ISSN: 0973-7510
E-ISSN: 2581-690X
Root endophytic freshwater hyphomycetes Anguillospora longissima and Cylindrocarpon aquaticum were evaluated for phosphate solubilization potential, on Pikovskaya media. Used endophytic fungi were isolated from the riparian plants Equisetum sp. and Eupatorium adenophorum Spreng., respectively, growing in the ravine areas of Kumaun Himalaya, India. Phosphate solubilizing potential was assessed by calculating solubilization index (SI) on PKV agar and by estimating solubilized phosphate through spectrometric analysis, at 827 nm wavelengths, using a double beam spectrophotometer. Both the isolated fungi were found potent to produce halo zones on PKV agar as well as to grow and solubilize the phosphate in PKV broth (phosphate rich medium). Further, decreased pH and increased fungal mycelial weights during the phosphate solubilization have proven the potential of used fungi to grow and solubilize the phosphate in PKV. Comparatively, fungus A. longissima was found much potent for phosphate solubilization (solubilization index (SI) = 1.53, solubilized phosphate 2.25 mg/L) than the fungus C. aquaticum (SI = 1.19 and 2.00 mg/L).
Freshwater fungi, root endophyte, phosphate solubilization, pH, mycelial weights, halo zones.
Phosphorus is an essential nutrient for the biochemical and physiological activities of plants but it is difficult to plants to obtain phosphorus from soil (Schachtman et al., 1998). A greater part of soil phosphorus, approximately 95–99% is present in the form of insoluble phosphates and cannot be utilized by the plants, only a little percentage of the applied phosphorus is available to plants, due the transformation of phosphorus into some complexes with aluminium or iron (Al or Fe) in acidic soils, and calcium (Ca) in calcareous soils (Sperber, 1958; Illmer and Schinner, 1992; Narsian et al., 1994; Nahas, 1996; Narsian and Patel, 2000). Phosphorus deficiencies are wide spread on soil throughout the world and phosphorus fertilizers represent major cost for agricultural production.
Soil is the most diverse terrestrial habitats for fungal biota that perform an important function in the soil ecosystem by decomposing plant residues, releasing nutrients and stimulating plant growth (Wardle and Giller, 1997; Rodriguez et al., 2004; Mittal et al., 2008). Many soil fungi are known to solubilize inorganic phosphates; these fungi play an important role in supplementing of phosphorus to the plants, allowing a sustainable use of phosphate fertilizers (Illmer and Schinner, 1992; Omar, 1998; Gyaneshwar et al., 2002). These fungi transform the insoluble phosphate into soluble form by solubilizing or dissolving the insoluble inorganic (Pinsol) phosphates, present in the soil, and make them available (Psol) to the plants (Oberson et al., 2001; Kang et al., 2002; Pradhan and Sukla, 2005). Furthermore, some phosphate solubilizing fungi have also been reported to increase the uptake of nutrient and yield in plants (Whitelaw, 2000; Vassileva et al., 2010 ). Some fungal strains are able to solubilize rock phosphate, aluminium phosphate and tricalcium phosphate (Kang et al., 2002). Some isolates of genus Aspergillus, from the rhizospheric soils, have also been reported for high solubilization of tricalcium phosphate (Reddy et al., 2002; Gyaneshwar et al., 2002).
The phosphate solubilizing fungi are also superior to bacteria for phosphate solubilization, both on precipitated agar and in liquid cultures (Kucey, 1983, 1987; Vazquez, 2000). The fungal hyphae attach to phosphate mineral particles and are able to reach at greater distance in soil, more easily than bacteria (Chabot et al., 1993). It has also been observed that phosphate solubilizing bacteria, upon repeated sub-culturing lose their phosphate solubilizing activity (Kucey, 1983; Illmer and Schinner, 1992) but such loses have not been observed in phosphate solubilizing fungi (Kucey, 1983). In general, phosphate solubilizing fungi (PSF) produce more acids and consequently exhibit greater phosphate solubilizing activity than bacteria (Banik and Dey, 1982; Venkateswarlu et al., 1984). On the basis of afore said account, it is quite clear that the magnificent of investigation on phosphate solubilization was carried out on the terrestrial or free living fungi isolated from other than endophytic habitats. Therefore, the aim of present study was to evaluate the phosphate solubilizing potential of isolated root endophytic freshwater fungi; Anguillospora longissima and Cylindrocarpon aquaticum.
Isolation of Root Endophytic Freshwater Fungi
The roots samples were collected from the riparian plants growing in the ravine area of Kilbury in Nainital, Kumaun Himalaya, India. The collected root samples were processed for the isolation of root endophytic freshwater hyphomycetous fungi (Singh and Sati, 2014). The isolates were identified with the help of relevant monographs and papers (Ingold, 1975).
Used Media
Pikovskaya media (agar and broth) were used to evaluate the phosphate solubilization potential of isolated root endophytic freshwater fungi. Malt Extract Agar (2% MEA, Hi-media) medium was used to the culture and to maintain the isolated fungi.
Determination of Dry Mycelium weights and pH
In liquid culture studies, the growing mycelium were separated by filtering the culture broths through Whatman paper No 1. Harvested mycelia were washed with distilled water and mycelial dried weights were estimated by evaporating the moisture contents in a oven at 67 °C for 24 hour (Singh and Singh, 2012). Cultures were filtered through Whatman paper No. 1 and culture filtrates were used to determine the pH of samples using µ pH System 361 (Systronics) pH meter.
