ISSN: 0973-7510
E-ISSN: 2581-690X
Plastic materials have become a necessity of human life especially in the packaging of food commodities and biomedical procedures. Bioplastic is emerging as an effective alternative to fossil oil-based materials to avoid the environmental hazards of the plastic industry. During this study, chicken feathers were used as a substrate to isolate keratin degrading bacteria. Among 14 identified isolates, Bacillus sp BAM3 was found to be the most promising isolate. Partial 16S rDNA analysis-based molecular characterization revealed it is a strain of Bacillus cereus. Bacillus sp BAM3 can grow and produce keratinase in feathers containing basal medium as the sole carbon and energy source. The maximum keratinase production (730U/ml) was achieved within 24 h under optimum reaction conditions. The optimized reaction pH and temperature were noted as 9.0 and 50 °C for crude keratinase activity, respectively. The chicken feathers were used as a substrate in 2, 5, and 10 wt% glycerol to synthesize keratin-based bioplastic with keratinolytic bacterium Bacillus cereus BAM3. Bioplastic prepared from keratin with 2% of glycerol was found to possess good mechanical properties. Therefore, the results present a novel keratinolytic isolate of Bacillus cereus BAM3, which may have potential biotechnological applications in keratin hydrolysis processes. The development of keratin-based bioplastics possessing superior crystalline morphology requires further investigations to substitute fossil oil-based materials.
Bacillus cereus, bio-plastic, keratinolytic bacteria, feather
The plastic carrier bags gained popularity in the late 1970s1. The environmental hazards of synthetic polymers have urged scientists to develop biodegradable alternatives from renewable polysaccharides2,3 proteins4,5 and lipids6. The limited stock and rising prices of petroleum enhance the utility of bioplastic in the future. The traditional plastic products manufactured from fossil oil are non-biodegradable and thus are considered hazardous for terrestrial and marine environments. The plastic material used for the food packaging ends up in landfills whereas only 5.7% is recycled7. The usage of unpalatable animal parts including, viscera, heads, bones, legs, and feathers is increasing in the manufacturing of various products8. Feathers are particularly a potent source of developing biodegradable commodities. Keratin waste is rich in protein (90%), nitrogen (15–18%), and sulfur (2-5%)9. A considerable amount of keratin waste especially feathers is produced during animal farming and trading. Keratin proteins are highly resistant to enzymatic lysis and chemical agents, as they contain multiple disulfide bonds (S–S)10. However, numerous keratinolytic microorganisms have been documented including the members of the genus Bacillus, Vibrio, Serratia, actinomycetes11,12, and recombinant strain of Bacillus13. Chrysosporia microfungi belonging to the genus Chrysosporium, also known as geophilic dermatophytes, are specialized in the degradation of keratin proteins. The occurrence and growth of keratin-degrading microorganisms depend upon the enrichment of keratin in the soil. The chicken feather-based keratin protein, either alone or in combination with synthetic or natural polymers, has diverse applications in manufacturing films, sponges, and fibers2,14,15,16,17. The keratin of chicken feather4,8,11,17 can be applied to develop biodegradable and environmentally safe bioplastic18. A recent study has reported that keratolytic bacteria degraded chicken feathers into keratin micro-particles to produce bioplastic19. The present study focuses to assess the mechanical properties and ability of keratinolytic bacterium to produce chicken feathers-based bioplastic film at various glycerol concentrations. The study can facilitate the development of commercial bioplastic films in the future.
Isolation of keratinolytic bacteria with chicken feathers
Isolation and screening of the keratolytic bacteria were performed by suspending the samples in feather meal broth medium as described by Agrahari and Wadhwa20. One gram of the soil sample and poultry or feather waste was added in 50 ml of the medium. All flasks were incubated at 37°C for 7-14 days. Then, 1 ml broth of each was transferred to Feather Meal Agar Medium plates and incubated at 37°C for 2 days. The developed colonies were purified by repeated streaking to obtain pure culture on Nutrient agar plates.
Chicken feathers were used as a substrate to isolate keratinolytic bacteria in keratin medium. Chicken feathers were obtained from the Al-Rayan poultry farm, Jeddah, Saudi Arabia. Feathers were prepared according to Tork et al., 201021 with minor modification. Feather-degrading bacteria were isolated and grown in feather meal medium (basic medium) containing (g LG1): K2HPO4 (1.4), MgSO4 (0.1), KH2PO4 (0.7), NaCl (0.5), ground keratin as sole carbon and nitrogen source, and 15-20 g agar for solidification. The pH of this medium was adjusted to 7.0 9,22. The feather meal plates were spread with 1mL diluted medium and incubated at 37°C for 2 days for general bacteria whereas incubation of 5-7 days was carried out for actinomycetes. Nutrient agar medium was used to maintain purified bacterial colonies at 4°C.
