ISSN: 0973-7510
E-ISSN: 2581-690X
Foot rot disease (Phytophthora capsici) causes severe economic losses to cultivators of black pepper. Fungicides used for managing the disease adversely affect the export potential of the product due to their residual toxicity. Endospore-forming bacterial strains, Bacillus amyloliquefaciens VLY24, Bacillus pumilus VLY17, and Bacillus velezensis PCSE10 were evaluated for growth promotion and disease suppression in black pepper nursery at two locations in Kerala, India. All the isolates could suppress Phytophthora capsici under in vitro conditions. Spraying detached leaves with a cell suspension of the bacterial isolates could delay the development and progression of lesion. In the in vivo trials, bacterized cuttings had a better establishment in the nursery when compared to uninoculated control. Plant growth parameters like the number of leaves, roots, and shoots were more in bacterized cuttings. Cuttings treated with Bacillus pumilus VLY17 when challenge inoculated with the pathogen, showed 84.74 percent reduction in the size of lesions on the leaves, and showed the least disease index (0.27) compared to pathogen inoculated control. It is proposed that endospore forming Bacillus spp. with anti-oomycetic activity could be potential biocontrol agents against foot rot disease of black pepper.
Black Pepper, Endospore-forming Bacteria, Phytophthora capsici, Biocontrol, Plant Growth Promotion
Black pepper (Piper nigrum L.), also called “black gold”, is a highly prized spice due to its versatility, as it can flavor food, serve as a preservative, and has several health benefits. Black pepper is indigenous to the tropical forests of the Malabar coast in southwest India. Kerala and Karnataka contribute to the lion’s share of the black pepper production in India. Black pepper cultivation thrives in the humid sub-mountainous regions of the Western Ghats as it is a tropical vine that prefers high humidity and rainfall. Phytophthora foot rot is the most destructive and pervasive disease affecting black pepper in all pepper-growing areas worldwide. The crop loss due to this disease is one of the setbacks faced by black pepper growers in Kerala, especially during the southwest monsoon season.1 The leaves, stems, and roots system of the plants are affected by the disease resulting in defoliation and, in extreme cases, wilting and complete death of the vines.
The standard management strategies include phytosanitary measures, routine preventive spraying of vines, and soil drenching with synthetic fungicides. However, synthetic fungicides have high costs, residual toxicity, and protracted degradation time which adversely affect human health.2 Furthermore, there is a growing global demand for organically grown spices. Biocontrol thus becomes vital in the organic production of black pepper.3 In high-value crops, microorganisms can be employed successfully as a secure alternative to traditional plant health management techniques.
Endophytic Bacillus spp. that produce endospores have garnered more interest recently as biocontrol agents because they possess a number of characteristics that make them suitable candidates for field-level application. The endospore-forming ability of Bacillus spp. can be utilized to develop formulations that give rise to stable products with an extended shelf life. Piper spp. harbor numerous endospore-forming bacterial endophytes, and Bacillus velezensis PCSE10, an isolate from Piper colubrinum, showed enhancement in growth parameters of black pepper cuttings and suppression of Phytophthora foot rot.4,5 This study was undertaken recognizing the importance of endospore-forming bacteria in plant growth promotion and their role in the management of Phytophthora foot rot.
Microbial cultures
Three Bacillus isolates with proven biocontrol and plant growth promotion activity, viz., Bacillus amyloliquefaciens VLY24, Bacillus pumilus VLY17, and Bacillus velezensis PCSE10, the oomycete pathogen, Phytophthora capsici, available at the Department of Agricultural Microbiology, College of Agriculture, Vellayani, Kerala were used. The pathogenicity of Phytophthora capsici was tested by the method outlined by Anith et al.6 Nutrient Agar (NA) was used for growing Bacillus spp. and Potato Dextrose Agar (PDA) for P. capsici.
Antagonism of Bacillus isolates against Phytophthora capsici
A dual culture plate assay was carried out on potato dextrose agar (PDA) medium to assess the percentage inhibition of P. capsici by the bacterial isolates.7
Percentage growth inhibition = [C – T / C] X 100
where C is the diameter (cm) of P. capsici growth on control plates, T is the diameter of P. capsici growth on dual culture plates. The experiment was laid out in Completely Randomised Design (CRD) with five replications.