Screening of Isolated Fungi for Phosphate Solubilization
The isolated root endophytic freshwater fungi were screened on PKV’s agar medium containing CaHPO4 as sole phosphate source. The Petri dishes with specific (PKV’s Agar) medium were inoculated with 5 mm fungal discs and incubated at 25 ±2 °C for 7 days. The formations of halo zones on the test medium around the growing fungal colonies were used as an indicator of phosphate solubilization. The solubilization index (SI) was determined by dividing the whole diameter (colony dia. + halo zone dia.) with the colony diameter following Fankem et al. (2006) as given below –
Solubilization Index = Colony diameter + halo zone diameter / Colony diameter
The calculating SI was used to classify the solubilization efficiency (SE) of isolated fungal strains as described by Hara and Oliveira (2005).
Quantification of Solubilized Phosphate in Liquid Medium
Conical flasks of 250mL were dispensed with 100 ml of PKV’s broth and supplemented with 0.50 mg of P2O5 (as extra source of TCP) then autoclaved at 121 °C (15 lb) for 18 minutes. Initial pH of media was recorded before the autoclaving. Autoclaved flasks were allowed to cool overnight in a laminar flow and then inoculated with 5 mm fungal discs (2 disc/flask) while for control experiments, uninoculated flasks were kept under the same condition. The inoculated flasks were incubated at 25 ±2 °C without shaking for 21 days in a B.O.D. incubator. The solubilized phosphate concentration was estimated three times after an interval of 7 days, during the total 21 days of study. The amount of P solubilized (Psol) was reported after deducting the values of soluble P concentration of the un-inoculated control samples (i.e., P released by autoclaving). The culture broths were filtered through Whatman paper No. 1 and centrifuged at 10,000 rpm, at 4 ±0.2 °C for 15 minutes. Supernatants were used for the estimation of solubilized phosphate at 827 nm wavelength, using a double beam spectrophotometer (Murphy and Riley, 1962).
Analysis of Data
Experiments were performed with the three sets of a replicate and average mean values with standard error mean (SEM) were used in phosphate solubilization assessments.
On the basis of morphological analyses, the isolated fungi were identified as Anguillospora longissima (Sacc. & Syd.) Ingold and Cylindrocarpon aquaticum (Nils.) Marvanova and Descals (Ingold, 1975; Singh and Sati, 2014).
Phosphate Solubilization Efficiency of Isolates on PKV Agar
Isolated root endophytic freshwater fungi; A. longissima and C. aquaticum were found positive for phosphate solubilization and produced clear halo zones around the growing fungal colony (Fig. 1 and 2; Table 1). Both the used fungi produced halo zones rapidly and followed almost same pattern for halo zones formation (Table 1).
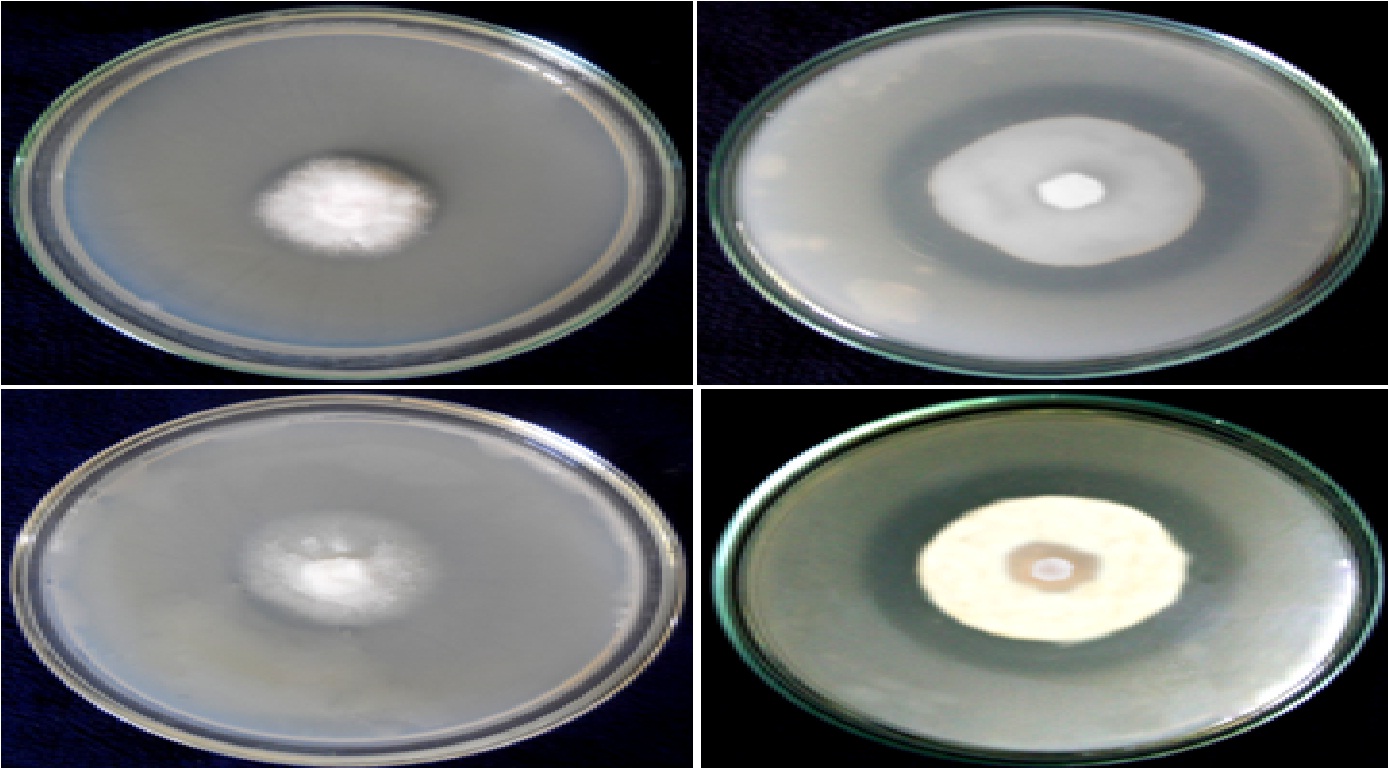
Fig. 1. Halo zones formation by the isolated root endophytic freshwater fungi A. longissima (A & B) and C. aquaticum (C & D) during the phosphate solubilization on PKV agar.
