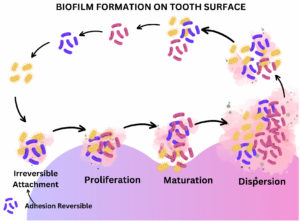
Biofilm in the oral cavity is a complex, organized 3-D structure with varied pathobionts, resulting in oral dental infections, mainly dental caries and periodontitis.13 Several studies pointed out the effect of persistent infections in connection with biofilm-like pneumonia in patients with cystic fibrosis, prostatitis, etc.9 Several reported bloodstream infections due to indwelling medical devices may also be due to biofilm formation.14 Bacterial species produce extracellular polysaccharides in the biofilm, which is the structural backbone of the biofilm. EPS aids in water retention prevents desiccation and attack by harmful agents, provides nutrient storage, and retains necessary extracellular enzymes. Most bacteria inhabiting the biofilm can synthesize and degrade the extracellular matrix.7 The primary step in establishing the dental biofilm is the attachment of planktonic populations into the oral cavity or indirect adherence to previously colonized bacterial cells.15 Specific bacterial species attach permanently to the substratum, start cell division, produce micro-colonies, secrete the polymeric substance EPS, and progress as a biofilm (Figure 2).16,17 Genetic exchange, quorum sensing, metabolic communication, and synthesis of inhibitory substances like bacteriocin are the primary regulatory mechanisms determining bacterial composition and metabolism.17-19 Therefore, the keen knowledge regarding biofilm formation and its molecular and biochemical mechanism with bacterial social interactions may render novel strategies for treating biofilm-associated infections.20,19
Figure 2. The primary step in establishing the dental biofilm is the reversible attachment of planktonic populations into the oral cavity or indirect adherence to previously colonized bacterial cells.15 Specific bacterial species then attach permanently to the substratum, start cell division, produce micro-colonies, secrete the polymeric substance EPS, and progress as a biofilm.16 17 Biofilm proliferates and matures and finally reaches the threshold, resulting in the distortion and dispersion of biofilm, releasing the bacterial population to initiate the next cycle of biofilm formation
In oral biofilm the ability of the oral pathogen to produce adhesive molecules is enhanced in the presence of sucrose.21 The biochemical composition of extracellular polysaccharides of oral pathogen biofilm constitutes mainly glucans and fructans. These polymers form a sticky matrix that holds the bacterial cells together in the biofilm. In oral pathogen biofilm the enzyme Glucosyltransferases catalyse the transfer of glucose from sucrose to form glucan polymers. Oral pathogen ferments dietary sugars to produce acids, decreasing pH within the biofilm and contributing to its Cariogenic Potential. The acidic environment created by oral pathogen biofilms causes enamel demineralization and progresses to the development of dental caries. Oral pathogen S. mutans uses autoinducer peptides for quorum sensing to coordinate gene expression within the biofilm. Streptococcus mutans secretes a peptide signal molecule called competence-stimulating peptide encoded by the comC gene.22 This communication contributes to biofilm development and virulence. The host immune response and antimicrobial factors in saliva play a role in shaping oral biofilms.23
Biofilm-forming pathogens like Pseudomonas aeruginosa, causing respiratory, and urinary tract infections, etc, are held together by a matrix of extracellular polymeric substances (EPS). This matrix consists of polysaccharides, proteins, and DNA, providing structural support to the biofilm Alginate is a significant polysaccharide component in P. aeruginosa biofilms. PEL (cationic exopolysaccharide) and Polysaccharide Synthesis Locus (PSL) are the two other EPS polysaccharides. It contributes to the structural integrity of the biofilm and provides protection. Beyond its structural role, PSL exhibits a distinctive function as an intercellular signaling molecule. PSL acts as a structural adhesive and functions as a signaling molecule.24 Communication through quorum sensing using the autoinducer AHL allows coordinated gene expression within the biofilm, enhancing virulence within the biofilm, P. aeruginosa cells aggregate into microcolonies. The EPS matrix surrounds and encases these microcolonies. The biofilm matures as more layers of EPS are produced, creating a three-dimensional Mushroom-Like Structures. Various proteins, including adhesins and enzymes, are found in the EPS matrix, contributing to biofilm adhesion and function.25
Immunological response in connection with oral biofilm
The oral cavity is a polymicrobial environment residence to millions of bacteria. The count of the bacterial population in the mouth has to be kept in control for the maintenance of oral health and the overall well-being of an individual. Immunological surveillance is the crucial factor that closely monitors the growing microbial population. The pathobionts dwelling in the oral cavity possess specific beneficial roles as well. Hence, the part of the immune response is not to eliminate the bacterial flora but to keep the microbial growth under control and not allow the transient flora to outgrow the resident flora. There are several mechanisms by which immune mechanisms trigger an inflammatory response concerning pathogenic oral microflora. Different molecular pathways may regulate this mechanism; unfortunately, the genes and proteins involved in such ways are not studied in detail. Table 3 lists the most critical immune molecules maintaining immune responses.26
Table (3):
Critical immune molecules involved in maintaining immune responses
No |
Immune compound |
Role played |
|---|---|---|
1 |
Hsp70 |
Heath shock chaperone protein |
2 |
oPMN |
Circulating neutrophils capable of rapid mobility |
3 |
MMp |
The enzyme involved in the destruction of extracellular matrix |
4 |
TNF |
Cytokine is involved in cell destruction and bone remolding |
5 |
IL-1 |
Interleukin is involved in tissue and bone destruction |
6 |
slgA |
Secretory IgA produced in saliva |
The secretory IgA is the potent antibody involved in providing mucosal immunity. IgA exists in two dimeric forms. IgA is present in saliva and is stimulated by cariogenic streptococci. IgA plays a pivotal role in maintaining the oral microbiota by selective elimination of pathogenic flora, preventing the overgrowth of resident flora. More studies must be conducted to rule out the connection between autoinducer 2 and IgA.