In-vitro screening of Bacillus spp. for disease suppression
The biocontrol potential of antagonistic bacterial isolates against P. capsici was investigated using detached leaf assay in the black pepper variety Karimunda. A single colony of Bacillus isolate was cross-streaked heavily on nutrient agar (NA) plates and incubated for 24 h. The bacterial cell suspension was prepared by adding 10 mL sterile distilled water to the plates and aseptically collected as described by Varkey et al.8 The suspension was sprayed on leaves detached from the vines of variety Karimunda. Excess moisture on the leaves was removed by placing them in a laminar airflow chamber. Phytophthora capsici was inoculated on the treated leaves. Untreated leaves inoculated with pathogen served as control. The time duration for lesion appearance on leaves and lesion size (cm) was recorded on the second and third day after inoculation to assess the effectiveness of bacterial endophytes.
Indole acetic acid production by Bacillus spp.
The plant growth promotion potential of the isolates was tested by estimating the IAA production.9 The isolates were cultured in nutrient broth, amended with or without tryptophan (0.1%), and incubated at 28°C for 48 hours with constant shaking at 100 rpm. Uninoculated nutrient broth was kept as a control. After five days of incubation, 2 mL of cell-free supernatant was transferred to a test tube, two drops of orthophosphoric acid and 4 mL Salkowski reagent was added to it, and incubated in the dark for 25 min to complete the reaction. The standard curve of IAA was prepared with different concentrations starting from 1 to 100 ppm. Spectrophotometric absorbance at 530 nm was compared with the standard curve to determine the concentration of IAA.
Assessment of growth promotion by the bacterial isolates on black pepper
The potential of individual Bacillus isolates and a consortium of the three isolates to promote the growth of black pepper plants (varieties Panniyur-1 and Karimunda) in two different regions of Kerala; Ambalavayal (high range area) and Balaramapuram (southern laterite lowland) was evaluated in vivo. These varieties were chosen as they are popular in their respective regions. The pot culture experiment was conducted using three-node cuttings of black pepper bacterized with a cell suspension of Bacillus spp. (107 CFU mL-1) as described earlier.10 Cuttings planted without bacterization served as control. The planted cuttings were maintained in a polyhouse and irrigated daily. Biometric observations of the cuttings were taken 120 days after planting (DAP). A Completely Randomised Design with four replications and 15 plants per replication was maintained.
Relative abundance of endospore-forming bacterial isolates in the rhizosphere
Endospore forming bacterial colonization in roots of treated plants was assessed at 120 DAP following the method described by Yashaswini et al.11 Two plants were randomly selected from each treatment and roots were tapped well to remove adhering soil. A two-step enrichment technique assessed the abundance of endospore formers. One gram root sample was oven dried at 35°C for 2 days to kill non-endospore formers. The samples were added to 9 mL sterile water, thoroughly vortexed and heated at 80°C for 10 minutes in a water bath. The samples were then serially diluted, plated in NA medium and incubated at 28°C. Colony count was recorded after 24 hrs of incubation. The relative abundance of spore formers was worked out using the formula.
Relative abundance (%) = Pt – Pc / Pc x 100
where Pt is population in treated plants, Pc is the population in plants without bacterization expressed in CFU g-1
In vivo screening of Bacillus spp. against foliar infection by P. capsici in black pepper nursery
In vivo disease suppression potential of the individual Bacillus isolates and their consortium was evaluated in black pepper cuttings raised in polybags. Bacterial suspension (107 CFU mL-1) was sprayed on leaves of intact rooted cuttings at 110 DAP. The pathogen Phytophthora capsici was challenge inoculated seven days after the foliar spray. The inoculated leaves were observed daily and occurrence and progression of lesion development were recorded. Pathogen-inoculated plants and uninoculated plants served as the positive and negative control. Plants sprayed with copper oxychloride (0.2%) were kept as chemical check.
The disease severity was scored on a 0-5 scale based on the leaf lesion size as proposed by Kollakkodan et al.4 Leaf scoring is as follows:
Score Lesion size (cm)
0 No Lesion
1 0.1 – 2.0
2 2.1 – 3.0
3 3.1 – 4.0
4 4.1 – 5.0
5 > 5.0
The Disease index = Sum of indvidual ratings / No. of leaves assessed x Maximum grade used
Statistical analysis
The data were analysed by ANOVA and one factorial CRD using KAU GRAPES software.12 The treatments were compared using least significant differences at 5% probability.
