ISSN: 0973-7510
E-ISSN: 2581-690X
The rising demand for sustainable industrial practices necessitates the development of environmentally benign alternatives to traditional volatile organic solvents. Ionic liquids (ILs), characterized by their unique properties, represent a promising class of such alternatives. This study conducted a comprehensive ecotoxicological assessment of four textile-relevant ILs: 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]), 1-allyl-3-methylimidazolium chloride ([AMIM][Cl]), choline chloride ([Ch][Cl]), and tetraoctylammonium bromide ([TOA][Br]) or [N8888][Br]. The antimicrobial activity of these imidazolium- and ammonium-based ILs was investigated against Escherichia coli DH5α and Staphylococcus aureus MTCC 3103, serving as model Gram-negative and Gram-positive bacteria, respectively, utilizing the agar well diffusion method. Key findings revealed that while the positive control, tetracycline, established strong inhibition zones (approximately 25 mm for S. aureus, 22 mm for E. coli), IL toxicity was highly dependent on both chemical structure and solvent. In aqueous solution, the imidazolium-based [BMIM][Cl] showed significant activity, producing inhibition zones of approximately 18 mm against both bacterial strains. [TOA][Br] exhibited a dramatic increase in toxicity when dissolved in ethanol, generating inhibition zones exceeding 25 mm, a potency surpassing that of tetracycline. Instead, the hydrophilic [Ch][Cl] showed minimal activity even in ethanol. Findings also highlight the complexity and major influence of solvent interactions on the ecotoxicity of ILs. This research provides essential data for the informed design and sustainable application of ILs in industrial processes, emphasizing the critical need for thorough environmental risk assessment that extends beyond their inherent non-volatility.
Ionic Liquids (ILs), Antimicrobial Activity, Ecotoxicology, Solvent Effect, Escherichia coli, Staphylococcus aureus
The search for sustainable manufacturing practices has intensified the demand for environmentally benign solvents, driven by the urgent need to mitigate the risks associated with conventional organic solvents, which are often toxic, flammable, and volatile.1 Ionic liquids (ILs), with their unique combination of physicochemical properties have emerged as promising alternatives, offering attractive solutions for cleaner manufacturing processes.2,3 These organic salts, liquid at standard conditions, possess negligible vapor pressure, tunable solvation capabilities, and remarkable thermal stability.4 This versatility has fueled their exploration in diverse fields, including catalysis, separations, biotechnology, materials science, and consumer products.1,5,6 For example, in textile industries imidazolium-based IL [BMIM][Cl] is utilized as a solvent for degumming Tasar silk7 to hydrolyze cellulose,8 wool/cellulose mix films9 and [AMIM][Cl] aids in the recycling of polyester-cotton/cellulose-nylon blends and the processing of cellulose.10 Additionally promising are ammonium-based ILs, such as [TOA][Br], which is used to produce antimicrobial silver nanoparticles on cotton,11 choline chloride ([Ch][Cl]), which has the potential to biodegrade azo dyes,12 and wool fiber that dissolves in deep eutectic solvents.13 However, considering that ILs are regarded as “green” solvents, a thorough understanding of their environmental fate and toxicity is essential in order to promote their broad usage and ensure its sustainability. Since recent studies have shown that many ILs can be extremely harmful,1,14 a comprehensive knowledge of their toxicity and environmental impact is necessary. This emphasizes the necessity of conducting thorough ecotoxicological assessments prior to the widespread use of ILs. Indeed, the increasing use of ILs in industrial processes raises concerns about their potential release into aquatic environments through wastewater streams, underscoring the importance of developing effective strategies for their removal and biodegradation.5 To effectively assess the potential ecological impact of ILs and address these concerns, a comprehensive evaluation of their toxicity across diverse organisms is essential. Recent studies have explored the effects of ILs on a wide range of species, encompassing bacteria, yeast, invertebrates, fish, and even mammalian cell lines.15-19