Recent findings from the study conducted by Ahmed A. et al., state that Secretory IgA, the primary form of IgA, plays a dynamic role in safeguarding the host from pathogens to support a balanced relationship between the host and microbiota.27 While the exact mechanism of how IgA distinguishes between different bacterial species is not fully understood, it is widely accepted that bacterial surface carbohydrate moieties significantly contribute to IgA selectivity across taxonomic species. Another critical mechanism by which Secretory IgA mediates the neutralization of pathogen is through a process called immune exclusion.28 Secretory IgA-mediated enchained growth is another mechanism that involves linking and segregating bacterial plasmid donor and recipient clones, preventing the transfer of conjugative plasmids. SIgAs also have a unique function known as ‘coating,’ which enhances bacterial translocation in Peyer’s patches. This improves resident dendritic cells (DCs) in antigen sampling and activation.29,30 These are among many mechanisms by which Secretory IgA maintains a selective targeting mechanism to maintain microbial homeostasis between resident flora by eliminating transient flora.
The coating of the SIgA structure allows bacteria to clump together and it seems to regulate the metabolism of coated bacteria. Additionally, an IgA coating could support resident bacterial colonization by bringing bacteria together, either with mucus or each other, encouraging the formation of biofilms, which are often regulated by quorum sensing.31 Autoinducer-2 plays a crucial role in safeguarding the existing microbial community by triggering the activation of genes responsible for alternative mechanisms when cell populations reach elevated densities. This proactive response is essential for preserving the balance of the resident flora, preventing the invasion of harmful pathogens, and facilitating the absorption of nutrients. In essence, Autoinducer-2 serves as a protective mechanism, ensuring the well-being of the normal microbial inhabitants and supporting their vital functions within the ecosystem.32
Hsp70 is a chaperone protein, also known as stress response protein, involved in cellular heat shock response. Recent research suggests the importance of Hsp70 in stimulating natural killer cells by enhancing phagocytosis and potent Défense mechanisms. The cytoprotective nature of Hsp70 is vital in establishing a healthy microbial niche in the oral cavity. Inflammatory mediators like interleukin 1 (IL1), Tumor Necrosis factor (TNF), and Matrix metalloproteinases (MMp) are released in response to bacterial LPS. Inflammatory mediators aid immune response by breaking the extracellular matrix, paving the way for tissue development and promoting leukocyte infiltration at the site of infection.33
Quorum sensing signaling
Further investigation on various bacterial social interactions showed the presence of certain compounds known as autoinducers, critical factors in gene regulation, and social interactions in a poly-microbial environment.34 The ability of a bacterial population to communicate among themselves depending on the recognition of extracellular signaling compounds is known as Quorum sensing. QS enables the bacterial population to change their behavioral pattern by the variations in physiological and biochemical parameters of the bacterial population in the nearby vicinity.35 Quorum sensing poses a significant threat in treating oro dental infections, as it renders microorganisms resistant to various drugs and treatment regimens. As stated by Saxena et al. pathogenic inhabitants of bacterial biofilm utilize quorum sensing mechanisms to initiate virulence and resist antimicrobial therapies.36
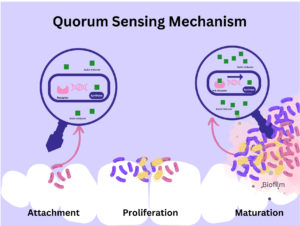
Furthermore, the quorum sensing mechanism is well established in Gram-positive and Gram-negative bacteria; however, maintaining the primary function, the induction of the QS mechanism in both Gram-positive and Gram-negative bacteria is different. With the rapidly growing bacterial population, these autoinducers accumulate to a certain threshold level, thereby enabling the diverse set of target genes to support the survival of bacteria in the changing environment.37 Various autoinducers that play vital roles in regulating the quorum sensing mechanism are listed in Table 4.38,39 The 3 classes of quorum sensing systems in bacteria are shown in Table 5.40,41 The mechanism involved in quorum sensing is shown in Figure 3.
Table (4):
Different autoinducers, enzymes, and regulators that play vital roles in controlling quorum sensing mechanisms
| Key signaling molecules and Quorum Sensing regulator types. | ||||
|---|---|---|---|---|
| Autoinducers | Specific examples of autoinducers in different bacterial species | Role of Autoinducer In in QS | Autoinducer synthase | Quorum Sensing Regulators |
| Autoinducer 193 | Acyl homoserine lactones | AHL binds to transcriptional factors and regulates gene expression, contributing to QS | AHL synthases AI-2 synthase |
LuxR-type regulators LuxP/Q-type regulator |
| Autoinducer 2 | Furanosyl borate di ester | Bacterial periplasmic receptor, LuxP binds to AI-2, initiating QS signal transduction. | ||
| Cyclic dipeptides | Cyclo(pro-tyr) | Acts as QS signaling molecules | ||
| Bradyoxetin94,95 | 2-{4-[[4-(3-aminooxetan-2-yl)phenyl](imino)methyl]phenyl}oxetan-3-ylamine | Involved in the Synthesis of branched Homoserine lactone | ||
Table (5):
The 3 classes of QS systems in bacteria. Different quorum sensing regulator systems and autoinducers are found in Gram-positive and Gram-negative bacteria
No |
Quorum sensing regulator |
Autoinducers |
Type of bacteria |
|---|---|---|---|
1 |
LuxI/LuxR-type Quorum Sensing |
Acylhomoserine lactones (AHL) |
Gram-Negative Bacteria |
2 |
Oligopeptide-two-component-type Quorum Sensing |
Small peptides |
Gram-Positive Bacteria |
3 |
Lux S-encoded (Autoinducer)-2 Quorum Sensing |
Autoinducer 2 |
Both Gram-Negative & Gram-Positive Bacteria |
Figure 3. Bacterial quorum sensing mainly focuses on producing, releasing, and detecting extracellular chemical signaling molecules -called autoinductors.57 These autoinducer molecules accumulate in the bacterial environment, and upon reaching equilibrium concentration, these signal enters the sensitive bacterial cell and bind with the receptor protein, resulting in the manipulation and expression of specific genes. These genes code for more signaling molecules, increasing bacterial density and forming biofilm
The quorum sensing mechanism can be considered a cell-to-cell communication among compatible bacterial species for a positive co-existence. It’s a bacterial communication system that controls and coordinates gene expression based on population density. Multiple quorum sensing mechanisms exist, of which the AHL quorum sensing system is the most studied. AHL stands for acyl-homoserine lactone, and it serves as a signaling molecule in Gram-negative bacteria. The two key proteins involved in AHL-based QS systems are LuxI-type and LuxR-type proteins. The LuxI-type protein functions as a cytoplasmic AHL synthase, responsible for synthesizing AHL molecules. LuxR-type protein, on the other hand, acts as an AHL-responsive DNA-binding transcriptional regulator. The process begins with bacterial cells producing AHL signals at a low basal rate. These AHL signals can diffuse through the cell membrane without the need for a specific receptor. As the bacterial population density increases, the concentration of AHL signals also rises. When the threshold concentration is reached, LuxR-type transcriptional regulator proteins bind to the AHL signals, forming a LuxR/AHL complex. This LuxR/AHL complex then binds to specific DNA sequences known as lux boxes in the promoter regions of target genes. The binding of LuxR/AHL complex to the lux box alters gene expression, leading to the coordinated regulation of various bacterial functions. This system allows bacteria to synchronize their biofilm forming activities based on the local population density.