In vitro screening of Bacillus isolates for suppression of Phytophthora capsici
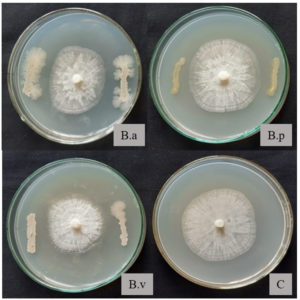
Dual culture plate assay indicated that all three test organisms suppressed the growth of P. capsici in-vitro. B. velezensis PCSE10 exhibited the maximum inhibition percentage (31.40%) (Table 1, Figure 1).
Table (1):
Inhibition of mycelial growth of Phytophthora capsici by endophytic Bacillus species in dual culture plate assay.
Bacillus species |
Diameter of P. capsici mycelial growth (cm) |
Percentage Inhibition (%)* |
|---|---|---|
B. amyloliquefaciens VLY24 |
4.45 ± 0.14c |
27.76 |
B. pumilus VLY17 |
5.10 ± 0.01b |
17.21 |
B. velezensisPCSE10 |
4.23 ± 0.12c |
31.33 |
Control |
6.16 ± 0.35a |
– |
SE(m) (±) |
0.11 |
– |
CD(0.05) |
0.40 |
– |
*Mean (± SD) of five replications. Values in a column followed by the same alphabet do not differ significantly (p≤0.05)
Figure 1. In-vitro inhibition of Phytophthora capsici by endospore-forming bacterial isolates (B.a – B. amyloliquefaciens VLY24, B.p – B. pumilus VLY17, B.v – B. velezensis PCSE10, C – control plate with P. capsici alone)
Detached leaf assay revealed the suppression of lesion development on leaves sprayed with bacterial suspension after challenge inoculation with the pathogen. While lesions developed in 24 h in leaves sprayed with sterile water, it was observed only after 48 h in B. pumilus VLY17 treated ones. Disease suppression of 97.20% was observed on the second day after inoculating P. capsici in B. pumilus VLY17 treated leaves. The average length of the lesion was the least in B. pumilus VLY17, which was found to be on par with the consortium treatment and other isolates except for the control (Table 2 and Figure 2).
Table (2):
Lesion development in detached leaf assay on pinprick inoculation with Phytophthora capsici.
| Treatments | Time taken for lesion appearance (hrs) |
Two days after inoculation | Three days after inoculation | ||
|---|---|---|---|---|---|
| Average length of lesion (cm) | Percent disease suppression | Average length of lesion (cm) | Percent disease suppression | ||
| B. amyloliquefaciens VLY24 | 36 | 0.56±0.20b | 76.40 | 1.60 ±0.52b | 78.41 |
| B.pumilus VLY17 | 48 | 0.10 ±0.10c | 97.20 | 0.46±0.25b | 93.66 |
| B.velezensis PCSE10 | 40 | 0.20±0.10c | 91.66 | 2.16±1.25b | 70.44 |
| Consortium of bacterial isolates | 40 | 0.12 ±0.03c | 95.12 | 0.35±0.31b | 95.22 |
| Control | 24 | 2.40±0.40a | – | 7.33±2.10a | – |
| SE(m)(±) | – | 0.11 | – | 0.46 | – |
| CD(0.05) | – | 0.40 | – | 1.58 | – |
*Mean (± SD) of four replications. Values in a column followed by the same alphabet do not differ significantly (p≤0.05)
Figure 2. Detached leaf assay showing lesion development two days after inoculation (2 DAI) and three days after inoculation (3 DAI) of P. capsici (B.a – B. amyloliquefaciens VLY24, B.p – B. pumilus VLY17, B.v – B. velezensis PCSE10, Consortium –all three Bacillus spp., Control –sterile water)
Assessment of growth promotion by the bacterial isolates on black pepper
All three isolates produced a significant amount of IAA, with B. velezensis PCSE10 (15.10 ppm) showing the highest IAA in media without tryptophan. Supplementation of L-Tryptophan in the growth medium resulted in a four-fold increase in IAA production by B. amyloliquefaciens VLY24 (23.34 ppm) (Table 3).
Table (3):
Indole acetic acid production by endophytic Bacillus species.
| Bacillus species | IAA (ppm)* | |
|---|---|---|
| Without L-Tryptophan | With L-Tryptophan | |
| B. amyloliquefaciens VLY24 | 5.98±0.023b | 23.34±0.60a |
| B. pumilus VLY17 | 4.72 ±0.28c | 9.34±0.15c |
| B. velezensis PCSE10 | 15.10 ±0.03a | 15.33±0.10b |
| SE(m)(±) | 0.09 | 0.20 |
| CD(0.05) | 0.33 | 0.69 |
*Mean (± SD) of three replications. Values in a column followed by the same alphabet do not differ significantly (p≤0.05)
Black pepper cutting with bacterization showed a better percentage establishment than the control (Table 4). The experiments conducted in two different black pepper growing locations revealed that the number of leaves, roots, shoot and root length, and root volume was significantly higher in plants treated with bacteria (Table 5). The fresh and dry weight of newly emerged shoots of bacterized plants increased significantly compared to the non-bacterized control (Table 6). Plants bacterized with B. pumilus VLY17 showed maximum biomass of newly emerged shoots and roots at both test locations.