Screening of keratolytic bacteria
Primary screening of keratolytic bacteria
Keratolytic bacteria were primarily screened with skimmed milk agar medium19. All the ingredients of the milk agar medium were sterilized in autoclave except milk powder. The sterile milk powder was separately added as the medium reached the tolerable temperature (45ºC). The sterilized medium was poured into sterile Petri dishes. The suspected bacteria isolates, maintained in starch agar medium and nutrient agar, were inoculated in milk agar plates. The plates were incubated at 37°C for two days and examined for the formation of clear zones on the skimmed milk agar. The bacterial isolates exhibiting clear zone formation on the agar medium were recorded as positive.
Secondary screening of keratolytic bacteria
The positive isolates of primary screening were subjected to secondary screening for the isolation of feather degrading bacteria. A modified basal liquid medium supplemented with a native chicken feather (5 cm) was placed in test tubes for secondary screening. The medium was sterilized and inoculated with selected bacterial isolates of primary screening. The test tubes were incubated at 37°C and examined on weekly basis for feather degradation.
Identification of Bacterial Isolate BAM3
The best keratinase-producing microorganism was identified based on cultural, morphological, and biochemical tests such as oxidase and catalase production assays. Molecular identification of the most promising isolate (BAM3) revealed it as keratinolytic Bacillus sp. BAM3. Molecular identification was carried out by extracting genomic DNA of bacteria grown to exponential phase in Luria-Bertani medium. Centrifugation of bacterial culture was carried out for 15 min at 12,000 rpm and supernatant (10 ml aliquot) was harvested. Sterile distilled water was used to wash the supernatant. Gene JET Genomic DNA Purification kit (Thermo Fisher Scientific, Massachusetts, USA) was used to extract the DNA. PCR amplification of 16SrDNA was carried out with two primers (16F27: 52 AGAGTTTGATCCTGGCTCAG 32 and 16R1522: 52 AAGGAGGTGATCCAGCCGCA 32 ) according to Buonaurio et al.23. A reaction mixture of 25 µL was prepared for the PCR amplification of the 16S rRNA gene. The reaction mixture contained 12.5 µL master mix (2x), 1 µL of each primer (10 pmoles/µl), 2 µL DNA, and 9.5 µL dH2O. PCR conditions were set as initial denaturation at 94 oC for 5 min, 35 denaturation cycles at 94 oC for 1 min, annealing at 55 oC for 1 min, extension at 72 oC for 2 min, and a final extension at 72 oC for 10 min. Sanger sequencing of PCR samples was carried out from Beijing Genomic Institute (BGI), Hong Kong, China. BLAST software of the NCBI database was used to compare the sequences. T-Caffee algorithm (https://www.ebi.ac.uk/, EMBL-EBI, Cambridgeshire, UK) was used to align the obtained sequences in Ugene 24. The phylogenetic tree was constructed in an interactive tree tool iTOL (http://itol.embl.de/index. shtml) 24.
Keratinase Assay
The method of Letourneau et al. 26 was followed to estimate Keratinase activity by using Keratin azure substrate (Sigma-Aldrich, USA). The substrate was frozen at -10°C followed by grinding into fine powder in Oscillating mil mm400 retch. Five-gram fine powder of the substrate was suspended in 1 ml of 50 mM Tris-HCl buffer (pH 8.0). The reaction mixture containing 1 ml of each, crude enzyme and keratin azure suspension, was placed in a water bath at 50°C for 30 min. 2 ml of 0.4 M trichloroacetic acid (TCA) was finally added to stop the reaction. The substrate was removed by centrifuging the mixture for 5 min at 3000×g. Spectrophotometry of the supernatant was carried out at 595 nm to measure the release of azo dye. The amount of enzyme causing 0.01 absorbance increase between the sample and control at 595 nm was considered as one-unit (U) of keratinase activity23,25.
