ISSN: 0973-7510
E-ISSN: 2581-690X
Urinary tract infection (UTI) is one of the most common complaints in the outpatient clinic and a major health problem owing to the emergence of antibiotic resistance and biofilm formation. The objective of this study was to isolate and identify the causative bacterial agent of UTI and detect in vitro biofilm formation by Escherichia coli and investigate its correlation with antibiotic resistance. Urine samples from 519 patients with suspected UTIs were collected and processed by conventional microbiological procedures. Antimicrobial susceptibility testing for E. coli isolates was performed on Mueller Hinton agar (MHA) plates using the Kirby-Bauer disk diffusion method. Biofilm production was evaluated using the tissue culture plate method. Of 519 urine samples, 115 (22.1%) showed significant bacteriuria. The most common isolate was E. coli (n=57, 49.6%), followed by Klebsiella spp. (n=23, 20%). All E. coli isolates were evaluated for their ability to form biofilms in vitro. Of 57 isolates, 50 (87.7%) were biofilm producers and 7 (12.3%) were non-biofilm producers. Antibiogram of E. coli isolates revealed the highest resistance to ampicillin (96.5%) and nitrofurantoin (91.2%), followed by amoxyclav (82.5%), ceftazidime (73.7%), cefepime (71.9%), and tetracycline (71.9%). A significant association (p<0.05) was observed between biofilm formation and resistance to amoxyclav, ceftazidime, cefepime, imipenem, and nitrofurantoin. A significant correlation was noted between biofilm production and antibiotic resistance. Hence, screening of all isolates of uropathogenic E. coli for biofilm production and studying their antibiogram would allow appropriate choice of antibiotic therapy.
UTI, Uropathogenic Escherichia coli, Biofilm
Urinary tract infection (UTI) is considered as the microbial invasion of any tissues extending from the renal cortex to the urethral meatus. The urinary system includes organs that collect, store, and release urine from the body. Accordingly, UTI is classified based on the site of infection as follows: bladder (cystitis), kidney (pyelonephritis), and urethra (bacteriuria)1.
UTI is one of the most frequently presented complaints in outpatient clinics, and most patients are in their reproductive age (18-37 years). UTI is one of the most common hospital-acquired infections, representing as high as 35% of nosocomial infections, and accounts for the second most common cause of bacteremia in patients admitted to hospitals2,3. It has been estimated that about 6 million patients have UTI per year worldwide, of which around 30,000 are treated in the wards. In India, UTI is the third most common cause of hospital admission, and its prevalence varies from 21.8 to 31.3 in different parts of the country4,5. Uropathogenic Escherichia coli (UPEC) is the most common cause of UTI, accounting for approximately 90% and 50% of community-acquired and hospital-acquired UTIs, respectively. E. coli is as an endogenous microorganism in the human bowel and is deemed harmless under natural conditions. E. coli from the intestine is present in the fecal matter. The passage of trace amounts of fecal matter through the urethral opening allows entry of the microorganism into the urinary tract, wherein it thrives, multiplies, and eventually causes an infection. Some common ways involved in the migration of E. coli through the urethral opening are as follows:
Sexual contact
A woman’s urethra is located next to the vagina and anus, making it easy for bacteria to move into the urinary tract during sexual intercourse and sexual contact.
Improper cleaning
Wiping from the back to front after excretion can drag E. coli directly into the urethra.
Holding urine
Frequent urination facilitates the continuous flushing of bacteria such as E. coli from the system. This is particularly important before and after intercourse.
Enlarged prostate gland
This exerts extra pressure on the bladder, thereby preventing it from properly emptying and flushing E. coli from the body6.
About 60% to 70% of UPEC have the ability to form biofilms7. Relapses and chronic infections by UPEC have been associated with the ability of pathogenic strains to form biofilms. Several studies have shown that 50%-90% of isolates collected from patients with relapsed infections were biofilm producers8. Drug resistance among bacteria causing UTIs is increasing and considered as a major hurdle in the treatment of UTI. Biofilms protect the bacteria from the host immune response and impede the effects of antibiotics. High antimicrobial concentrations are imperative to inactivate organisms growing in a biofilm, and this may increase antibiotic resistance by 1000-fold9. In this context, the present study aimed to determine the correlation between biofilm production and multidrug resistance in UPEC isolates.