The maximum solubilization index SI = 1.53 by A. longissima and SI = 1.19 by the fungus C. aquaticum, were recorded for the respective fungi, during the 7 days of incubation on PKV agar. Both the used isolates fungi were classified as low (SI <2) phosphate solubilizing fungal strains (Fig. 2 ABCD; Table 1).
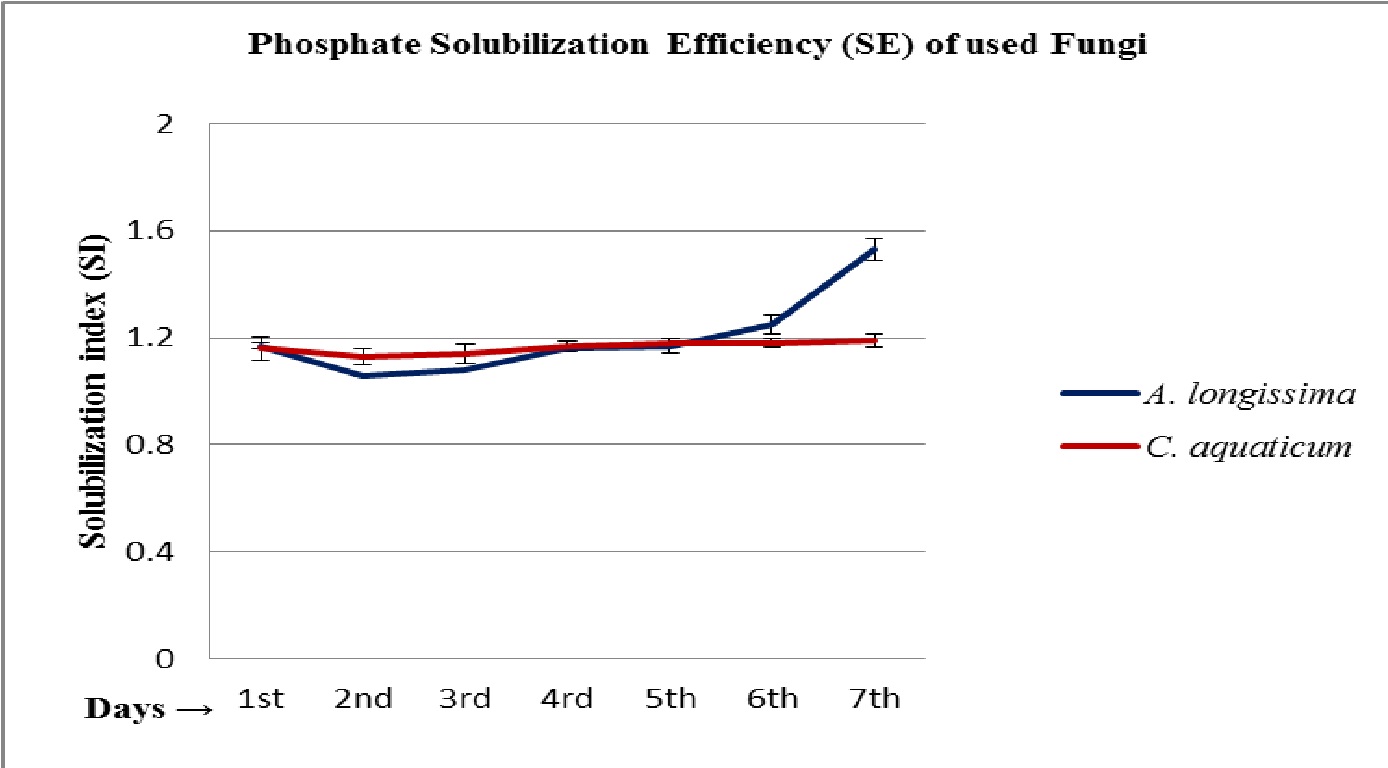
Fig. 2. Phosphate solubilization efficiencies of isolated root endophytic fungi A. longissima and C. aquaticum, on PKV agar
Table (1):
Phosphate solubilization index and efficiency isolated root endophytic freshwater fungi A. longissima and C. aquaticum on PKV agar.
| Days of Incubation | Solubilization Index (SI) | Solubilization Efficiency (SE) | |
|---|---|---|---|
| A. longissima | C. aquaticum | ||
| 1st | 1.17 (± 0.010) | 1.16 (± 0.043) | low (SI <2) |
| 2nd | 1.06 (± 0.003) | 1.13 (± 0.030) | low (SI <2) |
| 3rd | 1.08 (± 0.000) | 1.14 (± 0.035) | low (SI <2) |
| 4rd | 1.16 (± 0.012) | 1.17 (± 0.017) | low (SI <2) |
| 5th | 1.17 (± 0.025) | 1.18 (± 0.017) | low (SI <2) |
| 6th | 1.25 (± 0.034) | 1.18 (± 0.017) | low (SI <2) |
| 7th | 1.53 (± 0.043) | 1.19 (± 0.023) | low (SI <2) |
Quantification of Solubilized Phosphate in PKV Broth
The fungal isolates were found positive to grow and potent for phosphate solubilization, in the test medium (Table 2 and 3). The pH of the culture filtrates was found dropped significantly whereas it was remained almost constant in control (Fig. 5 and 6; Table 2 and 3). Further, the decreased pH of cultures filtrates and increased fungal mycelial weights in respect of days, clearly confirms the efficiency and ability of used fungi for phosphate solubilization in PKV broth. The fungus A. longissima solubilized slightly higher amount (2.25 mg/L) of phosphate, compare to the amount solubilized (2.00 mg/L) by secondary used fungus C. aquaticum, within the 21 days of study (Table 2 and 3). It was also noticed that the increases in amount of solubilized phosphate was directly proportional to the increases in fungal mycelial weights (Fig. 4; Table 2 and 3). Relying upon the data summarized in table 2 and 3, it is confirmed that both the used root endophytic freshwater fungi (A. longissima and C. aquaticum), are efficient to grow and to solubilize the phosphate in phosphate rich medium (PKV’s broth).