Effect of pH and temperature on keratinase production
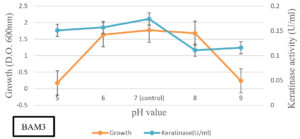
The flasks were incubated at different temperatures (25, 30, 37, 45, and 50°C) for 7 days at 200 rpm to assess optimum temperature. Keratin azure was used as a substrate at optimum growth temperature, and bacterial growth and keratinase activity were measured. The effect of different pH on keratinase production of the selected bacterial isolate was also estimated. The medium was prepared at pH 5, 6, 7, 8, and 9. Different sterile feather meal broths were separately placed in 250 ml Erlenmeyer flasks containing 50 ml of the medium and 2 ml of pre-culture of the selected bacterial culture. The flasks were incubated at 37ºC for 7 days at 200 rpm and bacterial growth and keratinase activity were evaluated.
Preparation of bioplastic film
The keratin-based bioplastic was prepared according to Ramakrishnan et al27 with slight modifications. Keratin solution (100 ml) was mixed with different concentrations of glycerol (2, 5, and 10 wt%) and subjected to constant magnetic stirring for 5 h at 60 °C. The aliquot was spread on circular aluminum weighing boat (43 mm diameter at top) and placed in the oven for 24 h at 60 °C. After 24 h, the mixture was allowed to cool down and dry. Then, bioplastic film was detached from the aluminum weighing boat and labeled. The process was repeated for all glycerol concentrations.
Statistical Analysis
The experiments were performed in triplicate. Means of replicates and standard deviation were compared by student t-test to differentiate between controls and treatments.
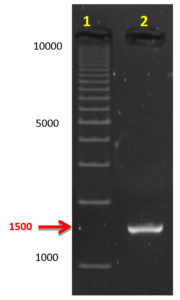
Fourteen bacterial isolates were found with varying levels of keratinase activity and BAM3 isolate was noted to be the most promising. The isolate was characterized as rod-shaped Gram-positive bacteria with white-colored irregular rough colonies on nutrient agar plates. This bacterial isolate was identified as Bacillus sp. based on biochemical and physiological characteristics. Molecular identification revealed the phylogenetic tree of this isolate, which contains only one cluster. Bacillus sp BAM3 was more related to one strain of Enterobacter cloacae , five strains of Bacillus cereus, one strain of Bacillus thuringiensis, one strain of Bacillus toyonensis, three strains of Bacillus paramycoides, two strains of Bacillus albus, two strains of Bacillus anthracis, one strain of Endophytic bacterium, one strain of Bacillaceae bacterium, and one strain of Bacillus subtilis. The partial 16S rDNA sequencing confirmed it as Bacillus cereus BAM3 sharing 98.9–100% identity and 100% coverage (Figure 1, 2).
Fig. 1. Partial 16S rDNA gene sequences based phylogenetic tree of Bacillus cereus BAM3 (acc. no. MK248483.1) and closely related strains
Fig. 2. The 1500 bp 16S rDNA amplicon separated on 0.8% agarose gel electrophoresis compared to DNA Ladder (1kb). Lane 1: 1kb DNA leader (Bio-Rad) and, Lane 2: Amplified gene from keratinolytic bacterial isolate
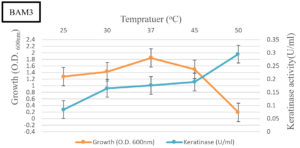
Effect of pH and temperature on keratinase production
BAM3 isolate completely degraded feather within 10 days (Figure 3). The keratinase production was significantly higher and noted as 730 U/ml-1. The growth and keratinase production ability of isolate BAM3 were confirmed using one feather in mineral broth medium at an initial pH of 7 and 50°C. The highest growth was noted at 37oC whereas optimum keratinase production was observed at 50°C. At 50°C, the highest level of keratinase activity (about 730Uml-1) was observed after 24 h. After 7 days of growth, an increase in the medium pH (above 9) was noted (Figures 4 and 5).
Fig. 3. (A) The flask on the left represents control whereas the flask on the right contains completely degraded feathers by bacterial isolate BAM3 after 10 days in mineral feather medium. (B) Degradation process in one row of the feather
Preparation of bioplastic film
At 2 wt% glycerol concentration, the mixture was better homogenized and depicted plasticization signs in the keratin matrix without single-phase morphology and separation. These findings are in line with the previous reports28. Undissolved particles were observed in bioplastic films at 5 and 10 wt% glycerol concentrations. 10 wt% glycerol bioplastic film depicted more voids and un-dissolved particles than 5 wt% glycerol bioplastic film. Another study 28 has reported that the phase separation and formation of empty voids is directly proportional to the glycerol content (Figure 6).