Gram-positive bacteria indulge in quorum sensing via activating small molecules called Autoinducer peptides (AIP). The pathogenesis of Bacillus cereus is associated with quorum sensing.42 In Gram-positive QS systems, small signal peptides undergo post-translational processing and are the primary communication agents. These peptide signals engage with the sensory component of a two-component histidine kinase signaling system.43 After being synthesized within the cell, Autoinducing Peptides (AIPs) undergo processing and bind to their corresponding membrane-bound two-component histidine kinase receptor at high extracellular concentrations. This binding event typically triggers the receptor’s kinase activity, leading to its autophosphorylation. The phosphorylated receptor transfers the phosphate group to a cognate cytoplasmic response regulator.44 Upon receiving the phosphate group, the response regulator becomes phosphorylated and subsequently activates the transcription of genes within the Quorum Sensing (QS) regulatory system. Biofilms, being impervious to several drugs, pose severe threats to the treatment regimen in combating infections. Quorum Sensing is pivotal in controlling gene regulation and metabolism of bacteria inhabiting the biofilm, enabling the bacteria to initiate virulence and drug resistance. Hence, the next logical progression is to inhibit the quorum sensing mechanism and disrupt the biofilm to minimize infections. Various compounds have been studied globally to analyze their quorum-sensing inhibitory effect. Among the multiple compounds studied, phytochemicals are widely accepted as Quorum Sensing inhibitors.
Quorum sensing inhibition strategies
The process by which the regulatory property of Quorum Sensing is inhibited in the biofilm via various bioactive compounds is called Quorum sensing inhibition (QSI). Disrupting quorum sensing is the current approach for encountering biofilm-related infections.45 QSI may also be called quorum quenching.41,46 In the present scenario of increasing microbial resistance, quorum quenching is undoubtedly an exciting area of study: it focuses mainly on reducing antibiotic resistance, minimizing biofilm-related infection, etc. Quorum quenching is a term used to define the mechanism to prevent quorum sensing. Molecules capable of quorum quenching can disrupt microbial communication and inhibit biofilm formation.47 A recent update on the quorum quenching mechanism uses a structural analogue of quorum sensing receptors (autoinducers). Interestingly, Jamuna Bai et al. also proposed that competitive binding of phytochemicals having structural similarity with autoinducers like AHL may bind to quorum sensing receptors, thereby controlling quorum sensing and bacterial virulence.48
Besides their ability to control infection, quorum-sensing inhibitors also participate in microbe-host interaction, microbial physiology, and microbe-microbe communication.49 An additional advantage of QSI, suggested by Borges et al., is that it may facilitate the use of a lower dose of antibiotics, increasing its effectiveness.50 Overall, QSI needs further investigation and studies as it can be used to combat multidrug-resistant organisms and improve general dental public health. Future studies will be designed to understand quorum sensing inhibitors’ dosage, drug delivery, efficacy, and cost-effectiveness, which will open new doors in the therapeutic regimen of oro-dental infections.51,49 Several studies carried out so far have identified different synthetic and phytochemical-based compounds that inhibit quorum sensing. Among them, phytochemicals are proven to be safer and more effective. In an interesting report by Basavaraju et al., quorum sensing inhibition occurs via enzyme inhibition and molecular mimics of quorum sensing signals by QS inhibitors. Specific small molecules (autoinducers) and their inhibitors and elements of the QS system are listed in Table 6.52,41,53 A novel advancement in QS inhibitor research is the development of a new bacterial strain to detect QS inhibitors in a given test sample. In this study by Rasmussen et al. two sets of QSI detector systems were made to identify QS inhibitors, i.e., an antibiotic-resistant gene incorporated system with a repressor and a gene bound to a LuxR-regulated promoter.54 Researchers across the globe studied and analyzed various methods involved in quorum sensing at the biochemical and molecular levels. The critical aspect of quorum sensing inhibition is to disrupt the cell-to-cell communication between the species inside a biofilm. Quorum sensing inhibition can be controlled by minimizing the effect of autoinducers either by enzymatic degradation or by inhibiting the synthesis of autoinducers. Competitive binding to receptor protein may down-regulate the target gene expression, disrupting communication signaling mechanisms.55 The gene expression in the manipulated system is noted to identify Quorum Sensing Inhibition.56
Table (6):
Certain small molecules (autoinducers), inhibitors of autoinducers, and the various vital elements contained in the quorum sensing mechanism
No |
Autoinducers |
QS Inhibitors |
Crucial elements of the QS system |
|---|---|---|---|
01 02 03 04 05 |
Acyl homoserine lactone Autoinducer 2 cyclic dipeptides AHL synthases AI-2 synthase |
Acyl homoserine lactone-acylase Acyl homoserine lactone-lactonase Paraoxonase l-Canavanine Furanone |
The autoinducers The Signal Synthase The Signal Receptor The Signal Response Regulator The regulated genes. |
Bacterial quorum sensing mainly focuses on producing, releasing, and detecting extracellular chemical signaling molecules-called autoinductors.57 These autoinducer molecules accumulate in the bacterial environment, and upon reaching equilibrium concentration, these signal enters the sensitive bacterial cell and bind with the receptor protein, resulting in the manipulation and expression of specific genes.58 Autoinducers are pivotal in quorum sensing inhibition and biofilm formation of pathogenic Pseudomonas aeruginosa. These signaling molecules help the bacteria overcome the host defense mechanisms and establish a biofilm. The varied range of quorum signaling molecules is identified in Pseudomonas species. 2-heptyl-3-hydroxy-4(1H)-quinolone also known as Pseudomonas Quinolone Signal (PQS), (S)-3-(S)-butyl-homoserine lactone (BHL), 2-heptyl-4(1H)- quinolone (HHQ) a PQS precursor, oxododecanoyl-homoserine lactone (OdDHL), etc. are of greater significance in establishing a well-structured biofilm.59