The study was carried out at the Department of Microbiology, Yenepoya Medical College and Hospital, after receiving ethical clearance from the Yenepoya Ethics Committee.
Inclusion criteria
Culture isolates from urine samples of patients from all age groups with a high colony count (>105 colony-forming units [CFU]/mL) were included.
Exclusion criteria
Colony count < 105 CFU/mL
Culture plates with multiple bacterial growth.
Methodology
Study design
Descriptive longitudinal study
Sampling technique
Convenience sampling
Sample collection
Freshly voided midstream urine samples were collected from patients with suspected UTI in a sterile, dry, wide-necked, leak-proof universal sterile container under aseptic conditions10.
Culture and identification
The well-mixed and non-centrifuged urine samples were inoculated by a wire loop to deliver 0.001 mL of the specimen onto 5% sheep blood agar, cysteine-lactose electrolyte-deficient agar, and MacConkey agar plates using the streak plate method following standard microbiological procedures. The plates were aerobically incubated at 37°C for 24 h and examined for the presence or absence of bacterial growth. Cultures that formed >105 CFU/mL were considered to have significant bacteriuria. All positive samples showing significant bacteriuria were further tested for physical characteristics such as colony morphology, odor, swarming, and presence of hemolysis on respective media using different biochemical reactions performed as per standard procedures. Thus, gram-negative rods were identified with the help of a series of biochemical tests such as triple-sugar iron agar, indole, Simmons citrate agar, oxidase, urease, and motility tests. Morphologically identical colonies of suspected strains were taken from agar plates, suspended in nutrient broth, and vortexed. The suspensions were inoculated into butts and slants of biochemical testing media. The inoculated media were aerobically incubated at 37°C for overnight, and bacteria were identified following the standard flow chart. Gram-positive cocci were identified based on their reactions in catalase and coagulase tests10.
Antibiotic susceptibility test
Antimicrobial susceptibility testing of E. coli isolates was performed on Mueller Hinton agar (MHA) using the Kirby-Bauer disk diffusion method as per the Clinical and Laboratory Standards Institute (CLSI) guidelines.(11) The following antibiotics were used: amoxyclav (AMC), cefepime (CPM), ceftazidime (CAZ), trimethoprim-sulfamethoxazole (COT), gentamicin (GEN), imipenem (IMP), nitrofurantoin (NIT), norfloxacin (NOR), and piperacillin-tazobactam (PTZ) (Table 1).
Table (1):
List of antibiotics used for antimicrobial susceptibility testing.
Antibiotics |
Potency (μg) |
|---|---|
Amikacin (AK) |
30 |
Ampicillin (AMP) |
10 |
Amoxyclav (AMC) |
20 /10 |
cefepime (CPM) |
30 |
Ceftazidime (CAZ) |
30 |
Trimethoprim sulfamethoxazole (COT) |
1.25/23.75 |
Gentamicin (GEN) |
10 |
Imipenem (IMP) |
10 |
Nitrofurantoin (NIT) |
300mcg |
Norfloxacin (NOR) |
10mcg |
Piperacillin-tazobactam (PTZ) |
100/10 |
Tetracycline (TE) |
30mcg |
Procedure
Each bacterial sample was emulsified in sterile saline in a test tube (mixed thoroughly so that no solid particles remained in the solution). The turbidity of the solution was evaluated by matching with turbidity standards (0.5 McFarland standard). The sterile swab was dipped into broth culture, and the excess fluid from the swab was removed by gently squeezing the swab against the wall of the test tube. Using the lawn culture method, the swab was streaked onto a sterile MHA plate, which was allowed to dry for a few minutes. Antibiotic disks (6 on each plate) were aseptically placed on MHA plate and the plate was incubated for 18-24 h at 37°C.