Table (4):
Establishment of black pepper cuttings on bacterization with Bacillus isolates.
Treatments |
Percent establishment (%)*(60 DAP) |
|---|---|
B. amyloliquefaciens VLY24 |
55.55± 16.77a |
B. pumilus VLY17 |
70.00±10.00a |
B. velezensis PCSE10 |
48.88±10.18a |
Consortium of bacterial isolates |
50.00±10.00a |
Control |
19.99±11.54b |
SE(m)(±) |
6.92 |
CD(0.05) |
21.80 |
*Mean (+ SD) of four replications (n=60). Values in a column followed by the same alphabet do not differ significantly (p≤0.05). DAP-Days After Planting
Table (5):
Effect of bacterization of black pepper cuttings on growth parameters in nursery.
| Treatments | No. of leaves/plant* | No. of roots/plant* | Shoot length*(cm) | Root Length* (cm) | Root volume*(cm3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A# | B# | A# | B# | A# | B# | A# | B# | A# | B# | |
| B. pumilus VLY17 | 8.66 ± 0.57a | 7.00±1.73a | 5.33 ± 0.57c | 6.75±2.63b | 56.00 ± 3.00 a | 42.33±8.62a | 18.16 ± 0.76b | 26.90 ± 1.93a | 5.16 ± 0.15a | 6.66±2.61a |
| B. amyloliquefaciens VLY24 | 4.66 ± 0.57c | 5.33±0.57ab | 16.00 ± 1.00a | 16.75±1.50a | 33.66 ± 3.21b | 21.00±0.86b | 25.33 ± 4.50a | 19.16 ± 0.28b | 3.36 ± 0.15c | 2.73±0.57bc |
| B. velezensis
PCSE10 |
3.00 ± 1.00d | 4.66±0.57b | 12.00 ± 1.73b | 15.25±5.85a | 27.33 ± 2.08c | 36.33±1.60a | 15.00 ± 1.00b | 9.16 ± 0.76c | 3.33 ± 0.15c | 2.40±0.52bc |
| Consortium | 6.66 ± 0.57b | 6.33±1.15ab | 10.33 ± 0.57b | 11.75±3.40ab | 37.33 ± 3.21b | 37.66±3.51a | 18.16 ± 0.76b | 24.60 ± 3.73a | 4.00 ± 0.20b | 4.13±1.60ab |
| Control | 1.33 ± 0.57e | 1.66±0.57c | 2.33 ± 0.57d | 9.00±4.10b | 18.00 ± 1.00d | 12.16±2.93c | 10.00 ± 2.00c | 7.50 ± 1.32c | 1.33 ± 0.57d | 0.73±0.30c |
| SE(m)(±) | 0.39 | 0.59 | 0.57 | 1.89 | 1.58 | 2.56 | 1.32 | 1.15 | 0.17 | 0.82 |
| CD(0.05) | 1.24 | 1.87 | 1.81 | 5.70 | 4.81 | 8.07 | 0.64 | 3.64 | 0.54 | 2.58 |
*Mean (± SD) of four replications (n=60). Values in a column followed by the same alphabet do not differ significantly (p≤0.05). All readings taken at 120 days after planting.
# A- experiment conducted at Ambalavayal, Wayanad, Kerala with variety Panniyur 1 and B- experiment conducted at Balaramapuram, Thiruvananthapuram, Kerala with variety Karimunda
Table (6):
Effect of bacterization of black pepper cuttings on biomass.