Phytochemicals as quorum-sensing inhibitors
As per the literature review, natural bioactive compounds, especially those derived from plants, have immense potential as pharmaceutical compounds. These bioactive compounds can be exploited in new treatment strategies owing to their unique medicinal properties and effectiveness in combating infections. Medicinal plants, which are as old as humankind, were used in traditional folk medicines.60 This review aims to highlight the relevance of phytochemicals as quorum-sensing inhibitors, which could be used along with antibiotics as co-therapy molecules, enhancing treatment efficacy. Also, we propose using phytochemicals to overcome biofilm resistance to antimicrobials and promote plant-based products as quorum-sensing inhibitors. During the last few decades, a relatively higher incidence of antimicrobial resistance has attracted the scientific community’s attention to an effective alternative to overcome multidrug resistance.61 Even though quorum-sensing inhibitors are studied globally, our knowledge regarding the exact potential of these bioactive compounds is still at the introductory level. Recent studies revealed plant-based phytochemicals’ ability as excellent Quorum sensing inhibitors due to their chemical complexity and diverse biological ability.62
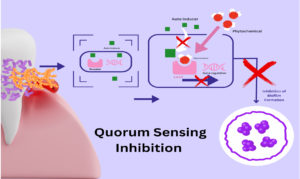
From studies worldwide, the inability of pathobionts to resist plant-based bioactive compounds is evident, which makes them a therapeutic alternative in bacterial infections. In this sense, we have focused on the different antibacterial mechanisms and therapeutic targets of various flavonoids. Ciric et al. stated that phytochemicals’ quorum sensing inhibition mechanism is blocking autoinducers like AHL, autoinducers, and autoinducers type 2.63 The process of quorum sensing inhibition is schematically represented in Figure 4.
Figure 4. Cell-to-cell communication between bacteria is enabled by synthesizing and releasing autoinducer molecules, which bind with the receptor protein. The quorum sensing inhibition mechanism by phytochemicals blocks autoinducers like AHL by competitive binding to the receptor protein, resulting in the downregulation of target gene expression
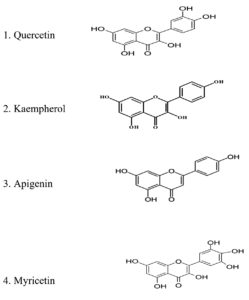
Upon extensive literature review, the following flavonoids were found to potently manage various physiological conditions and prevent microbial infections. Some major flavonoids studied include Quercetin Kaempferol, Rutin, Myricetin, Luteolin, Catechin, Rosmarinic acid, etc.76 Quercetin is a potent bioactive compound with many pharmacological properties like antibacterial, anti-inflammatory, anticancer, oxidant, etc. Interestingly, quercetin recently gained GRAS (Generally Recognized As Safe) status by the United States Food and Drug Organization.64 The antimicrobial ability of quercetin is connected with the cell wall disruption of the bacterial cell.65 This Lan et al. stated that quercetin had a more remarkable ability to inhibit gram-negative than gram-positive bacteria.64 This variation in the mode of action of quercetin connects with the difference in cell wall composition of Gram-negative and positive bacteria.66 Even though quercetin is found to be bioactive, its solubility and bioavailability are questioned.67
Biofilm forming bacteria typically utilize the AHL, LuxR/I-type quorum sensing (QS) system. Phytochemicals like flavonoids exert their quorum quenching potential against the Gram-negative bacteria in three ways: first, by inhibiting the production of signalling molecules through the LuxI synthase; second, by hindering the activity of AHL-producing enzymes; and third, by releasing enzymes that degrade signals. They may also target the LuxR signal receptor by either blocking or mimicking signals. To interrupt the reception of signals, flavonoid compounds can compete with AHLs by having a similar structure or non-competitively binding site on the LuxR receptor other than the AHL binding site.68 In simpler terms, these plant compounds disrupt the communication of biofilm forming bacteria by halting the production of signaling molecules, blocking their receptors, or imitating signals. This interference aids in controlling bacterial behavior.
An exciting study by Dimitry et al. revealed that small side -OH or -OCH3 groups on the B-ring of flavones positively impact their quorum sensing (QS) inhibitor activity. The study about the structure-activity relationship analyses by Grabski et al., highlighted the crucial role of two hydroxyl moieties in the flavone A-ring, which is the backbone for potent inhibition of AHL. Specifically, the hydroxyl group in ring A of quercetin was identified as necessary for interaction with AHL expression.69 These findings shed light on the specific structural features of flavones that contribute to their effectiveness as inhibitors in quorum sensing, providing valuable insights for future research and development in this area.70 The bioactive potential of quercetin is mainly due to the functional phenolic hydroxyl groups and double bonds.71
The minimal inhibitory concentration of quercetin varies with bacterial species. Shu et al. investigated the extent of antibacterial activity of quercetin against the major oral pathogens, including Streptococcus mutans, Streptococcus sanguis, Streptococcus sobrinus, Prevotella intermedia, and Actinobacillus actinomycetemocomitans, with MIC ranging between 1–8 mg/mL.72 At a MIC value of 500 µg/mL, growth and proliferation of S. mutans on adhesive–dentin interfaces fixed with quercetin were inhibited. At a MIC value of 20 mcg/mL, quercetin inhibited the growth of Staphylococcus aureus, Pseudomonas aeruginosa.64,73 Quercetin also showed antibacterial activity against Micrococcus luteus and Shigella sonei at MIC of 25 mcg/mL; the antibacterial effect of quercetin against Methicillin-resistant Staphylococcus aureus (MRSA), Methicillin-sensitive S. aureus (MSSA) and Standard Enterococcus was well documented by Ngyuemnet et al.45 Many researchers around the globe reported the anti-adhesive and anti-biofilm properties of Psidium guajava leaf extract.74 Paluch et