Observation and interpretation
The diameter of the zone of inhibition for each antibiotic was recorded using a metric ruler. Results were interpreted as sensitive, moderately sensitive, and resistant, as per the CLSI guidelines11.
Biofilm production12
Biofilm production was determined using the tissue culture plate (TCP) method.
Procedure
A colony from an overnight grown culture of isolates on MacConkey agar plate was inoculated into a 3 mL brain heart infusion (BHI) broth with 1% glucose prepared in different dilutions (1:20, 1:40, 1:80, 1:100). Then, 0.2 mL of inoculated broth was loaded into a 96-well flat bottom microtiter plate. Plates were covered and incubated for 24 h at 37°C under aerobic conditions. The contents of the wells were removed after incubation; the wells were washed four times with 0.2 mL phosphate-buffered saline, treated with sodium acetate (2%) for 30 min, and then stained with crystal violet for 1 min. The wells were treated with 0.2 mL ethanol and their optical density was measured at 570 nm wavelength using an enzyme-linked immunosorbent assay (ELISA) plate reader. The test was performed with appropriate controls in duplicates (Fig. 1).
Statistical analysis
Statistical analysis was carried out using InStat software. A chi-square (χ2) test was performed and a value of p<0.05 was considered statistically significant.
A total of 519 urine samples were processed during the study period of 1 year, of which 115 (22.1%) samples showed significant bacterial growth (>105 CFU/mL). There were more female patients (n=75; 65.2%) than male patients (n=40; 34.7%). Patients were divided into nine age groups. The incidence of UTI was the highest among women from the 21-30 year age group followed by women from the 31-40 year age group and was the lowest for women 70 years or older. Among men, the incidence of UTI was the highest among those from the 41-50 year age group, followed by men from the 51-60 year age group (Table 2).
Table (2):
Age wise distribution among male and female patients.
Age group |
Females (n 75) |
Males (n 40) |
|---|---|---|
0-10 |
5 |
3 |
11-20 |
4 |
2 |
21-30 |
17 |
2 |
31-40 |
15 |
6 |
41-50 |
6 |
12 |
51-60 |
11 |
9 |
61-70 |
9 |
4 |
71-80 |
4 |
1 |
Above 81 |
4 |
1 |
Bacteriology of UTI
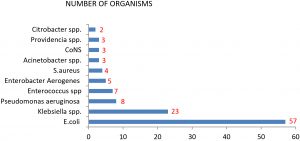
Among the 115 samples, 101 (87.82%) were detected with gram-negative bacilli and 14 (12.17%) were positive for gram-positive cocci. E. coli (n=57; 49.6%) was the most common species isolated during the study, followed by Klebsiella spp. (n=23; 20%), Pseudomonas aeruginosa (n=08; 7%), Enterococcus spp. (n=07; 6.1%), Enterobacter aerogenes (n=05; 4.3%), Staphylococcus aureus (n=04; 3.5%), Acinetobacter species (n=03; 3.5%), CoNS (n=03;3.5%), Providencia species (n=03; 3.5%), and Citrobacter koseri (n=02; 1.7%) (Fig. 2).
Biofilm formation
Among the 57 isolates tested for in vitro biofilm formation ability, 50 were deemed to be biofilm producers. Of them, 08 (14%) strains were strong biofilm producers, 42 (73.7%) were moderate biofilm producers, and 07 (12.3%) strains were non-biofilm producers (Table 3).
Table (3):
Biofilm producers and non biofilm producers.
Mean OD values |
Adherence |
Biofilm formation |
N=57, (%) |
|---|---|---|---|
<0.120 |
None |
None/weak |
07 (12.3) |
0.120-0.240 |
Moderate |
Moderate |
42 (73.7) |
≥0.240 |
Strong |
High |
8 (14) |
The overall resistance pattern of UPEC was evaluated and the highest resistance was confirmed toward ampicillin (96.5%) and nitrofurantoin (91.2%), followed by amoxyclav (82.5%). Medium resistance was observed for ceftazidime (73.7%), cefepime (71.9%), tetracycline (71.9%), co-trimoxazole (66.7%), piperacillin/tazobactam (49.1%), and gentamicin (45.6%) and minimum resistance was observed for norfloxacin (17.5%), followed by amikacin (22.8%) and imipenem (33.3%) (Table 4).