| Treatments | Fresh weight* (g) of newly emerged shoots | Fresh weight* (g) of roots | Dry weight* (mg) of newly emerged shoots | Dry weight* (mg) of roots | ||||
|---|---|---|---|---|---|---|---|---|
| A# | B# | A# | B# | A# | B# | A# | B# | |
| B. pumilus VLY17 | 16.53 ± 0.75a | 13.10 ± 2.84a | 3.24 ± 0.10a | 3.22 ± 1.59 | 2514.33 ± 217.14a | 2274.86 ± 470.13a | 560.00 ± 115.33a | 450.20 ± 164.51a |
| B. amyloliquefaciens VLY24 | 7.53 ± 0.31c | 7.73 ± 1.03b | 1.82 ± 0.16b | 2.27 ± 0.47 | 1499.00 ± 52.50c | 1520.73 ± 255.72ab | 388.33 ± 8.02b | 340.76 ± 37.91ab |
| B. velezensis PCSE10 | 4.43 ± 0.21d | 5.66 ± 2.61bc | 1.30 ± 0.13c | 1.64 ± 0.55 | 1223.00 ± 11.53d | 1244.63 ± 359.80b | 182.00 ± 13.12cd | 182.66 ± 71.21b |
| Consortium | 9.50 ± 0.64b | 9.03 ± 5.45ab | 1.83 ± 0.05b | 2.62 ± 0.82 | 1999.66 ± 112.00b | 2161.80 ± 613.50a | 234.33 ± 15.04c | 405.90 ± 168.84a |
| Control | 3.08 ± 0.56e | 2.63 ± 0.39c | 0.98 ± 0.07d | 0.88 ± 0.06 | 633.33 ± 152.75e | 400.62 ± 294.28c | 103.00 ± 11.35d | 152.20 ± 28.80b |
| SE(m) (±) | 0.31 | 1.51 | 0.06 | 0.50 | 75.68 | 242.05 | 30.42 | 64.76 |
| CD (0.05) | 0.96 | 4.56 | 0.21 | NS | 238.481 | 762.73 | 95.89 | 204.10 |
*Mean (± SD) of four replications (n=60). Values in a column followed by the same alphabet do not differ significantly (p≤0.05). All readings taken at 120 days after planting.
# A- experiment conducted at Ambalavayal, Wayanad, Kerala with variety Panniyur 1 and B- experiment conducted at Balaramapuram, Thiruvananthapuram, Kerala with variety Karimunda
Abundance of root colonization by endospore-forming bacterial isolates
B. pumilusVLY 17 inoculated plants showed better root colonization by endospore formers. The consortium also showed a significantly higher percentage abundance of Bacillus spp. (Table 7.).
Table (7):
Population of endospore-forming bacteria in the rhizosphere of black pepper plants.
Treatments |
Log CFU g-1 |
Percentage abundance* |
|---|---|---|
B. pumilus VLY17 |
5.67 ± 0.02a |
41.75 |
B. amyloliquefaciens VLY24 |
5.04 ± 0.10d |
26.00 |
B. velezensis PCSE10 |
5.12 ± 0.02c |
28.12 |
Consortium of bacterial isolates |
5.40 ± 0.01b |
34.75 |
Control |
4.00 ± 0.00e |
0 |
SE(m)(±) |
0.02 |
|
CD(0.05) |
0.10 |
*Mean (± SD) of three replications. Values in a column followed by the same alphabet do not differ significantly (p≤0.05)
In vivo screening of Bacillus spp. against foliar infection incited by Phytophthora in black pepper nursery
Challenge inoculation of P. capsici on leaves of pepper cuttings showed variation depending on the treatment. The lesion size was the least in B. pumilus VLY17 treated plants (0.63 cm) when observed one week after inoculation. The chemical check and uninoculated control did not develop lesions (Table 8). B. pumilus VLY17 treatment showed an 87% reduction in lesion size compared to pathogen inoculated control. A disease index of 0.95 was observed in pathogen inoculated control, while B. pumilus VLY17 showed the least disease index of 0.27 among the Bacillus treatment (Figure 3).
Table (8):
Effect of bacterization on disease development in Phytophthora capsici inoculated leaves in black pepper nursery.