al. highlighted the need for combinatorial therapy in combating microbial invasion. They suggested the combination of quorum quenchers with antibiotics. This strategy of therapeutic application can aid in reducing drug resistance, which is the need of hour 47. Frasinetti et al. studied the ability of Cannabis Sativa L. seed extract to control the biofilm formation by Staphylococcus aureus, including MRSA strains.75 Nostro et al., suggested the possible benefit of using polyphenols in preventing infections caused by Pseudomonas aeruginosa. The anti-biofilm effect of flavonoids against Candida albicans was reported by Arora and Onsare.76 A study conducted by Onsare and Arora, 2015 compared the efficacy of Moringa oleifera seed coat extract against the biofilm formation of Staphylococcus aureus, Pseudomonas aeruginosas, and Candida albicans. There was an 88% reduction of biofilm formation in Staphylococcus aureus and Pseudomonas aeruginosa biofilm after 24 hours of incubation with the Moringa oleifera seed coat.77 Harjai et al. and Lu et al. pointed out the effectiveness of garlic extract in minimizing the autoinducers of Pseudomonas and Vibrio spp, henceforth reducing biofilm formation.78,79 The antibacterial mechanism of Quercetin is mainly by the destruction of bacterial cell wall integrity and inhibition of nucleic acid synthesis. Studies also showed the ability of quercetin to denature protein, to disrupt plasma membrane, destroying bacterial cell wall and cell membranes and changing cell morphology. Thus quercetin act as bactericidal agent.80 A recent finding from the study of An-Ping Li et al., provides valuable insight into the antibacterial mechanism of Kaempferol. Kaempferol mainly functions at the molecular level of bacteria and acts as a bacteriostatic agent, gradually decreasing the bacterial energy metabolism, distorting the cellular integrity and leakage of contents, and progressing to cell death.81 Yan Zeng et al. compared the antibacterial efficacy of quercetin and Kaempherol against the oral pathogen Streptococcus mutans.82 Their study reports more or less similar effectiveness for quercetin and kaempferol antibacterial activity. The findings from this study demonstrated the inhibitory activity of quercetin and kaempferol against oral pathogens’ biofilms. This suggests that quercetin and kaempferol could be explored as potential alternative anti-caries agents, offering promising prospects in the search for novel therapeutics to combat dental caries.83 Quercetin and kaempferol are more or less similar in structure except for one additional OH group in quercetin. Nur Farisya et al. revealed that ring A and B hydroxylation is crucial for flavonoids’ antibacterial efficacy.84 Šmejkal and colleagues suggested that having a hydroxyl group at C5 enhanced the antibacterial activity of flavonoids.85
In silico analysis done by Susana Fernandes et al., they compared the efficacy of curcumin and 10-undecenoic acid against two quorum signaling mechanisms, i.e., LuxS/autoinducer-2 (AI-2) from Bacillus subtilis and LasI/LasR from pseudomonas aeruginosa respectively—the former quorum sensing molecule as the universal QS system and the later as a specific QS system. Their study showed curcumin and 10 undecenoic acids’ potential ability to minimize quorum sensing. Curcumin at a concentration of 1.25–5 µg/mL triggered the reduction of LuxS/AI-2 QS system by 33–77% and 10-undecenoic acid at a concentration of 12.5–50 µg/mL reduced the signaling mechanisms LuxS/AI-2 QS system by 36–64%. There was 21% Inhibition of the LasI/LasR QS system by curcumin at 200 µg/mL and 10–54% by 10-undecenoic acid at a concentration of 15.625–250 µg/mL, respectively.86 In the in silico analysis by Susasna Fernandes et al., identified curcumin and 10-undecenoic acid as potential alternatives for addressing bacterial pathogenicity and virulence. These compounds offer advantages in terms of availability, and toxicity compared to traditional approaches like industrial disinfection and antibiotics. This suggests a potential avenue for developing new strategies to combat bacterial infections. This approach aims to avoid the selective pressure often associated with conventional disinfection and antibiotics, which can contribute to developing resistant strains. The study supports the co-administration of phytochemical-based plant products along with the present treatment approaches. Combining curcumin and 10-undecenoic acid with existing antimicrobial agents such as antibiotics or biocides might contribute to restoring or enhancing their effectiveness against microbes.87,86 Aswathanarayan and Vittal studied the biofilm inhibitory properties of berberine against Pseudomonas aeruginosa and Salmonella typhimurium. The phytochemical berberine is an isoquinoline alkaloid with tremendous inhibitory action on biofilm and, simultaneously, an excellent antibacterial and anti-infective compound. The study by Jamuna Bai et al. compared the antibiofilm effect of phytochemicals against gram-negative pathogens P. aeruginosa PA01 and S. enterica sv Typhimurium. Berberine hydrochloride, a protoberberine alkaloid, is extensively investigated for its pharmacologically essential activities, including antimicrobial, anticancer, antifungal, anti-inflammatory, and antimalarial properties. Due to its low cytotoxicity, the compound has been used in therapeutical applications.48 Berberine disrupted the biofilm formed by Enterococcus faecalis.88 Due to its antibacterial efficacy, berberine can be exploited as an endodontic irritant against polymicrobial endodontic pathogens.89
Dental caries and oral infections (Periodontitis) caused by pathogens like Porphyromonas gingivalis, Enterococcus faecalis, Streptococcus species, etc., are the significant pathobionts in oral biofilm, and their pathogenicity is mainly due to quorum sensing. Several methods of QSI, along with scaling and polishing with daily hygiene practices, may minimize the risk of periodontal infection. With their ability to quench quorum, plant-based bioactive compounds undoubtedly improve the efficacy of treatment scenarios in oral and dental infections.90 The three widely studied mechanisms of phytochemicals related to biofilm are depicted in Figure 1.50 Some phytochemicals with reported QSI action, their structure, and reference are listed in Table 7.