However, traditional ecotoxicological methods often present significant hurdles.6 For example, determining minimum inhibitory concentrations (MIC) and minimum bactericidal/fungicidal concentrations (MBC/MFC) relies on planktonic susceptibility assays and necessitates considerable microbiological expertise for the preparation of test cultures, media, and dilution series under sterile conditions.20-24 These conventional methods are often costly, labor-intensive, and require specialized expertise.6 This highlights the urgent need for simpler, more rapid, and cost-effective toxicity screening methods that can be readily implemented. Microbial assays emerge as a particularly powerful approach for the early identification and elimination of potentially harmful ILs, given their inherent advantages of rapid growth, ease of cultivation, and minimal resource requirements. Building upon the advantages of microbial assays, this study employs agar diffusion tests as a streamlined and readily accessible approach for preliminary ecotoxicological assessments.1,14,25 Agar diffusion tests provide a rapid and cost-effective alternative for evaluating the toxicity of ILs in solid media. This widely employed methodology, frequently utilized to assess the susceptibility of microorganisms to diverse chemical compounds, including ionic liquids, offers several advantages: it is procedurally simple, economically viable, requires minimal preparation and no specialized equipment, utilizes small quantities of the test compounds, and can be readily implemented by researchers with fundamental microbiological skills.1,14 By connecting the sensitivity and adaptability of microorganisms, these assays enable researchers to efficiently screen and prioritize ILs, paving the way for the development of truly sustainable and environmentally benign solvents.
Main focus of the study is ecotoxicological evaluation of four ionic liquids (ILs) exhibiting promising applicability in textile processing. Imidazolium-based ILs, such as 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]) and 1-allyl-3-methylimidazolium chloride ([AMIM][Cl]), Tetraoctylammonium bromide ([TOA][Br]) or [N8888][Br], Choline chloride ([Ch][Cl]). Despite the growing interest in these ILs for textile applications, their potential impact on microbial communities and their biodegradability in natural environments remain largely unexplored. This knowledge gap hinders the ability to fully assess the environmental risks associated with their use and to develop effective bioremediation strategies.
Therefore, this study aims to address this critical knowledge gap by conducting a comprehensive ecotoxicological evaluation of the four selected ILs on Escherichia coli and Staphylococcus aureus, serving as representative microbial models and providing a preliminary assessment of their potential toxicity (IL toxicity on E. coli and S. aureus). E. coli serves as a reliable indicator of water contamination and provides insights into potential impacts on aquatic ecosystems, whereas S. aureus, a prevalent human pathogen, highlights possible threats to mammalian health and allows for the assessment of potential risks to human health, should these ILs be released into the environment. Both organisms are well-characterized, easy to cultivate, and sensitive to a range of toxicants, making them suitable for this ecotoxicological evaluation. This approach seeks to contribute to a more comprehensive understanding of the environmental fate of ILs and to promote their sustainable application in various industrial processes.
Chemicals and bacterial strains
Four ionic liquids (ILs) were selected for this study: two imidazolium-based ILs (1-butyl-3-methylimidazolium chloride, [BMIM][Cl]; 1-allyl-3-methylimidazolium chloride, [AMIM][Cl]) and two ammonium-based ILs (choline chloride, [Ch][Cl]; tetraoctylammonium bromide, [TOA][Br] or [N8888][Br]). [AMIM][Cl], [Ch][Cl], and [TOA][Br] were purchased from Ottokemi (Maharashtra, India), while [BMIM][Cl] was purchased from Sisco Research Laboratories Pvt. Ltd. (SRL) (Mumbai, India). Their purities were as follows: [AMIM][Cl] (97%), [Ch][Cl] (99%), [TOA][Br] (98%), and [BMIM][Cl] (98%). All ILs were in solid state at room temperature (20 °C). Model microorganisms Escherichia coli DH5α (donated by Dr. Anil Kumar, Central University of Jharkhand) and Staphylococcus aureus MTCC3103 (procured from the Microbial Type Culture Collection and Gene Bank (MTCC) at the Institute of Microbial Technology (IMTECH), Chandigarh, India) were chosen for this study. Both strains are well-characterized in antimicrobial susceptibility testing and are relevant to environmental (E. coli) and human health (S. aureus) concerns. Stock cultures were maintained at 4 °C on Luria-Bertani (LB) agar slants. Prior to each experiment, overnight cultures were prepared by inoculating a single colony into LB broth and incubating at 37 °C with shaking at 120 rpm for 24 hours.