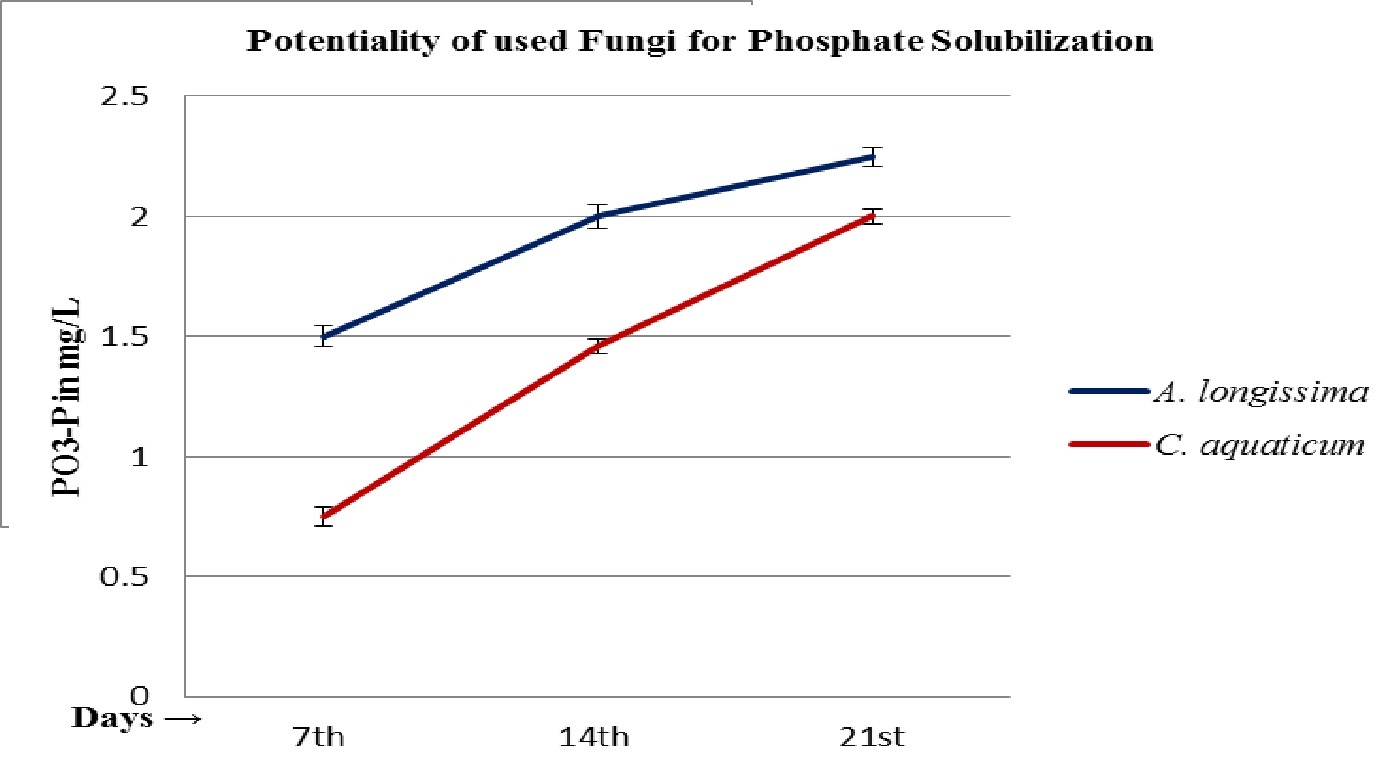
Fig. 3. Phosphate solubilization potential of isolated root endophytic fungi A. longissima and C. aquaticum, in PKV broth
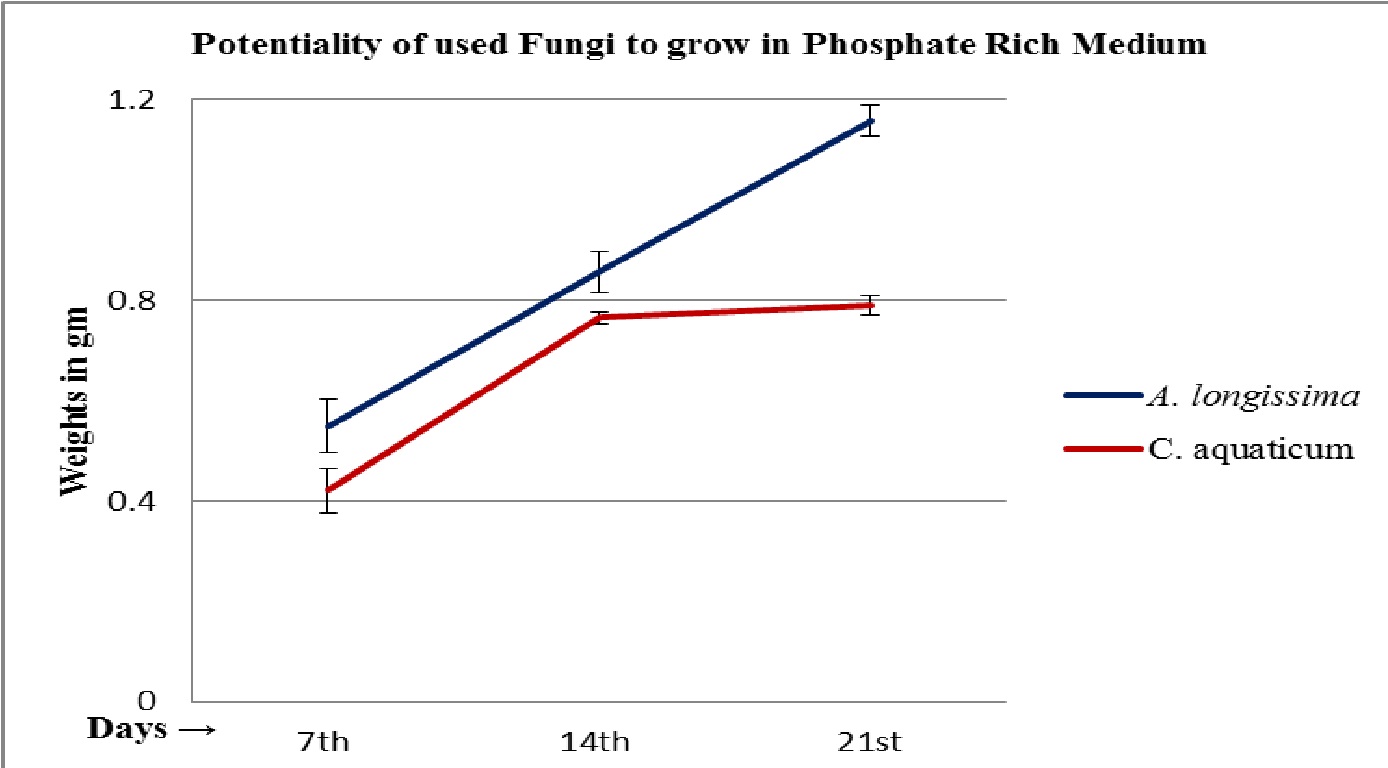
Fig. 4. Increased mycelial weights of isolated root endophytic fungi A. longissima and C. aquaticum during phosphate solubilization