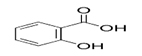
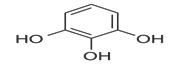
Table (7):
Selected phytochemicals with Quorum Sensing Inhibitory action, their structure and reference
No |
Phytochemical |
Structure |
Reference |
|---|---|---|---|
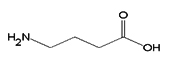
01 |
GABA |
96 |
|
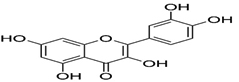
02 |
Flavonoids
eg- quercetin |
97 |
|
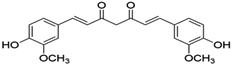
03 |
Curcumin |
98 |
|
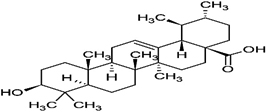
04 |
Ursolic acid |
99 |
|
05 |
Rosmarinic acid |
62 |
|
06 |
Salicylic acid |
100 |
|
07 |
Pyrogallol |
101 |
As per the literature review, flavonoids in various phytochemicals are essential in managing different medical conditions. Flavonoids are polyphenolic compounds distributed widely in nature. Four classes of flavonoids have been identified so far based on their structural difference and molecular complexity: Flavanones, Flavones, Catechins, and Anthocyanins.91,92 Different flavonoid compounds and their natural source are listed in Table 8.92 Different pharmacological and biochemical attributes of flavonoids are listed in Table 9.92 The chemical structure of the most widely studied flavonoids is shown in Figure 5. Flavonoids are proven bioactive compounds owing to their pharmacological and biochemical activities. Henceforth, flavonoids can be exploited as an excellent compound for various medical and dental applications.
Table (8):
Natural source of various flavonoid categories
No |
Flavonoid category |
Compounds present |
Natural source |
|---|---|---|---|
1. |
Flavones/Flavanols |
Quercetin Kaempferol Rutin Myricetin Luteolin |
Onion, Kale Broccoli Hot pepper Berries, apples Celery, parsley |
2. |
Flavanones |
Fisetin Hesperetin |
Citrus fruit Strawberries |
3. |
Anthocyanins |
Delphinidin Epicatechin Epigallocatechin gallate |
Cherries Tea Grapes |
4. |
Catechins |
Catechins |
Red wine |
5. |
Isoflavones |
Genistein daidzein |
Soy beans Legumes |
Table (9):
Different pharmacological and biochemical attributes of flavonoids
| Flavonoids | |
|---|---|
| Pharmacological importance | 1. Antibacterial 2. Antifungal 3. Anti-viral 4. Anti-inflammatory 5. Anti-tumor 6. Anti-osteoporotic 7. Anti-thrombogenic 8. Anti-atherosclerotic |
| Biochemical effect | 1. On enzymes 2. On hormones |
Using phytochemicals as combinatorial drugs paves the way for modernizing the treatment regimen for effectively reducing biofilm-associated infections. Phytochemicals are promising candidates in the therapeutic scenario of plaque-associated dental ailment. The use of phytochemicals as drugs dates back to ancient times. With the advancement in modern technologies, the road is not so far that newer addition of bioactive compounds as combinatorial drugs may find their place in the successful treatment of various clinical conditions. Different research proved the ability of multiple phytochemicals to overcome infection and increase the health and survival of the global population. Even though numerous studies highlight the importance of phytochemicals in medical conditions, we are still in the pre-clinical stage. Hence, the present study suggests the relevance and significance of more scientific investigation on natural products used as effective compounds in inhibiting quorum sensing, thereby minimizing the incidence of biofilm-associated infections. Additionally, a better understanding of the possibility of phytochemicals inhibiting Quorum Sensing activity may contribute to developing unique and novel anti-quorum sensing compounds against bacterial infection. Thus, applying plant-based anti-pathogenic compounds in combating diseases may minimize the emergence of drug-resistant bacterial strains in contrast with antimicrobial agents, which may accelerate drug resistance.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Popova C, Dosseva-Panova V, Panov V. Microbiology of periodontal diseases. A review. Biotechnol Biotechnol Equip. 2013;27(3):3754-3759.
Crossref - Asfour HZ. Anti-Quorum Sensing Natural Compounds. J Microsc Ultrastruct. 2018;6(1):1-10.
Crossref - Saboon, Chaudhari SK, Arshad S, Amjad MS, Akhtar MS. Natural Compounds Extracted from Medicinal Plants and Their Applications. In: Akhtar MS, Swamy MK, Sinniah UR, eds. Natural Bio-Active Compounds. Springer Singapore; 2019:193-207.
Crossref - Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26(3):229-242.
Crossref - Larsen T, Fiehn NE. Dental biofilm infections-an update. Apmis. 2017;125(4):376-384.
Crossref - Jose M, Arya S, Thankam FG. Oro-dental regeneration. Tissue Engineering. Elsevier; 2022:53-76.
- Saini R, Saini S, Sharma S. Biofilm: A dental microbial infection. J Nat Sci Biol Med. 2011;2(1):71-75.
Crossref - Berglundh T, Giannobile WV, Sanz M, Lang NP. Lindhe’s Clinical Periodontology and Implant Dentistry. John Wiley & Sons. 2021.
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318-1322.
Crossref - Brown JL, Johnston W, Delaney C, et al. Polymicrobial oral biofilm models: simplifying the complex. J Med Microbiol. 2019;68(11):1573-1584.
Crossref - Zhang Q, Ma Q, Wang Y, Wu H, Zou J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int J Oral Sci. 2021;13(1):30.
Crossref - Ben Amara H, Song HY, Ryu E, et al. Effects of quorum-sensing inhibition on experimental periodontitis induced by mixed infection in mice. Eur J Oral Sci. 2018;126(6):449-457.
Crossref - Gurenlian JR. The Role of Dental Plaque Biofilm in Oral Health. J Dent Hyg. 2007;81(5):11.
- Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33(8):1387-1392.
Crossref - Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88(11):982-990.
Crossref - Rath S, Bal SCB, Dubey D. Oral biofilm: development mechanism, multidrug resistance, and their effective management with novel techniques. Rambam Maimonides Med J. 2021;12(1):e0004.
Crossref - Sonawane JM, Rai AK, Sharma M, Tripathi M, Prasad R. Microbial biofilms: Recent advances and progress in environmental bioremediation. Sci Total Environ. 2022;824:153843.
Crossref - Wang Y, Bian Z, Wang Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl Microbiol Biotechnol. 2022;106(19-20):6365-6381.
Crossref - Li YH, Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12(3):2519-2538.
Crossref - Polizzi A, Donzella M, Nicolosi G, Santonocito S, Pesce P, Isola G. Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis. Pharmaceutics. 2022;14(12):2740.
Crossref - Rezaei T, Mehramouz B, Gholizadeh P, et al. Factors associated with Streptococcus mutans pathogenicity in the oral cavity. Biointerface Res Appl Chem. 2023;13(4):368.