Antibiotic Susceptibility Test for the Selection of Positive Control
To establish a baseline for bacterial sensitivity and identify a potent antimicrobial agent to serve as a positive control for subsequent assays, an antibiotic susceptibility test using the Kirby-Bauer disk diffusion method was conducted. This widely recognized technique allows for the assessment of bacterial susceptibility to a panel of antibiotics by measuring the zones of inhibition produced by antibiotic-impregnated disks on agar plates.26,27 Both E. coli and S. aureus were challenged with a diverse array of antibiotics, including erythromycin (15 µg/disc), amoxicillin/clavulanate (30 µg/disc), tetracycline (30 µg/disc), vancomycin (30 µg/disc), penicillin-G (10 µg/disc), clindamycin (2 µg/disc), ampicillin/sulbactam (20 µg/disc), and ceftazidime (30 µg/disc). Sterile Luria-Bertani (LB) (Composition in gL-1 Tryptone: 10 g/L, Yeast extract: 5 g/L, Sodium chloride: 10 g/L). Agar plates were evenly spread with bacterial suspensions adjusted to an optical density
(OD600) of 0.5 to ensure uniform bacterial growth across the surface. Antibiotic discs were then carefully placed on the inoculated agar surface, and a 0.9% (w/v) NaCl solution was included as a negative control to monitor any potential effects of the solvent alone. Following incubation at 37 °C for 24 hours, the diameters of the resulting zones of inhibition were precisely measured using a Vernier caliper.1 Each antibiotic was tested in triplicate to ensure reproducibility. The antibiotic demonstrating the most pronounced inhibitory effect against both E. coli and S. aureus, as evidenced by the largest zone of inhibition, was selected as the positive control for subsequent ecotoxicity assays.
Ecotoxicity Study by Agar Well Diffusion Assay
Following the establishment of a baseline for bacterial sensitivity by antibiotic susceptibility test, the agar well diffusion method was employed to assess the inhibitory effects of the selected ionic liquids (ILs) on microbial growth. This standardized assay, widely utilized in antimicrobial susceptibility testing, involves measuring the zone of inhibition surrounding a diffusion source as a quantitative indicator of antimicrobial activity.1,28,29 Tetracycline, identified as the most potent inhibitor in the preceding susceptibility tests, served as the positive control. An aqueous solution of 0.9% (w/v) NaCl served as the negative control to account for any potential effects of the solvent vehicle. Two bacterial species, Escherichia coli DH5α and Staphylococcus aureus MTCC3103, were selected as target organisms based on their contrasting morphologies, well-characterized sensitivities to antimicrobial agents, and relevance in assessing potential environmental and human health risks. Stock cultures were meticulously maintained under optimal growth conditions (4 °C in LB broth) to ensure consistent viability and reproducibility. The assay proceeded as follows: actively growing cultures of the target microorganisms, established in Luria-Bertani medium, were standardized and uniformly spread (1 mL) onto sterile glass plates. Wells (6 mm diameter) were aseptically punched into the agar surface within a laminar flow chamber. 100 µl of each IL was added to the well (if IL was in solid state than solvent was added dropwise to convert it in liquid state).1,30 Following incubation at 37 °C for 24 hours, the diameters of the resultant zones of inhibition were precisely measured using a vernier caliper.1 All ILs were tested in triplicate, and the reported zone of inhibition represents the average of three replicates, with the standard deviation calculated to assess inter-replicate variability.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0.1.244. A two-way analysis of variance (ANOVA) was conducted to assess the effects. Data are presented as mean ± standard deviation (SD) or standard error of the mean (SEM), as specified. A p-value of <0.05 was considered statistically significant.