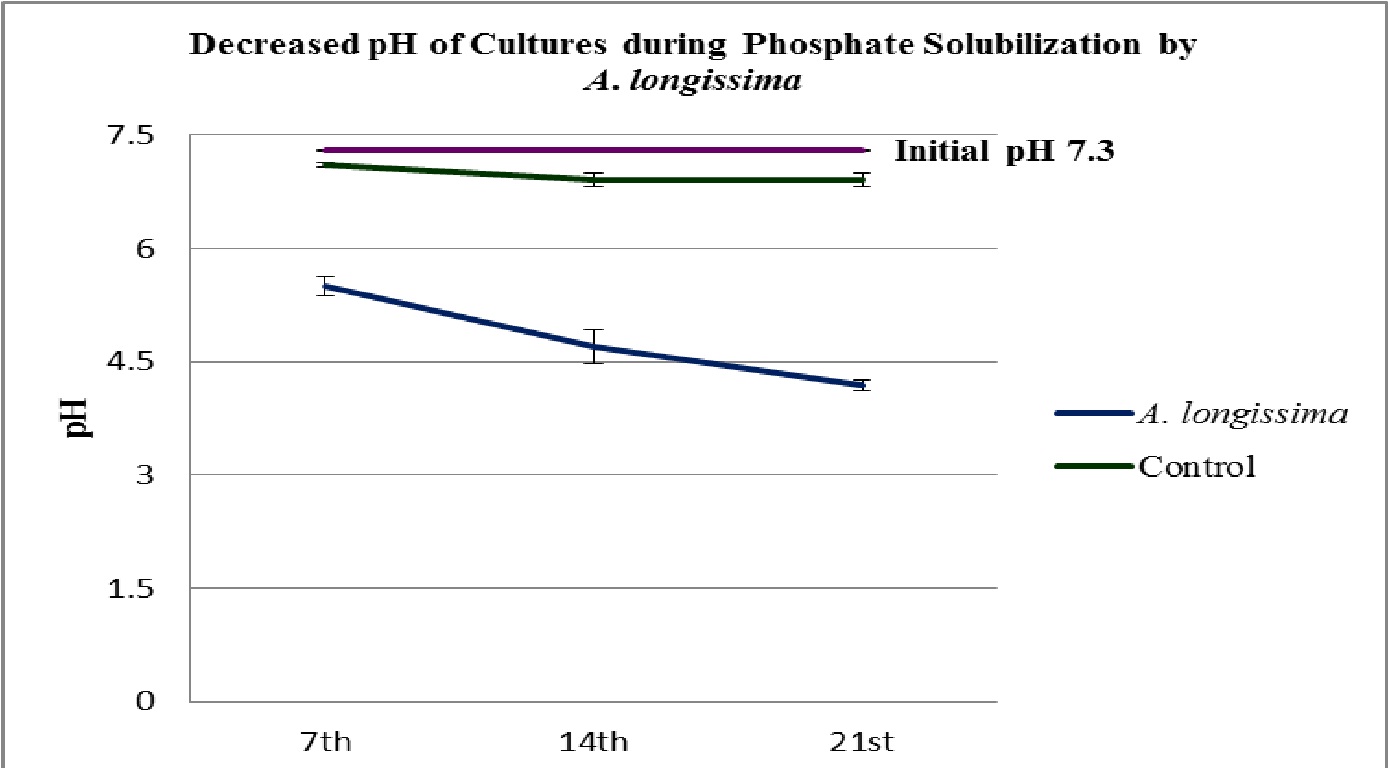
Fig. 5. Decreased pH of during the phosphate solubilization by the isolated root endophytic fungus A. longissima
Table (2):
Estimation of solubilized phosphate at 827 nm, pH and mycelial weight of isolated root endophytic fungus A. longissima during the phosphate solubilization, in PKV broth.