Treatments |
Lesion size* (cm) (7 DAI) |
Percentage reduction in lesion size over control (%)* (7 DAI) |
Disease Index* (7 DAI) |
|---|---|---|---|
B. pumilus VLY17 |
0.63 ± 0.10d |
87.74 |
0.27 ± 0.04d |
B. amyloliquefaciens VLY24 |
3.66 ± 0.15c |
29.03 |
0.54 ± 0.07c |
B. velezensis PCSE10 |
4.20 ± 0.28b |
19.35 |
0.62 ± 0.00b |
Consortium of bacterial isolates |
3.60 ± 0.10c |
30.32 |
0.54 ± 0.02c |
COC (0.2%) spray |
0.00e |
100 |
0.00 e |
Pathogen inoculated control |
5.20 ± 0.28a |
0 |
0.95 ± 0.02a |
Uninoculated control |
0.00e |
100 |
0.00 |
SE(m)(±) |
0.10 |
0.02 |
|
CD(0.05) |
0.30 |
0.05 |
*Mean (± SD) of three replications, each having independent 9 observations (n=27). Values in a column followed by the same alphabet do not differ significantly (p≤0.05). DAI-Days After Inoculation
Figure 3. Lesion development on representative leaf samples of black pepper plants treated with endospore-forming bacterial isolates after challenge inoculation with Phytophthora capsici (B.a – B. amyloliquefaciens VLY24, B.p – B. pumilus VLY17, B.v – B. velezensis PCSE10, Consortium –all three Bacillus spp., Control – sterile water)
The potential of any biological agents with antagonism can be initially screened by in vitro trials.7,13 The zone of inhibition generated in the dual culture experiment indicates the biocontrol capability of bacterial antagonists against P. capsici.14,15 The percentage inhibition can be used as a metric for determining antagonistic potential. Plant-associated bacteria isolated from several habitats showed in vitro inhibition of P. capsici growth, exhibiting the diversity of antagonistic bacteria across the living system. Competition, antibiosis, and mycoparasitism may be some of the disease suppressing features of antagonists.16-19 However, in the current screening approach, antibiosis as a pathogen inhibitory mechanism was given priority. In vitro inhibition of P. capsici by the isolates used in the study could be due to the generation of inhibitory material.
The dual culture approach did not include three interacting components involved in any disease development, viz., the host plant, pathogen, and antagonist. Anith et al.14 proposed that an experiment involving the interaction of the three, which more closely approximate the field settings, would transmit a strict basis for antagonism. Hence a detached leaf assay would be better than the dual culture plate assay. In our study, we observed a delay in the disease appearance when the leaves were treated with bacterial suspension (36-48 hrs) compared to the control (24 hrs). A similar delay in the development of symptoms was observed in earlier studies where antagonistic bacteria was sprayed on leaves.5,10 Bacillus cereus BT8 was previously reported to induce resistance against P. capsici in cacao in a detached leaf assay.20 The bacterial isolates used in the present study were able to control the disease and reduce the extent of the lesion development due to direct antagonism.
Indole 3-acetic acid (IAA) stimulates cell division, nodule formation, seed germination, root and shoot development, enhances the photosynthetic rate, and confers resistance to biotic and abiotic stress on plants. It is the main auxin produced using tryptophan as a starting material. Microorganisms can make IAA from IPA, indole-3-acetamide, tryptamine, and indole-3-acetaldehyde, by oxidizing the tryptophan side chain. As a result, the tryptophan-added medium may encourage bacteria that produce IAA to produce it more quickly. An L-tryptophan modified specific broth can be used to assess the IAA generation by bacterial isolates. IAA production has a good impact on root growth and development, which improves nutrient uptake.21 In our study, IAA production was observed in all three endospore-forming bacterial isolates in both cases. The amendment of nutrient broth with L-tryptophan enhanced the IAA production by B. pumilus VLY17 and B. amyloliquefaciens VLY24 indicating their potential for increasing the growth of plants in vivo.
Endospore-forming bacteria also deliver a range of growth-promoting benefits to crops. In a vegetatively propagated crop like black pepper, the bacterial antagonist must survive on the cuttings for 30 to 45 days until root initiation.14 In black pepper, endospore-forming bacteria were introduced into the plant prior to planting the cuttings in nursery.22 This type of bacterization with endospore-forming bacteria prevents Phytophthora infection and could be used to propagate healthy plants.
Major growth indices of black pepper plantlets were found to be improved significantly in plants bacterized with endospore-forming bacterial isolates. Tilak and Reddy23 reported that Bacillus spp. increased growth of corn, wheat and pigeon pea. In our study number of leaves and roots per plant increased on bacterization. According to Amaresan et al.,24 synthesis of IAA (59.22 g mL-1) by B. pumilus considerably increased root and shoot biomass of tomato and chili under greenhouse conditions. Wang et al.25 reported that bacterization of Capsicum annum with Bacillus spp. significantly affected plant growth aspects compared to untreated control. Fresh weight and dry weight of newly emerged shoot and root increased significantly on bacterized plants compared to control. There are similar reports on increased plant growth promotion by bacterization.5,10 Sziderics et al.26 reported the growth promotion effects of Bacillus strain EZB8 in Capsicum annuum in terms of increased fresh weight of roots, shoots and leaves. Vyshakhi and Anith27 found many endospore-forming Bacillus spp. conferring growth-promoting properties in vegetable seedlings in vitro. Several studies related to our work highlighting the growth-promoting potential of endospore-forming B. pumilus, B. amyloliquefaciens, and B. velezensis isolated from crop plants such as amaranth, chili, and pepper growing in similar environments are available.11,28,29 In most plant growth promotion aspects tested, B. pumilus treated plants showed the maximum increase among all the treatments. Anith30 evaluated the effect of Bacillus pumilus cultured in coconut water on growth parameters in black pepper cuttings and found significant improvement over the uninoculated control.