Antibiotic susceptibility and selection of positive control
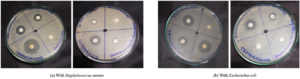
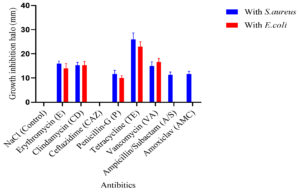
To establish a baseline for bacterial sensitivity and identify a potent antimicrobial agent to serve as a positive control for subsequent assays, antibiotic susceptibility testing was conducted on Escherichia coli DH5α and Staphylococcus aureus MTCC3103 using the Kirby-Bauer disk diffusion method.26 This standardized assay evaluates the susceptibility of bacteria to a panel of antibiotics by measuring the zones of inhibition formed around antibiotic-impregnated disks on agar plates. Eight antibiotics from diverse classes were tested, and the resulting zones of inhibition for E. coli and S. aureus are shown in Figure 1. The bacterial species (p <0.0001), ionic liquid treatment (p <0.0001), and their combination (p <0.0001) all had statistically significant effects on antimicrobial activity, according to a two-way ANOVA (Figure 2). For S. aureus, the maximum inhibition zone was observed with tetracycline, while the minimum was observed with ampicillin/sulbactam (A/S) and no inhibition zone with ceftazidime. In the case of E. coli, the maximum inhibition zone was observed with tetracycline, the minimum with penicillin-G, and no inhibition zone was observed with ceftazidime (CAZ), ampicillin/sulbactam (A/S), and amoxicillin/clavulanate (AMC). Based on these results, tetracycline, exhibiting the largest inhibition zone against both bacterial species, was selected as the positive control for subsequent ecotoxicity assessments.
Figure 2. Selection of positive control using antibiotic susceptibility test against Staphylococcus aureus and Escherichia coli
Ecotoxicity assessment of ionic liquids
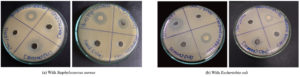
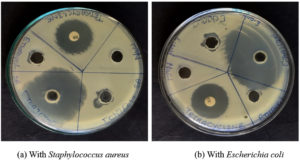
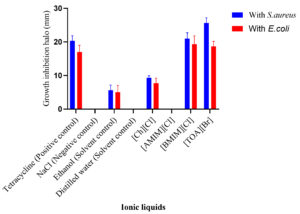
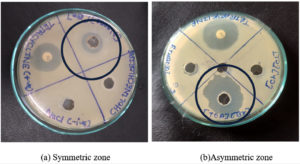
The ecotoxicological impact of four selected ionic liquids ([Ch][Cl], [AMIM][Cl], [BMIM][Cl], and [TOA][Br]) was rigorously evaluated using the agar well diffusion method. This standardized assay, widely employed in antimicrobial susceptibility testing, involves measuring the zone of inhibition surrounding a diffusion source as a quantitative indicator of antimicrobial activity.1,14,29 Tetracycline, exhibiting a pronounced inhibitory effect in prior antibiotic susceptibility testing, served as the positive control. An aqueous solution of 0.9% (w/v) NaCl functioned as the negative control. Among the studied ILs, the imidazolium-based ILs ([AMIM][Cl] and [BMIM][Cl]) demonstrated complete solubility in water, whereas the ammonium-based ILs ([TOA][Br] and [Ch][Cl]) exhibited limited water solubility. When dissolved in water, [BMIM][Cl] exerted the most significant inhibitory effect against both S. aureus and E. coli, followed by [TOA][Br], while [Ch][Cl] and [AMIM][Cl] showed no discernible inhibition (Figure 3). However, upon dissolving [TOA][Br] and [Ch][Cl] in ethanol to enhance their solubility, a marked shift in inhibitory potency was observed (Figure 4).6 [TOA][Br] exhibited the most pronounced inhibitory effect, surpassing even the positive control (tetracycline), indicating its heightened toxicity in this solvent. Conversely, [Ch][Cl] exhibited