| Days | Initial pH | Reduction in pH | Soluble phosphate(mg/L) | Mycelial dry weight (gm) | |
|---|---|---|---|---|---|
| In sample | In control | ||||
| 7th | 7.3 (± 0.010) | 5.5 (± 0.120) | 7.1 (± 0.033) | 1.50 (± 0.042) | 0.550 (± 0.053) |
| 14th | 7.3 (± 0.011) | 4.7 (± 0.226) | 6.9 (± 0.088) | 2.00 (± 0.051) | 0.857 (± 0.041) |
| 21st | 7.3 (± 0.011) | 4.2 (± 0.068) | 6.9 (± 0.088) | 2.25 (± 0.040) | 1.159 (± 0.031) |
The results summarized in table 1-3, clearly indicate that both the used root endophytic aquatic or freshwater fungi are efficient for phosphate solubilization. The maximum solubilization index (SI = 1.53 and 1.19) was recorded onto the 7th days of incubation (Fig. 3; Table 1). It has been observed that the production of halo zone by phosphate solubilizing fungi depends upon the medium components and potentiality of involved strains (Beever and Burns, 1981; Gadd 1999). In the present study, the used fungi belongs to a specific group of fungi (i.e. freshwater fungi) that were isolated from a special niche (as root endophytes), might be with different physiology for their activities on used medium (PKV agar) and showed a varied solubilization index (SI). These fungi are well known to mineralize the leaf litters in freshwater streams (Ingold 1975) and therefore, comparatively be envisaged for their active phosphate solubilization efficiencies that not studied earlier. The findings of present study confirm the potentiality of used fungi in phosphate solubilization and the recorded solubilizing index was observed due to the rapid utilization of nutrients from the phosphorus rich medium as well as due to the production of some organic acid that solubilized or mineralized the complex structure of phosphate (Fig. 1 and 2; Table 1). The recorded higher solubilization index on first day suggests that solubilization of phosphate not only depends upon the production of organic acid to dissolve P but also indicates the involvement of some enzymatic reaction in biological phosphate solubilization.
In liquid culture studies, both the used root endophytic fungi significantly dropped the initial pH of growing cultures (Fig. 6 and 7; Table 2 and 3). Illmer and Schinner (1995) reported a significant decline in the pH of the culture medium by phosphate solubilizing microbes, due to the production of organic acids. The production of organic acids or proton by the phosphate solubilizing fungi has also been reported earlier by the workers (Kucey, 1987; Cunningham and Kuiack, 1992; Pradhan and Sukla, 2005). The present study supports the earlier findings and suggests that some organic acids or protons were secreted by the used root endophytic freshwater or aquatic fungi which lowered the pH, during the phosphate solubilization while decreases in pH were not recorded for un-inoculated samples (Fig. 6 and 7; Table 2 and 3). Cunningham and Kuiack (1992) also reported decrease in pH during the fungal solubilization of calcium phosphate (CaHPO4) by the fungus Penicillium bilaiae. Vazquez et al., (2000) also observed a remarkable drop in pH of culture media, supplemented with tricalcium phosphate, during the phosphate solubilization by the fungus Aspergillus niger.
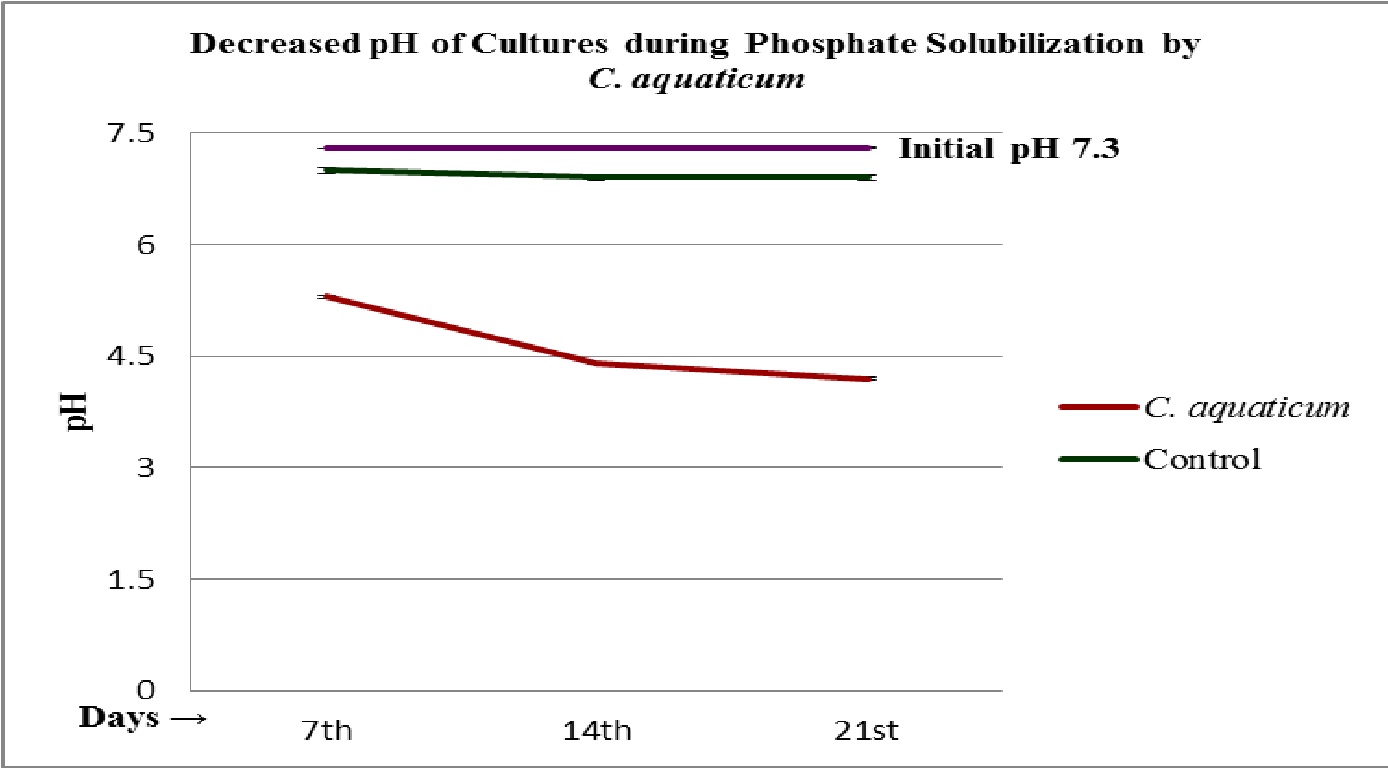
Fig. 6. Decreased pH of during the phosphate solubilization by the isolated root endophytic fungus C. aquaticum
Table (3):
Estimation of solubilized phosphate at 827 nm, pH and mycelial weight of isolated root endophytic fungus C. aquaticum during the phosphate solubilization, in PKV broth.