Feather degrading keratinolytic microorganisms and keratinase enzymes could improve the digestibility of feather keratin9,10,11 to use as bioplastic12,13. A promising Keratinolytic bacterium was successfully isolated and characterized as Bacillus cereus BAM3. Some of the other isolated bacteria belonged to the Bacillus group whereas a few were Gram-negative. The findings of this study are in line with previous reports. Degradation of keratin by Gram-positive (Bacillus, Streptomyces) and Gram-negative bacteria has been reported14,15. Keratinase-producing strain of Bacillus cereus had also been isolated30. Bacillus cereus BAM3 efficiently degraded keratin in intact feathers. Contrarily, the literature reveals the keratin degradation mostly by Bacillus cereus strains16. In this study, complete feather degradation was achieved by the aerobic growth of inoculated isolates, which used it as a primary source of nitrogen, carbon, and energy. Complete degradation of feathers in 30 days has been previously reported17. The results depict that optimized bacterial growth conditions significantly increased the keratin degradation18. The optimum bacterium growth rate temperature increased the production of a total enzyme (730 U/ml). Increased pH values were observed during feather degradation that is closely related to other microorganisms possessing higher keratinolytic activity19, 31. The pH has been reported as a key factor in enzymatic feather degradation due to the collaborative function of keratinase and alkaline conditions. Keratin acts as a carbon and energy source for bacteria, hence a higher feather concentration might significantly enhance the growth and keratinase production32, 33,34. The combination of 2 wt% of glycerol and keratin produced bioplastic with good surface morphology and mechanical properties. These results are comparable to the findings of Ramakrishnan et al27. They mixed extracted keratin solution with a range of glycerol solution (2-10wt%) and found that the bioplastic film produced by 2wt% glycerol possessed better mechanical and biodegradable properties35.
This study led to the successful synthesize of bioplastic by using keratin of chicken feathers. The potent Bacillus cereus BAM3 isolate could be used for the efficient biodegradation of poultry feathers. The higher concentrations of glycerol reduce maximum tensile strength in the keratin films of the chicken feathers. The results revealed that bioplastic made from 2 wt% glycerol and keratin had the best mechanical properties. This is a remarkable finding as these bioplastic films can substitute fossil oil-based environmentally harmful materials. The conditions optimized during this study for the growth of B. cereus BAM3 isolate and release of the enzyme can facilitate the industrial production of keratinase. These conditions should be further worked out to improve the productivity of the strain and enzyme. The keratin-based bioplastic films should also be characterized to determine their thermal stability and crystallinity for successful industrial applications.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors have made a substantial, direct, and intellectual contribution to the work and approve it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in this manuscript.
- Accinelli C, Saccà ML, Mencarelli M, Vicari A. Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere. 2012;89:136-143.
Crossref - Khot SN, Lascala JJ, Can E et al. Development and application of triglyceride-based polymers and composites. J Appl Polym Sci. 2001;82:703-723.
Crossref - Zhang Y, Rempel C, Liu Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit Rev Food Sci Nutr. 2014;54:1353-1370.
Crossref - Rocha Plácido Moore G, Maria Martelli S, Gandolfo C, José do Amaral Sobral P, Borges Laurindo J. Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocoll. 2006;20:975-982.
Crossref - Mauri AN, Añón MC. Effect of solution pH on solubility and some structural properties of soybean protein isolate films. J Sci Food Agric. 2006;86:1064-1072.
Crossref - Almeida A, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478-490.
Crossref - Zhao R, Torley P, Halley PJ. Emerging biodegradable materials: starch- and protein-based bio-nanocomposites. J Mater Sci. 2008;43:3058-3071.
Crossref - Reddy N, Chen L, Yang Y. Biothermoplastics from hydrolyzed and citric acid Crosslinked chicken feathers. Mater Sci Eng C. 2013;33:1203-1208.
Crossref - Dou Y, Zhang B, He M, Yin G, Cui Y. Preparation and Physicochemical Properties of Dialdehyde Starch Crosslinked Feather Keratin/PVA Composite Films. J Macromol Sci Part A. 2014;51:1009-1015.
Crossref - Zhu G-Y, Zhu X, Wan X-L, et al. Hydrolysis technology and kinetics of poultry waste to produce amino acids in subcritical water. J Anal Appl Pyrolysis. 2010;88:187-191.
Crossref - Jin E, Reddy N, Zhu Z, Yang Y. Graft Polymerization of Native Chicken Feathers for Thermoplastic Applications. J Agric Food Chem. 2011;59:1729-1738.
Crossref - Mokrejs P, Svoboda P, Hrncirik J, Janacova D, Vasek V. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manag Res. 2011;29:260-267.