a smaller inhibition zone when dissolved in ethanol. Figure 5 results revealed varying degrees of inhibition zones of both bacterial species to the tested ionic liquids, where the results were analyzed by two-way ANOVA showing that the type of ionic liquid (p <0.0001), the species of bacteria (p <0.0001), and their combination (p = 0.0010) had a significant impact. These observations have shown the great influence of solvent on the biological activity of ILs and highlight the critical importance of considering solubility when assessing their ecotoxicity. Furthermore, the agar well diffusion assays showed two distinct patterns of inhibition zones: symmetric and asymmetric. Symmetric inhibition zones, as shown in Figure 6a, characterized by a uniform, circular zone of inhibition around the well, indicative of consistent diffusion of the IL through the agar medium. In contrast, asymmetric inhibition zones, such as those observed in
Figure 6b exhibits an irregular or non-uniform shape, showing uneven diffusion of the IL through the agar. The reason for asymmetry may be because of factors, including inconsistencies in the agar medium, interactions between the IL and agar components, the inherent properties of the IL (e.g., viscosity, surface tension), and bacterial motility.1,14,30
Figure 3. Inhibition zone of ionic liquids in distilled water against (a) Staphylococcus aureus and (b) Escherichia coli
Figure 4. Inhibition zones of [TOA][Br] and [Ch][Cl] ionic liquids in ethanol against (a) Staphylococcus aureus and (b) Escherichia coli
Figure 5. Antimicrobial activity of selected ionic liquids against Staphylococcus aureus and Escherichia coli
This study presents a comprehensive ecotoxicological assessment of four textile-relevant ionic liquids (ILs)-1-butyl-3-methylimidazolium chloride ([BMIM][Cl]), 1-allyl-3-methylimidazolium chloride ([AMIM][Cl]), choline chloride ([Ch][Cl]), and tetraoctylammonium bromide ([TOA][Br])-using a comprehensive approach involving antibiotic susceptibility testing and ecotoxicological assessment. These ILs, representing imidazolium- and ammonium-based classes, were examined for their antimicrobial effects on E. coli DH5α and S. aureus MTCC3103.
Antibiotic susceptibility testing of E. coli DH5α and S. aureus MTCC3103, two model organisms with contrasting cell wall architectures, was conducted using the Kirby-Bauer disk diffusion method.26,27 Among the eight antibiotics tested, tetracycline consistently produced the most significant zones of inhibition in both strains, indicating its broad-spectrum efficacy. As illustrated in Figure 1, for S. aureus, tetracycline yielded a mean inhibition zone of approximately 25 mm, while for E. coli, it was approximately 22 mm. In contrast, ampicillin/sulbactam showed minimal inhibition against S. aureus (approx. 10 mm), and no inhibition was observed with ceftazidime for S. aureus. For E. coli, penicillin-G resulted in minimal inhibition (approx. 8 mm), and ceftazidime, ampicillin/sulbactam, and amoxicillin/clavulanate elicited no inhibition zone. Given its superior inhibitory effect on both bacterial species, tetracycline was selected as the positive control for subsequent ecotoxicity assays, with 0.9% (w/v) NaCl serving as the negative control.