The success of using biocontrol agents to combat diseases caused by plant pathogens is highly dependent on the isolates’ action on the host plant, which in turn is dependent on the delivery of bacterial isolates. Seed treatment,31 bacterization of propagating plant material,15 soil application,32 and foliar spray have been proposed as methods to deliver bacterial biocontrol agents to host plants.33 In the current study, disease progression on plants treated with bacterial suspension was compared with COC-treated plants and pathogen-inoculated control. As observed in many other studies, a significant decrease in disease progression was observed in bacterized plants compared to control.5,10,11 Enrichment technique for endospore formers revealed that bacterization of cuttings had a positive effect on root colonization indicating plant growth promotion and disease suppression due to endospore-forming Bacillus spp. It is in consensus with the findings of Yashaswini et al.11 The early establishment and survival of microbial inoculants in plants is crucial for plant growth promotion and disease suppression attributed to them. The ability of the isolates used in the present study to form endospores enhances the chances for their survival in soil and plant, even under unfavorable conditions.
Foot rot disease, caused by P. capsici is the most damaging and debilitating of the numerous diseases that afflict black pepper. Despite the use of many chemical and biological techniques, the disease continues to spread, demanding a shift in management strategy. The knowledge gained from this study about endospore-forming bacterial isolates can aid in developing improved management strategies with increased biocontrol potential against Phytophthora-induced foot rot in black pepper. Antagonistic endospore-forming Bacillus spp. significantly increase growth and suppress Phytophthora foot rot disease, paving the way for developing possible antagonists as bioinoculants in black pepper.
ACKNOWLEDGMENTS
The authors would like to thank Kerala Agricultural University for providing facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KNA conceptualized and supervised the study. RP arranged resources. ABA and RP performed the experiments. SAR performed data analysis. CN, SAR and VIS wrote the manuscript. CN, VIS and KNA reviewed the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Sarma YR, Anandaraj M, Rajan PP. Phytophthora, A threat to black pepper: Present status and future strategies of disease management. Spice India 1994;7:10-13.
- Meena VS, Bahadur I, Maurya BR, et al. Potassium-Solubilizing Microorganism in Evergreen Agriculture: An Overview. Potassium Solubilizing Microorganisms for Sustainable Agriculture. 2016;1-20.
Crossref - Nysanth NS, Divya S, Nair CB, Anju AB, Praveena R, and Anith KN. Biological control of foot rot (Phytophthora capsici Leonian) disease in black pepper (Piper nigrum L.) with rhizospheric microorganisms. Rhizosphere. 2022;100578.
Crossref - Kollakkodan N, Anith KN, Radhakrishnan NV. Diversity of endophytic bacteria from Piper spp. with antagonistic property against Phytophthora capsici causing foot rot. J Trop Agric. 2017; 55:63-70.
- Kollakkodan N, Anith KN, and Nysanth NS. Endophytic bacteria from Piper colubrinum suppress Phytophthora capsici infection in black pepper (Piper nigrum L.) and improve plant growth in the nursery. Arch. Phytopathol. Plant Protect. 2021;54(1-2):86-108.
Crossref - Anith KN. Management of nursery wilt of black pepper (Piper nigrum L.) with antagonistic bacteria. Curr Sci. 2002;83(5):561-562.
- Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Trans Br Mycol Soc. 1971;57(1):25-39.
Crossref - Varkey S, Anith KN, Narayana R, Aswini S. A consortium of rhizobacteria and fungal endophyte suppress the root-knot nematode parasite in tomato. Rhizosphere. 2018;5:38-42.
Crossref - Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26(1):192-195.
Crossref - Paul T, Nysanth NS, Yashaswini MS, Anith KN. Inoculation with bacterial endophytes and the fungal root endophyte, Piriformospora indica improves plant growth and reduces foliar infection by Phytophthora capsici in black pepper. J Trop Agric. 2021;59(2);224-235.