Crossref - Lasekan A, Abu Bakar F, Hashim D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013;33:552-565.
Crossref - Sharma S, Gupta A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Brazilian Arch Biol Technol. 2016;59.
Crossref - Boakye M, Rijal N, Adhikari U, Bhattarai N. Fabrication and Characterization of Electrospun PCL-MgO-Keratin-Based Composite Nanofibers for Biomedical Applications. Materials (Basel). 2015;8:4080-4095.
Crossref - Flores-Hernández C, Colín-Cruz A, Velasco-Santos C, et al. All Green Composites from Fully Renewable Biopolymers: Chitosan-Starch Reinforced with Keratin from Feathers. Polymers (Basel). 2014;6:686-705.
Crossref - Khosa MA, Wu J, Ullah A. Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv. 2013;3:20800-20810.
Crossref - Saravanan S, Sameera DK, Moorthi A, Selvamurugan N. Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int J Biol Macromol. 2013;62:481-486.
Crossref - Riffel A, Brandelli A. Keratinolytic bacteria isolated from feather waste. Brazilian J Microbiol. 2006;37:395-399.
Crossref - Agrahari S, Wadhwa N. Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site ay Ghazipur poultry processing plant. Int J Poult Sci. 2010; 5: 482-489.
Crossref - Tork S, Aly MM, Nawar L. Biochemical and Molecular Characterization of a New Local Keratinase Producing Pseudomomanas sp., MS21. Asian J Biotechnol. 2010;2:1-13.
Crossref - Lin X, Lee C-G, Casale ES, Shih JCH. Purification and Characterization of a Keratinase from a Feather-Degrading Bacillus licheniformis Strain. Appl Environ Microbiol. 1992;58:3271-3275.
Crossref - Buonaurio R, Stravato VM, Cappelli C. Brown spot caused by Sphingomonas sp. on yellow Spanish melon fruits in Spain*. Plant Pathol. 2001;50:397-401.
Crossref - Okonechnikov K, Golosova O, Fursov M. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28:1166-1167.
Crossref - Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39(suppl):W475-W478.
Crossref - Letourneau, Soussotte, Bressollier, Branland, Verneuil. Keratinolytic activity of Streptomyces sp. S .K 1–02/ : a new isolated strain. Lett Appl Microbiol. 1998;26:77-80.
Crossref - Ramakrishnan N, Sharma S, Gupta A, Alashwal BY. Keratin based bioplastic film from chicken feathers and its characterization. Int J Biol Macromol. 2018;111:352-358.
Crossref - Alashwal BY, Saad Bala M, Gupta A, Sharma S, Mishra P. Improved properties of keratin-based bioplastic film blended with microcrystalline cellulose: A comparative analysis. J King Saud Univ – Sci. 2020;32:853-857.
Crossref - Fenollar O, García D, Sánchez L, López J, Balart R. Optimization of the curing conditions of PVC plastisols based on the use of an epoxidized fatty acid ester plasticizer. Eur Polym J. 2009;45:2674-2684.
Crossref - Silverajah VSG, Ibrahim NA, Yunus WMZW, Hassan HA, Woei CB. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. Int J Mol Sci. 2012;13:5878-5898.
Crossref - Edwards A, Jarvis D, Hopkins T, Pixley S, Bhattarai N. Poly(å-caprolactone)/keratin-based composite nanofibers for biomedical applications. J Biomed Mater Res Part B Appl Biomater. 2015;103:21-30.
Crossref - Cao L, Tan H, Liu Y, Xue X, Zhou S. Characterization of a new keratinolytic Trichoderma atroviride strain F6 that completely degrades native chicken feather. Lett Appl Microbiol. 2008;46:389-394
Crossref - Ahmadpour F, Yakhchali B, Musavi MS. Isolation and identification of a keratinolytic Bacillus cereus and optimization of keratinase production. J Appl Biotechnol Reports. 2016;3:507-512.
- Cheng S-W, Hu H-M, Shen S-W, Takagi H, Asano M, Tsai Y-C. Production and Characterization of Keratinase of a Feather-degrading Bacillus licheniformis PWD-1. Biosci Biotechnol Biochem. 1995;59:2239-2243.
Crossref - Bach E, Daroit DJ, Corrêa APF, Brandelli A. Production and properties of keratinolytic proteases from three novel Gram-negative feather-degrading bacteria isolated from Brazilian soils. Biodegradation. 2011;22:1191-1201
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.