Table (4):
Antibiogram of E.coli.
Antibiotics |
Sensitive n, (%) |
Resistant n, ( %) |
|---|---|---|
AK |
44(77.1) |
13(22.8) |
AMC |
10(17.5) |
47(82.4) |
AMP |
2(3.5) |
55(96.4) |
CPM |
16(28) |
41(71.9) |
CAZ |
15(26.3) |
42(73.6) |
COT |
19(33.3) |
38(66.6) |
GEN |
31(54.3) |
26(45.6) |
IMP |
38(66.6) |
19(33.3) |
NIT |
5(8.7) |
52(91.2) |
NOR |
47(82.4) |
10(17.5) |
PTZ |
29(50.8) |
28(49.1) |
TE |
16(28) |
41(71.9) |
Association between antimicrobial resistance and biofilm formation
In comparison with non-biofilm producers, biofilm-producing isolates showed stronger resistance to antibiotics. The highest level of resistance was reported for ampicillin (82%) followed by nitrofurantoin (78%) and amoxyclav (72%), while the least resistance was conferred toward norfloxacin (6%) (Table 5). There was a significant association between resistance to amoxyclav, ceftazidime, cefepime, imipenem, and nitrofurantoin and biofilm formation (p<0.05). E. coli isolates resistant to three or more classes of antibiotics were categorized as multidrug-resistant (MDR) strains.
Table (5):
Comparison of antibiotic resistance with biofilm production.
| Antibiotics | Biofilm formation | P value | ||||
|---|---|---|---|---|---|---|
| Biofilm Producers (n=50), n (%) | Non biofilm producer (n=7), n (%) | |||||
| Resistant | Sensitive | Resistant | Sensitive | |||
| AK | 12 (24) | 38 (76) | 1 (14) | 6 (86) | 0.5662 | |
| AMC | 44 (88) | 6 (12) | 3 (43) | 4 (57) | 0.0032 | |
| AMP | 49 (85) | 1(15) | 6 (86) | 1 (14) | 0.0980 | |
| CPM | 39 (68) | 11 (22) | 2 (28) | 5 (72) | 0.0064 | |
| CAZ | 39 (68) | 11 (22) | 3 (43) | 4 (57) | 0.0480 | |
| COT | 35 (70) | 15 (30) | 3 (43) | 4(57) | 0.1536 | |
| GEN | 23 (46) | 27 (54) | 3 (43) | 4 (57) | 0.8768 | |
| IMP | 19 (38) | 31 (62) | 0 (0) | 7 (100) | 0.0457 | |
| NIT | 47 (94) | 3 (6) | 5 (72) | 2 (28) | 0.0482 | |
| NOR | 9 (18) | 41(82) | 1 (14) | 6 (86) | 0.8088 | |
| PTZ | 26 (52) | 24 (48) | 2 (28) | 5 (72) | 0.0612 | |
| TE | 37 (74) | 13 (26) | 4 (57) | 3 (43) | 0.3526 | |
In the present study, among the 50 biofilm producers, approximately 10%, 8%, 32%, 14%, 18%, 12%, and 6%, were resistant to 12, 11, 10, 8, 9, and 7 drugs, respectively. Among seven non-biofilm producers, only one isolate was MDR that showed resistance to 8 of 12 antibiotics. The other six isolates were sensitive to most of antibiotics tested.
UTI is one of the most common health problems affecting millions of people worldwide and is a leading cause of morbidity and high healthcare expenditures in people of all ages5. In the present study, the incidence of UTI was higher in female patients than in male patients. Our results are in line with those by Momoh et al.13 and Ahmed et al,14. who reported UTIs in 60.2% and 73% women and 39.8% and 23% men, respectively. The difference in the female:male ratio may be related to different clinical components. Women remain at a much higher risk of UTI (compared to men) owing to shorter urethra, which permits bacterial entry and infection in the bladder. In addition, hormonal changes may influence the beneficial bacteria that are responsible for competing with harmful microorganisms in the urinary tract.