| Days | Initial pH | Reduction in pH | Soluble phosphate(mg/L) | Mycelial dry weight (gm) | |
|---|---|---|---|---|---|
| In sample | In control | ||||
| 7th | 7.3 (± 0.000) | 5.3 (± 0.021) | 7.0 (± 0.033) | 0.75 (± 0.038) | 0.421 (± 0.045) |
| 14th | 7.3 (± 0.010) | 4.4 (± 0.006) | 6.9 (± 0.033) | 1.46 (± 0.030) | 0.766 (± 0.012) |
| 21st | 7.3 (± 0.010) | 4.2 (± 0.020) | 6.9 (± 0.033) | 2.00 (± 0.030) | 0.790 (± 0.019) |
The principle mechanism for phosphate solubilization is the production of organic acids, results in acidification of the microbial cell and its surroundings (FNCA, 2006). The organic acids secreted by these fungi, can either directly dissolve the mineral phosphate (through anion exchange of phosphate) or can chelate with iron (Fe) and aluminium (Al) ions associated with phosphate (Sperber, 1958; Whitelaw, 2000). Reyes et al. (1999) suggested that phosphorous release is a complex phenomenon that depends on many factors such as nutrition, physiology and growth conditions of the culture as well as potential of involved strain. Sayer and Gadd (2001) reported the production of gluconic and citric acid, in the presence of Co3(PO4)2 and Zn3(PO4)2, respectively by the fungus A. niger. Pradhan and Sukla (2005) reported that absence of soluble phosphate in media induces the acid production. Thus, the phosphate solubilization potential of these fungi may also depend upon the medium’s components of test media. The inorganic phosphate solubilization is associated mainly with the acidification of the medium by organic acid production. Majority of these fungi produce organic acids (Banik and Day, 1982; Cunningham and Kuiack, 1992; Gyaneshwar et al., 2002). Sperber (1958) identified organic acid metabolites as the primary means of inorganic P solubilization by fungi which act as chelating agents to acidify the surrounding environment. The production of citric and gluconic acid by the fungi Penicillium rugulosum and P. bilaiae was recorded in the presence of citrate confirms the chelating of citric acid with Ca of CaHPO4 (Cunningham and Kuiack, 1992). Fomina et al. (2004) also reported secretion of succinic and acetic acid by the majority of the fungal strains used in the solubilization of zinc phosphate. Although, the mechanism of phosphate solubilization is not clear but it is confirms that it was due to the production of organic acids by the used fungi. The studies on the bio-prospecting of these freshwater fungi are still less informative while they have proven to be good synthesizers of antimicrobial agents (Sati and Singh, 2014).
The available literature indicates that this is the first report on the phosphate solubilizing potential of root endophytic freshwater hyphomycetous fungi. It is also interesting to add that both the used fungi are basically freshwater inhabiting fungi which were recovered as endophyte from the living roots of riparian plants. The used fungi may be utilized as promising fungal strains to supply the phosphorus in an eco-friendly way.
ACKNOWLEDGMENTS
Authors are thankful to the Head, Department of Botany, to provide the necessary facilities during the work. We are also thankful to Dr. Shah Raj Ali, Department of Botany, to provide some required chemicals for his the study.
- Banik, S., Day, B.K. Available phosphate content of an alluvial soil as influenced by inoculation of some isolated phosphate solubilizing microorganisms. Plant Soil., 1982; 69(3): 353–364.
- Beever, R.E., Burns, D.J.W. Phosphorus uptake storage and utilization by fungi. Adv. Bot. Res., 1981; 8: 127–219.
- Chabot, R., Antoun, H., Cescas, M.P., Stimulation de la croissance du mais et de la laitue romaine par des microorganisms dissolvant le phosphore inorganique. Can. J. Microbiol., 1993; 39: 941–947.
- Cunningham, J.E., Kuiack, C. Production of citric acid and oxalic acid and solubilization calcium phosphate by Penicillium billai. Appl. Environ. Microbiol., 1992; 58: 1451–1458.
- F.N.C.A. Forum for Nuclear Cooperation in Asia. Biofertilizer Project Group. Biofertilizer Manual., 2006.
- Fankem, H., Nwaga, D., Deubel, A., Dieng, L., Merbach, W., Etoa, F.X. Occurrence and functioning of phosphate solubilizing microorganisms from oil palm tree (Elaeis guineensis) rhizosphere in Cameroon. Afr. J. Biotechnol., 2006; 5(24): 2450–2460.
- Fomina, M., Alexander, I., Hillier, S., Gadd, G.M. Zinc phosphate and pyromorphite solubilization by soil plant-symbiotic fungi. Geomicrobiology J., 2004; 21: 351–366.