Crossref - Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev. 2018;54(1):22-29.
Crossref - Vestby LK, Gronseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics. 2020;9(2):59.
Crossref - Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623-633.
Crossref - Irie Y, Roberts AEL, Kragh KN, et al. The Pseudomonas aeruginosa PSL Polysaccharide Is a Social but Noncheatable Trait in Biofilms. mBio. 2017;8(3):e00374-17.
Crossref - Niazy AA. LuxS quorum sensing system and biofilm formation of oral microflora: A short review article. Saudi Dent J. 2021;33(3):116-123.
Crossref - Yang Y, Palm NW. Immunoglobulin A and the microbiome. Curr Opin Microbiol. 2020;56:89-96.
Crossref - Moor K, Diard M, Sellin ME, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544(7651):498-502.
Crossref - Bunker JJ, Flynn TM, Koval JC, et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity. 2015;43(3):541-553.
Crossref - Abokor AA, McDaniel GH, Golonka RM, et al. Immunoglobulin A, an Active Liaison for Host-Microbiota Homeostasis. Microorganisms. 2021;9(10):2117.
Crossref - Uhlig F, Hyland NP. Making Sense of Quorum Sensing at the Intestinal Mucosal Interface. Cells. 2022;11(11):1734.
Crossref - Zeng X, Yue H, Zhang L, et al. Gut microbiota-derived autoinducer-2 regulates lung inflammation through the gut-lung axis. Int Immunopharmacol. 2023;124:110971.
Crossref - Rijkschroeff P, Loos BG, Nicu EA. Oral Polymorphonuclear Neutrophil Contributes to Oral Health. Curr Oral Health Rep. 2018;5(4):211-220.
Crossref - Lazar V. Quorum sensing in biofilms-how to destroy the bacterial citadels or their cohesion/power? Anaerobe. 2011;17(6):280-285.
Crossref - Jayaraman A, Wood TK. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng. 2008;10:145-167.
Crossref - Saxena P, Joshi Y, Rawat K, Bisht R. Biofilms: architecture, resistance, quorum sensing and control mechanisms. Indian J Microbiol. 2019;59(1):3-12.
Crossref - Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69(5):3431-3434.
Crossref - Muras A, Otero-Casal P, Blanc V, Otero A. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited. Sci Rep. 2020;10(1):9800.
Crossref - Biradar B, Devi P. Quorum sensing in plaque biofilms: challenges and future prospects. J Contemp Dent Pr. 2011;12(6):479-485.
Crossref - Muras A, Mayer C, Otero-Casal P, et al. Short-chain N-acylhomoserine lactone quorum-sensing molecules promote periodontal pathogens in in vitro oral biofilms. Appl Environ Microbiol. 2020;86(3):e01941-19.
Crossref - Basavaraju M, Sisnity VS, Palaparthy R, Addanki PK. Quorum quenching: Signal jamming in dental plaque biofilms. J Dent Sci. 2016;11(4):349-352.
Crossref - Deep A, Chaudhary U, Gupta V. Quorum sensing and Bacterial Pathogenicity: From Molecules to Disease. J Lab Physicians. 2011;3(1):4-11.
Crossref - Prazdnova EV, Gorovtsov AV, Vasilchenko NG, et al. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms. 2022;10(2):350.
Crossref - Whiteley M, Diggle SP, Greenberg EP. Bacterial quorum sensing: the progress and promise of an emerging research area. Nature. 2017;551(7680):313-320.
Crossref - Ahmed SA, Rudden M, Smyth TJ, Dooley JS, Marchant R, Banat IM. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl Microbiol Biotechnol. 2019;103(8):3521-3535.
Crossref - Hemmati F, Salehi R, Ghotaslou R, et al. Quorum Quenching: A Potential Target for Antipseudomonal Therapy. Infect Drug Resist. 2020;13:2989 3005.
Crossref - Paluch E, Rewak-Soroczyńska J, Jędrusik I, Mazurkiewicz E, Jermakow K. Prevention of biofilm formation by quorum quenching. Appl Microbiol Biotechnol. 2020;104(5):1871-1881.
Crossref - Aswathanarayan JB, Vittal RR. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018;8(63):36133-36141.
Crossref - Borges A, Simoes M. Quorum sensing inhibition by marine bacteria. Mar Drugs. 2019;17(7):427.
Crossref - Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simões M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules. 2016;21(7):877.
Crossref - Kenganora M, Rudraswamy S, Hombarvalli JSP, Doggalli N. Phytochemicals A Novel Therapeutic Approach to Control Oral Biofilm. Pharmacogn J. 2021;13(3).
Crossref - Kalia VC, Patel SK, Kang YC, Lee JK. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2019;37(1):68-90.
Crossref - Nazzaro F, Fratianni F, Coppola R. Quorum Sensing and Phytochemicals. Int J Mol Sci. 2013;14(6):12607-12619.
Crossref - Zhou L, Zhang Y, Ge Y, Zhu X, Pan J. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front Microbiol. 2020;11:589640.
Crossref - Santos CA, Lima EMF, Franco BDG de M, Pinto UM. Exploring phenolic compounds as quorum sensing inhibitors in foodborne bacteria. Front Microbiol. 2021;12:735931.
Crossref - Rasmussen TB, Bjarnsholt T, Skindersoe ME, et al. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol. 2005;187(5):1799-1814.
Crossref - Whiteley M, Diggle SP, Greenberg EP. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551(7680):313-320.
Crossref - Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR. Bacterial quorum sensing and microbial community interactions. MBio. 2018;9(3):e02331-17.
Crossref - Gupta MK, Singh R, Rangan L. Phytochemical screening, antibacterial, anti-biofilm and quorum sensing inhibiting activity of Alpinia nigra leaf extract against infectious pathogen Pseudomonas aeruginosa PAO1. Food Control. 2023;143:109327.
Crossref - Ta CAK, Arnason JT. Mini Review of Phytochemicals and Plant Taxa with Activity as Microbial Biofilm and Quorum Sensing Inhibitors. Molecules. 2016;21(1):29.
Crossref - Yap PSX, Yiap BC, Ping HC, Lim SHE. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J. 2014;8:6-14.
Crossref - Vattem DA, Mihalik K, Crixell SH, McLean RJ. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78(4):302-310.