- Yashaswini MS, Nysanth NS, Anith KN. Endospore-forming endorhizosphere bacteria from Amaranthus spp. improve plant growth and suppress leaf blight (Rhizoctonia solani Kuhn) disease of Amaranthus tricolor L. Rhizosphere. 2021;19,100387.
Crossref - Gopinath PP, Parsad R, Joseph B, Adarsh VS. GrapesAgri1: Collection of shiny apps for data analysis in agriculture. J Open Source Softw. 2021;6:3437.
- Lemessa F, Zeller W. Screening rhizobacteria for biological control of Ralstonia solanacearum in Ethiopia. BioControl. 2007;42(3): 336-344.
Crossref - Anith KN, Radhakrishnan NV, Manomohandas TP. Screening of antagonistic bacteria for biological control of nursery wilt of black pepper (Piper nigrum). Microbiol Res. 2003;158(2):91-97.
Crossref - Aravind R, Kumar A, Eapen SJ, Ramana KV. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol. 2009;48(1):58-64.
Crossref - Sreeja K, Anandaraj M, Bhai RS. In vitro evaluation of fungal endophytes of black pepper against Phytophthora capsici and Radopholus similis. J Spices Aromat Crops. 2016;25(2):113-122.
- Narisawa K, Usuki F, Hashiba T. Control of Verticillium yellows in Chinese cabbage by the dark septate endophytic fungus LtVB3. Phytopathol. 2004;94(5):412-418.
Crossref - Bailey BA, Bae H, Strem MD, Crozier J, Thomas SE, Samuels GJ, Vinyard BT, Holmes KA. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. BioControl. 2008;46(1):24-35.
Crossref - Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev. 2012;26(2-3):73-83.
Crossref - Melnick RL, Suarez C, Bailey BA, Backman PA. Isolation of endophytic endospore-forming bacteria from Theobroma cacao as potential biological control agents of cacao diseases. BioControl. 2011;57(3):236-245.
Crossref - Khalid A, Arshad M, and Zahir ZA. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol. 2004:96(3):473-480.
Crossref - Aravind R, Kumar A, Eapen SJ. Pre-plant bacterization: A strategy for delivery of beneficial endophytic bacteria and production of disease-free plantlets of black pepper (Piper nigrum L.). Arch Phytopathol Plant Prot. 2012;45(9):1115-1126.
Crossref - Tilak KVBR, Reddy, BS. Bacillus cereus and B. circulans-novel inoculants for crops. Curr Sci. 2006;90(5):642-644.
- Amaresan N, Jayakumar V, Kumar K, Thajuddin, N. Endophytic bacteria from tomato and chilli, their diversity and antagonistic potential against Ralstonia solanacearum. Arch Phytopathol Plant Prot. 2012;45(3):.344-355.
Crossref - Wang W, Wu Z, He Y, Huang Y, Li X, Ye BC. Plant growth promotion and alleviation of salinity stress in Capsicum annuum L. by Bacillus isolated from saline soil in Xinjiang. Ecotoxicol Environ. Safety. 2018;164:520-529.
Crossref - Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol. 2007;53(11):1195-1202.
Crossref - Vyshakhi AS, Anith KN. Co-inoculation with the root endophytic fungus Piriformospora indica and endophytic bacteria improves growth of solanaceous vegetable seedlings. Int J Veg Sci. 2021;27(6):536-51.
Crossref - Athira S, Anith, KN. Plant growth promotion and suppression of bacterial wilt incidence in tomato by rhizobacteria, bacterial endophytes and the root endophytic fungus Piriformospora indica. Indian Phytopathol. 2020;73(12):629-642.
Crossref - Uppala S. Potentiality of endophytic microorganisms in the management of leaf blight disease of amaranth (M.Sc. Thesis), Kerala Agricultural University. 2007:50.
- Anith KN. Mature coconut as a bio-fermentor for multiplication of plant growth promoting rhizobacteria. Curr Sci. 2009:97(11):1647-1653.
- Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 2016;6:1360.
Crossref - Bressan W, Borges MT. Delivery methods for introducing endophytic bacteria into maize. BioControl. 2004;49(3): 315-322.
Crossref - Kalita P, Bora LC, Bhagabati KN. Phylloplane microflora of citrus and their role in management of citrus canker. Indian Phytopathol. 1996;49(3):234-237.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.