- Gadd, G.M. Fungal production of citric and oxalic acid: Importance in metal speciation, physiology and biogeochemical processes. Adv. Micro. Physiol., 1999; 4: 47–92.
- Gyaneshwar, P., Kumar, N.J., Pareka, L.J., Podle, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil., 2002; 245 (1): 83–93.
- Hara, F.A.S., Oliveira, L.A. Physiological and ecological characteristics of rhizobia isolates from acid soils of Iranduba, Amazonas. Pesqui. Agropecu. Bras., 2005; 40: 667–672.
- Illmer, P., Schinner, F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Bio. Biochem., 1992; 24: 389-395.
- Ingold, C.T. An illustrated guide to aquatic and water borne Hyphomycetes (Fungi Imperfecti) with notes on their biology. Freshwater Bio. Assoc. Scient. Publ. No. 30 England., 1975; pp–96.
- Kang, S.C., Ha, C.G., Lee, T.G., Maheswari, D.K. Solubilization of insoluble inorganic phosphate by a soil inhabiting fungus Fomitopsis sp. Current Science., 2002; 82: 439–442.
- Kucey, R.M.N. Increased phosphorus uptake by wheat and field beans inoculated with a phosphorus solubihzing Penicillium bilaji strain and vesiculararbuscular mycorrhizal fungi. Appl. Environ. Microbiol., 1987; 53: 2699–2703.
- Kucey, R.M.N. Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can. J. Soil Sci., 1983; 63: 671–678.
- Mittal, V., Singh, O., Nayyar, H., Kaur, J., Tewari, R. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol. Biochem., 2008; 40(3): 718–727.
- Murphy, Y., Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Ann. Chem. Acta., 1962; 27: 31–36.
- Nahas, E. Factors determining rock phosphate solubilization by microorganisms isolated from soil. World J. Microbiol. Biotechnol., 1996; 12: 567–572.
- Narsian, V., Patel, H. Aspergillus aculeatus as a rock phosphate solubilizer. Soil Biol. Biochem., 2000; 32: 559–565.
- Narsian, V., Thakkar, J., Patel, H.H. Isolation and screening of phosphate solubilizing fungi. Indian J. Microbiol., 1994; 34: 113–113.
- Oberson, A., Friesen, D.K., Rao, I.M., Buhler, S., Frossard, E. Phosphorus transformations in an oxisol under contrasting land-use system: The role of the microbial biomass. Plant Soil., 2001; 237: 197–210.
- Omar, A.S. The role of rock-phosphate-solubilizing fungi and vesicular- arbuscular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock rhosphate, World J. Microbiol. Biotechnol., 1998; 14: 211–218.
- Pradhan, N., Sukla, L.B. Solubilization of inorganic phosphates by fungi isolated from agriculture soil, Afr. J. Biotechnol., 2005; 5(10): 850–854.
- Reddy, S.M., Kumar, S., Babita, K., Reddy, M.S. Bio-solubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger, Biores. Technol. 2002; 84: 187–189.
- Reyes, I., Bernier, L., Simard, R.R., Antoun, H. Effect of nitrogen source on solubilization of different inorganic phosphates by an isolate of Pencillium rugulosum and two UV-induced mutants. FEMS Microbiol. Ecol., 1999; 28: 281– 290.
- Rodriguez, R.J., Redman, R.S., Henson, J.M. The role of fungal symbioses in the adaptation of plants to high stress environments. Mitigation Adap. Strateg. Glob. Change., 2004; 9: 261–272.
- Sati, S.C., Singh, L. Bioactivity of root endophytic freshwater hyphomycetes Anguillospora longissima (Sacc. & Syd.) Ingold. The Scientific World Journal., 2014: 14: 5 pages.
- Sayer, J.A., Gadd, G.M. Binding of cobalt and zinc by organic acids and culture filtrates of Aspergillus niger grown in the absence or presence of insoluble cobalt or zinc phosphate. Mycol. Res., 2001; 105: 1261–1267.
- Schachtman, D.P., Reid, R.J., Ayling, S.M. Phosphorus uptake by plants: from soil to cell. Plant Physiol., 1998; 116: 447–453.
- Singh, L., Sati, S.C. Bio-prospecting of root endophytic aquatic fungus Cylindrocarpon aquaticum as antibacterial potential. J. Pure Appl. Microbiol., 2014; 8: 4903–4908.
- Singh, L., Singh, V.P. Microbial decolourization of textile dyes by the fungus Trichoderma harzianum. J Pure Appl. Microbiol., 2012; 6(4): 1829–1833.
- Sperber, J.I. Solution of apatite by soil microorganisms producing organic acids. Aust. J. Agri. Res., 1958; 9: 778–781.
- Vassileva, M., Serrano, M., Bravo, V., Jurado, E., Nikolaeva, I., Martos, V., Vassilev, N. Multifunctional properties of phosphate-solubilizing microorganisms grown on agro-industrial wastes in fermentation and soil conditions. Appl. Microbiol. Biotechnol., 2010; 85: 1287–1299.
- Vazquez, P., Holguin, G., Puente, E.M., Lopez-Cortes, A., Bashan, Y. Phosphate- solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils., 2000; 30: 5–6.
- Venkateswarlu, B., Rao, A.V., Raina, P. Evaluation of phosphorous solubilization by microorganisms isolated from arid soils. J. Indian Soc. Soil Sci., 1984; 32(3): 273–277.
- Wardle, D.A., Giller, K.E. The quest for a contemporary ecological dimension to soil biology. Soil Biol. Biochem., 1997; 28: 1549–1554.
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate solubilizing fungi. In: Advances in Agronomy (Ed. Donald L. Sparks). Academic press., 2000; 69: 99–151.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