The ecotoxicological analysis of the selected ILs highlights the complex interchange between their chemical structure, solubility, and interactions with microbial systems. The agar well diffusion method, a standardized assay widely utilized in antimicrobial susceptibility testing, revealed varying degrees of antimicrobial activity (Figure 3).1,14,29 When the ILs were assessed using water as a solvent (or water was added dropwise just to convert solid ILs in liquid state),30 significant differences in toxicity were observed (Figure 3). The imidazolium-based IL, [BMIM][Cl], demonstrated the most potent inhibitory effect against both S. aureus and E. coli, with an average inhibition zone of approximately 18 mm for both strains. This is consistent with previous findings that link increased alkyl chain length and hydrophobicity in imidazolium cations to enhanced membrane disruption in microbial cells.20-24 Since [BMIM][Cl] is hydrophobic, it probably integrates more easily into bacterial membranes, changing permeability and causing cell death. In addition, [TOA][Br] exhibited some inhibitory activity when dissolved in water, with inhibition zones for both bacteria measuring nearly 10-12 mm. Conversely, in aqueous solution (about 0-5 mm), [Ch][Cl] and [AMIM][Cl] showed little to no evidence of inhibition. The high hydrophilic nature of [Ch][Cl], restricts its ability to interact with the bacterial cell membrane. One important observation was that the solvent used significantly affected the performance of the ionic liquids, particularly for [TOA][Br] and [Ch][Cl]. The volume of ILs that had an impact on the bacteria depended on what they were dissolved in (Figure 4). While [TOA][Br] displayed minimal activity in aqueous form, its dissolution in ethanol markedly enhanced its inhibitory potency. When dissolved in ethanol, [TOA][Br] exhibited the most pronounced inhibitory effect among all tested ILs, generating extensive inhibition zones exceeding 25 mm for both S. aureus and E. coli. This significantly surpassed the positive control, tetracycline, which showed inhibition zones of approximately 25 mm for S. aureus and 22 mm for E. coli. This heightened toxicity of [TOA][Br] in ethanol suggests that ethanol likely enhances IL diffusion in the agar, thereby increasing its bioavailability and toxicity-an effect previously reported for hydrophobic or poorly water-soluble ILs.6 Conversely, [Ch][Cl] showed a smaller inhibition zone (approx. 5-7 mm) when dissolved in ethanol, whereas it had no activity in water. These observations underscore the profound influence of solvent on the biological activity of ILs and highlight the critical importance of considering solubility when assessing their ecotoxicity.
Furthermore, the agar well diffusion assays revealed two distinct patterns of inhibition zones: symmetric and asymmetric. Symmetric inhibition zones, exemplified by Figure 6a, are characterized by a uniform, circular zone, indicative of consistent diffusion of the IL through the agar medium. In contrast, asymmetric inhibition zones, such as those observed with (Figure 6b), exhibited an irregular or non-uniform shape, suggesting uneven diffusion. This asymmetry can arise from several factors, including inconsistencies in the agar medium, interactions between the IL and agar components, the inherent properties of the IL (e.g., viscosity, surface tension), and bacterial motility.1,6,14 The comparative antimicrobial assessment of ILs also emphasized the structural importance of both cation and anion components. While cations significantly influenced toxicity, particularly imidazolium and tetraalkylammonium types, the anion contribution appeared secondary but still relevant, especially in the ethanol-solubilized [TOA][Br].
This ecotoxicological assessment of four ionic liquids (ILs) commonly used in textile processing provides critical insights into their antimicrobial activity against Escherichia coli and Staphylococcus aureus, demonstrating a significant interplay between IL structure and solvent type. Findings, derived from the simple and effective agar well diffusion method, reveal distinct toxicity profiles. In aqueous solution, the imidazolium-based [BMIM][Cl] exhibited the highest inhibitory effect against both bacterial strains, producing average inhibition zones of approximately 18 mm. In contrast, [TOA][Br] showed moderate inhibition (10-12 mm), while [Ch][Cl] and [AMIM][Cl] displayed negligible or no activity (0-5 mm). Mainly, the solvent impacted IL effectiveness, particularly for less water-soluble compounds. When dissolved in ethanol, [TOA][Br] emerged as a remarkably potent inhibitor, generating extensive inhibition zones exceeding 25 mm for both S. aureus and E. coli, a performance that significantly surpassed the positive control, tetracycline (25 mm for S. aureus, 22 mm for E. coli). This highlights a critical solvent-dependent potentiation of antimicrobial action for certain ILs. Furthermore, the observation of both symmetric and asymmetric inhibition zones suggests varied diffusion characteristics of these ILs within the agar, offering valuable initial insights into their potential environmental fate and behaviour in microbial systems.