Crossref - Bankier C, Matharu RK, Cheong YK, Ren GG, Cloutman-Green E, Ciric L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci Rep. 2019;9(1):16074.
Crossref - Nguyen TLA, Bhattacharya D. Antimicrobial activity of quercetin: an approach to its mechanistic principle. Molecules. 2022;27(8):2494.
Crossref - Wang S, Yao J, Zhou B, et al. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J Food Prot. 2018;81(1):68-78.
Crossref - Osonga FJ, Akgul A, Miller RM, et al. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega. 2019;4(7):12865-12871.
Crossref - Hooda H, Singh P, Bajpai S. Effect of quercitin impregnated silver nanoparticle on growth of some clinical pathogens. Mater Today Proc. 2020;31(4):625-630.
Crossref - Bodede O, Shaik S, Chenia H, Singh P, Moodley R. Quorum sensing inhibitory potential and in silico molecular docking of flavonoids and novel terpenoids from Senegalia nigrescens. J Ethnopharmacol. 2018;216:134-146.
Crossref - Grabski H, Hunanyan L, Tiratsuyan S, Vardapetyan H. Interaction of quercetin with transcriptional regulator LasR of Pseudomonas aeruginosa: Mechanistic insights of the inhibition of virulence through quorum sensing. bioRxiv. 2017:239996.
Crossref - Deryabin D, Galadzhieva A, Kosyan D, Duskaev G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. Int J Mol Sci. 2019;20(22):5588.
Crossref - Magar RT, Sohng JK. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. 2020;30(1):11-20.
- Shu Y, Liu Y, Li L, et al. Antibacterial activity of quercetin on oral infectious pathogens. Afr J Microbiol Res. 2011;5(30):5358-5361.
Crossref - Jaisinghani RN. Antibacterial properties of quercetin. Microbiol Res. 2017;8(1):6877.
Crossref - Mishra R, Panda AK, De Mandal S, Shakeel M, Bisht SS, Khan J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front Microbiol. 2020;11:566325.
Crossref - Frassinetti S, Gabriele M, Moccia E, Longo V, Di Gioia D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. Lwt. 2020;124:109149.
Crossref - Arora DS, Onsare JG. In vitro antimicrobial potential, biosafety and bioactive phytoconstituents of Moringa oleifera stem bark. World J Pharm Res. 2014;3:2772-2788.
- Onsare JG, Arora DS. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol. 2015;118(2):313-325.
Crossref - Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2010;58(2):161-168.
Crossref - Lu R, Sun J, Qiu Y, et al. The quorum sensing regulator OpaR is a repressor of polar flagellum genes in Vibrio parahaemolyticus. J Microbiol. 2021;59(7):651-657.
Crossref - Yang D, Wang T, Long M, Li P. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. 2020;8825387
Crossref - Li AP, He YH, Zhang SY, Shi YP. Antibacterial activity and action mechanism of flavonoids against phytopathogenic bacteria. Pestic Biochem Physiol. 2022;188:105221.
Crossref - Zeng Y, Nikitkova A, Abdelsalam H, Li J, Xiao J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch Oral Biol. 2019;98:9-16.
Crossref - Periferakis A, Periferakis K, Badarau IA, et al. Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int J Mol Sci. 2022;23(23):15054.
Crossref - Shamsudin NF, Ahmed QU, Mahmood S, et al. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules. 2022;27(4):1149.
Crossref - Treml J, Smejkal K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr Rev Food Sci Food Saf. 2016;15(4):720-738.
Crossref - Fernandes S, Borges A, Gomes IB, Sousa SF, Simoes M. Curcumin and 10-undecenoic acid as natural quorum sensing inhibitors of LuxS/AI-2 of Bacillus subtilis and LasI/LasR of Pseudomonas aeruginosa. Food Res Int. 2023;165:112519.
Crossref - Puertas-Martín S, Banegas-Luna AJ, Paredes-Ramos M, et al. Is high performance computing a requirement for novel drug discovery and how will this impact academic efforts? Expert Opin Drug Discov. 2020;15(9):981-985.
Crossref - Chen L, Bu Q, Xu H, et al. The effect of berberine hydrochloride on Enterococcus faecalis biofilm formation and dispersion in vitro. Microbiol Res. 2016;186-187:44-51.
Crossref - Xie Q, Johnson BR, Wenckus CS, Fayad MI, Wu CD. Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. J Endod. 2012;38(8):1114-1117.
Crossref - Yada S, Kamalesh B, Sonwane S, Guptha I, Swetha R. Quorum sensing inhibition, relevance to periodontics. J Int Oral Health JIOH. 2015;7(1):67-69.
- Neelakantan P, Romero M, Vera J, et al. Biofilms in endodontics—current status and future directions. Int J Mol Sci. 2017;18(8):1748.
Crossref - Agrawal AD. Pharmacological activities of flavonoids: a review. Int J Pharm Sci Nanotechnol. 2011;4(2):1394-1398.
Crossref - Czajkowski R, Jafra S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol. 2009;56(1).
Crossref - Sun SJ, Liu YC, Weng CH, et al. Cyclic Dipeptides Mediating Quorum Sensing and Their Biological Effects in Hypsizygus Marmoreus. Biomolecules. 2020;10(2):298.
Crossref - Bogino PC, Nievas FL, Giordano W. A review: Quorum sensing in Bradyrhizobium. Appl Soil Ecol. 2015;94:49-58.
Crossref - Chevrot R, Rosen R, Haudecoeur E, et al. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci. 2006;103(19):7460-7464.
Crossref - Truchado P, Gimenez-Bastida JA, Larrosa M, et al. Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J Agric Food Chem. 2012;60(36):8885-8894.
Crossref - Rudrappa T, Bais HP. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J Agric Food Chem. 2008;56(6):1955-1962.
Crossref - Ren D, Zuo R, Gonzalez Barrios AF, et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol. 2005;71(7):4022-4034.
Crossref - Yuan ZC, Edlind MP, Liu P, et al. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci. 2007;104(28):11790-11795.
Crossref - Inchagova KS, Duskaev GK, Deryabin DG. Quorum sensing inhibition in Chromobacterium violaceum by amikacin combination with activated charcoal or small plant-derived molecules (pyrogallol and coumarin). Microbiology. 2019;88(1):63-71.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.