In our study, the frequency of UTI was the highest in women from 21 to 30 years of age and men between 41 and 50 years of age, consistent with the results of Santosh John thattil et al15. and Fatima S. et al.16 that reported the highest incidence of UTI in women from 26 to 35 years and men from 46 to 60 years of age.
In our study, the most common isolate was E. coli (49.5%), consistent with the observation reported by Kaur et al.17 (71.7%) and George et al.18 (69.8%). Thus, the host fecal flora may be a source of E. coli that spreads via the perineal, vaginal, and periurethral areas to the lower urinary tract, wherein it is established. Some common ways for the migration of E. coli include sexual contact, improper cleaning, holding urine (especially before and after intercourse), and enlarged prostate gland6.
We investigated the biofilm formation ability of UPEC. Among 57 isolates, 87.7% were positive for biofilm formation in vitro, which is in line with the results of Suman et al.19, Poursina F et al.20 and Yadav et al.21, showing 92, 80, and 76% E. coli isolates to be biofilm producers, respectively.
The correlation between biofilm production and resistance to amoxyclav, ceftazidime, cefepime, imipenem, and nitrofurantoin was found to be statistically significant (p<0.05); no significant correlation was observed (p>0.05) with amikacin, ampicillin, tetracycline, cotrimoxazole, piperacillin/tazobactam, gentamicin, and norfloxacin. The antibiotics found to be effective against biofilm-producing E. coli isolates were norfloxacin, amikacin, imipenem, and piperacillin/tazobactam.
A significant correlation was observed between multidrug resistance and biofilm formation. Approximately 90% biofilm producers were resistant to more than three classes of antibiotics. The results of our study are in agreement with those reported by Deotale et al.22 and Sevanan et al.23. where in biofilm-producing organisms were more resistant to antibiotics than non-biofilm-producing isolates. The correlation between antibiotic resistance and biofilm formation may be associated with multiple factors such as restricted penetration of drugs through the biofilm matrix or longer time needed to penetrate the biofilm than treatment duration. The expression of efflux pumps is considered as a mechanism underlying antimicrobial resistance not only in planktonic cells but also in biofilm structures21. It has been demonstrated that biofilm-producing microorganisms can tolerate up to 100-1000 times higher concentrations of antibiotics and disinfectants than planktonic cells, and biofilm-producing isolates showed increased resistance against phagocytosis and other host defense mechanisms9.
Limitations of the Study
In this study, other virulence factors such as hemagglutination, gelatinase production, and extended-spectrum beta-lactamase Amp C were not evaluated. The biofilm-producing capability of UPEC may differ in vivo. Further studies regarding the in vivo biofilm-forming capacity of uropathogens are warranted in case of treatment failure.
UTI was found to be more common in women than in men. The most common isolate was E. coli. Biofilm producers showed higher resistance to antibiotics than non-biofilm producers. Biofilm formation by UPEC may pose a health problem, as these bacteria are difficult to treat and increase the chances of chronic UTI. Norfloxacin, amikacin, imipenem, piperacillin, and tazobactam antimicrobials are particularly effective against biofilm-producing E. coli. These antibiotics may be used in the empirical therapy of UTI caused by biofilm-producing UPEC. A significant correlation was observed between biofilm production and antibiotic resistance in our study. Hence, screening of all isolates of UPEC for biofilm production and studying their antibiogram may help in providing an appropriate antibiotic therapy.
ACKNOWLEDGMENTS
We are thankful to all the faculty and technicians of Department of
Microbiology- Yenepoya Medical College and hospital for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest
AUTHORS’ CONTRIBUTION
All the authors substantially contributed to the conception, design, analysis and interpretation of data, checking and approving final version of manuscript.
FUNDING
None
ETHICS STATEMENT
The study was carried out after obtaining approval from institutional ethics committee; Protocol number 2018/091 dated 28.05.2018.
AVAILABILITY OF DATA
All the datasets analyzed during the study are included in manuscript.