This study emphasizes that without a thorough context-specific ecotoxicological evaluation, simple “green solvent” labelling for ILs is inappropriate. For the microbiology community, these findings emphasize the need for a fine approach to IL application and remediation. Designing ILs with reduced solvent-enhanced toxicity or figuring out the metabolic pathways utilized by IL-degrading bacteria, evaluating their wider IL degradation capacities, and optimizing bioremediation strategies to mitigate IL contamination biodegradability screening protocols should be the main goals of future research. Moreover, a deeper investigation into the long-term ecological impacts of ILs on complex microbial communities, coupled with the design of novel, less toxic, and more biodegradable ILs, is paramount for ensuring their sustainable utilization in industrial processes.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the Department of Life Sciences, Central University of Jharkhand, for providing the necessary laboratory facilities and support throughout the study and Dr. Anil Kumar, Assistant Professor, Central University of Jharkhand, for kindly providing us Escherichia coli bacterial stain used in this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
TL conceptualized the study, designed the experiments, performed data analysis and wrote the manuscript. AS supervised the study, reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Ventura SPM, de Barros RLF, Sintra T, Soares CMF, Lima AS, Coutinho JAP. Simple screening method to identify toxic/non-toxic ionic liquids: Agar diffusion test adaptation. Ecotoxicol Environ Saf. 2012;83:55-62.
Crossref - Rebros M, Gunaratne HQN, Ferguson J, Seddon KR, Stephens G. A high throughput screen to test the biocompatibility of water-miscible ionic liquids. Green Chemistry. 2009;11(3):402-440.
Crossref - Bubalo MC, Radosevic K, Redovnikovic IR, Slivac I, Sreek VG. Toxicity mechanisms of ionic liquids. Arh Hig Rada Toksikol. 2017;68(3):171-179.
Crossref - Zhang C, Wang H, Malhotra SV, Dodge CJ, Francis AJ. Biodegradation of pyridinium-based ionic liquids by an axenic culture of soil Corynebacteria. Green Chem. 2010;12(5):851-885.
Crossref - Gomez-Herrero E, Tobajas M, Polo A, Rodriguez JJ, Mohedano AF. Toxicity and inhibition assessment of ionic liquids by activated sludge. Ecotoxicol Environ Saf. 2020;187:109836.
Crossref - Wood N, Stephens G. Accelerating the discovery of biocompatible ionic liquids. Physical Chemistry Physics. 2010; 12(8): 1670.
Crossref - Vyas SK, Shukla SR. Degumming of Tasar silk using imidazolium-based ionic liquids. J Text Inst. 2020;111(9):1364-1370.
Crossref - Xing L, Wu Z, Gong G. Dissolution of Cotton Cellulose with Ionic Liquids 1-Butyl-3-Methylimidazolium Chloride and 1-Allyl-3-Methylimidazolium Chloride to Prepare Reducing Sugar. J Energy Eng. 2014;140(2):121.
Crossref - Hameed N, Guo Q. Blend films of natural wool and cellulose prepared from an ionic liquid. Cellulose. 2010;17(4):803-813.
Crossref - Yuan J, Wang Q, Fan X, Wang P. Enhancing Dye Adsorption of Wool Fibers with 1-butyl-3-methylimidazolium Chloride Ionic Liquid Processing. Textile Research Journal. 2010;80(18):1898-1904.
Crossref - Tarimala S, Kothari N, Abidi N, Hequet E, Fralick J, Dai LL. New approach to antibacterial treatment of cotton fabric with silver nanoparticle-doped silica using sol-gel process. J Appl Polym Sci. 2006;101(5):2938-2943.