- Otajevwo FD. Urinary tract infection among symptomatic outpatients visiting a tertiary hospital based in midwestern Nigeria. Glob J Health Sci. 2013;5(2):187-199. PMID: 23445708; PMCID: PMC4776789.
Crossref - Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010;60(3):213-216.
- Taher Aseel M. Mohamed Al-Meer F, Ghaith Al-Kuwari M, Ismail MF. Prevalence and Predictors of Asymptomatic Bacteriuria among Pregnant Women Attending Primary Health Care in Qatar. Middle East J Fam Med. 2009;7:10-13.
- Bano K, Khan J, Begum H, et al. Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani population. Afr J Microbiol Res. 2012;6(2):414-420(1).
Crossref - Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-284.
Crossref - Moreno E, Andreu A, Perez T, Sabate M, Johnson JR, Prats G. Relationship between Escherichia coli strains causing urinary tract infection in women and the dominant faecal flora of the same hosts. Epidemiol Infect. 2006;134(5):1015-1023.
Crossref - Stamm WE. Scientific and clinical challenges in the management of urinary tract infections. Am J Med. 2002:113(Suppl 1A):1S-4S. PMID: 12113865.
Crossref - Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105-107.
Crossref - Macia MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect. 2014;20(10):981-990.
Crossref - Cheesebrough M. District Laboratory Practice in Tropical Countries Part II. 2nd ed. London: Cambridge.University Press; 2006:105-114.
Crossref - Performance standards for antimicrobial susceptibility testing clinical and laboratory standard institute 2017; M100.27th Ed:1-148.
- Mathur T, Singhal S, Khan S, Upadhyay D J, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 2006;24:25-29.
Crossref - Momoh AR. The antibiogram types of Escherichia coliisolated from suspected urinary tract infection samples. J Microbiol Biotech Res. 2011;1:57-65.
- Ahmed SS, Shariq A, Alsalloom AA, Babikir IH, Alhomoud BN. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int J Health Sci (Qassim). 2019;13(2):48-55.
- Thattil SJ, Santhosh S. Prevalence of UTI in different age groups in a tertiary care hospital and their Antibiogram. International Journal of Contemporary Medical Research. 2018;5(Issue)ICV:77.83.
- Fatima S, Muhammad IN, Usman S, Jamil S, Khan MN, Khan SI. Incidence of multidrug resistance and extended-spectrum beta-lactamase expression in community-acquired urinary tract infection among different age groups of patients. Indian J Pharmacol. 2018;50(2):69-74.
Crossref - Kaur, Rupinder, Geeta Walia and Manika Mehta. “Prevalence of Urinary tract infections in children and their sensitivity to various antibiotics.” J Acad Indus Res. 2012;1(4):161–163.
- George CE, Norman G, Ramana GV, Mukherjee D, Rao T. Treatment of uncomplicated symptomatic urinary tract infections: Resistance patterns and misuse of antibiotics. J Family Med Prim Care.2015;4(3):416-421.
Crossref - Suman E, Jose J, Varghese S, Kotian M S. Study of biofilm production in Escherichia colicausing urinary tract infection. Indian J Med Microbiol 2007;25(3):305-306.
Crossref - Poursina F, Sepehrpour S, Mobasherizadeh S. Biofilm Formation in Nonmultidrug-resistant Escherichia coliIsolated from Patients with Urinary Tract Infection in Isfahan, Iran. Adv Biomed Res. 2018;7:40.
Crossref - Yadav M, Khumanthem S, Kshetrimayum M, Damrolien S. Biofilm production and its correlation with antibiogram among clinical isolates of uropathogenic Escherichia coli. International Journal of Advances in Medicine. 2018;5(3):638-643.
Crossref - Deotale VS, Attal R, Joshi SH, Bankar N. Correlation between Biofilm Formation and Highly Drug Resistant Uropathogens (Hdru). Int J Cur Res Rev. 2015;7(2):61-65.
- Sevanan M, Pongiya U, John N. Antimicrobial Susceptibility Pattern of Biofilm Producing Escherichia coli of Urinary Tract Infections. Current Research in Bacteriology. 2011;4(2):73-80.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.