Crossref - Sekar S, Surianarayanan M, Ranganathan V, MacFarlane DR, Mandal AB. Choline-based ionic liquids-enhanced biodegradation of azo dyes. Environ Sci Technol. 2012;46(9):4902-4908.
Crossref - Wang D, Tang RC. Dissolution of wool in the choline chloride/oxalic acid deep eutectic solvent. Mater Lett. 2018;231:217-220.
Crossref - Missoun F, de los Rios AP, Ortiz-Martinez V, Salar-Garcia MJ, Hernandez-Fernandez J, Hernandez-Fernandez FJ. Discovering low toxicity ionic liquids for Saccharomyces cerevisiae by using the agar well diffusion test. Processes. 2020;8(9).8091163.
Crossref - Bernot RJ, Kennedy EE, Lamberti GA. Effects of Ionic Liquids on the Survival, Movement, and Feeding Behavior of the Freshwater Snail. Physa Acuta. 2005;24(7):1759-1965.
Crossref - Pretti C, Chiappe C, Pieraccini D, et al. Acute toxicity of ionic liquids to the zebrafish (Danio rerio). Green Chem. 2006;8(3):238-240.
Crossref - Ranke J, Cox M, Muller A, Schmidt C, Beyersmann D. Sorption, cellular distribution, and cytotoxicity of imidazolium ionic liquids in mammalian cells – Influence of lipophilicity. Toxicol Environ Chem. 2006;88(2):273-285.
Crossref - Cho CW, Pham TPT, Zhao Y, Stolte S, Yun YS. Review of the toxic effects of ionic liquids. Sci Total Environ. 2021;786:47309.
Crossref - Goncalves ARP, Paredes X, Cristino AF, Santos FJV, Queiros CSGP. Ionic liquids-a review of their toxicity to living organisms. Int J Mol Sci. 2021;22(11):5612.
Crossref - Pernak J, Sobaszkiewicz K, Mirska I. Anti-microbial activities of ionic liquids. Green Chem. 2003;5(1):52-56.
Crossref - Pernak J, Goc L, Mirska I. Anti-microbial activities of protic ionic liquids with lactate anion. Green Chem. 2004; 6: 323-329.
Crossref - Demberelnyamba D, Kim KS, Choi S, et al. Synthesis and antimicrobial properties of imidazolium and pyrrolidinonium salts. Bioorg Med Chem. 2004;12(5):853-857.
Crossref - Megaw J, Busetti A, Gilmore BF. Isolation and Characterisation of 1-Alkyl-3-Methylimidazolium Chloride Ionic Liquid-Tolerant and Biodegrading Marine Bacteria. PLoS One. 2013;8(4):806.
Crossref - Luczak J, Jungnickel C, Lacka L, Stolte S, Hupka J. Antimicrobial and surface activity of 1-alkyl-3-methylimidazolium derivatives. Green Chem. 2010;12(4):593-60.
Crossref - Samori C, Pasteris A, Galletti P, Tagliavini E. Acute Toxicity of Oxygenated and Nonoxygenated Imidazolium-Based Ionic Liquids To Daphnia magna And Vibrio fischeri. Environ Toxicol Chem. 2007;26(11):2379-2382.
Crossref - Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin Infect Dis. 2009;49(11):1749-1755.
Crossref - Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. 2009. https://asm.org/protocols/kirby-bauer-disk-diffusion-susceptibility-test-pro
- Fernandes MM, Carvalho EO, Correia DM, et al. Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia coli. ACS Appl Bio Mater. 2022;5(11):5181-5189.
Crossref - Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M. Evaluation of Different Detection Methods of Biofilm Formation in the Clinical Isolates. Braz J Infect Dis. 15(4)305-311.
Crossref - Wood N, Ferguson JL, Gunaratne HQN, Seddon KR, Goodacre R, Stephens GM. Screening ionic liquids for use in biotransformations with whole microbial cells. Green Chemistry. 2011;13(7):1843-1851.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.