ISSN: 0973-7510
E-ISSN: 2581-690X
Clostridioides difficile is a principal cause of hospital-acquired gastrointestinal infections, with sporulation and toxin production being key determinants in the disease pathogenesis. Although infections have been escalating and the complications can be life-threatening, the narrow pipeline of approved therapeutics has not witnessed an equivalent surge. With the unfolding of worrisome mutations and antimicrobial resistance, attention has been drawn to either discovering new therapeutics, or even better, repurposing already available ones. Consequently, this study was undertaken to assess the anti-clostridial activity of auranofin, an anti-rheumatic FDA-approved therapeutic; and baicalin, a natural flavone glycoside with reported anti-microbial potential. In comparison with vancomycin, the in vitro efficacy of auranofin and baicalin was tested against hypervirulent C. difficile (BAA-1870TM). Broth suspensions were prepared with and without the three agents and anaerobically incubated. At 24- and 48-hours post-incubation, serial dilutions were prepared and inoculated onto agar plates. Viable cell counts and viable spore counts were then quantified. Meanwhile, toxin production was assessed via ELISA. At a concentration as low as 3 μg/mL, auranofin demonstrated a potent anti-clostridial activity. Both auranofin and baicalin exhibited a remarkable reduction in C. difficile viable cell counts (P-value 0.03 for each) and spore counts (P-values 0.023 and 0.045 respectively). While auranofin and baicalin proved to be non-inferior to vancomycin as inhibitors of C. difficile growth, both drugs proved to be superior to vancomycin in decreasing the spore counts 48-hours post inoculation. Additionally, auranofin markedly reduced C. difficile toxin production (P-value 0.021); a feature that was deficient in both baicalin and vancomycin. To enrich the currently limited repertoire of anti-clostridial drugs, further research is encouraging to compare between the in vivo efficacy of auranofin and that of baicalin. Both agents represent promising approaches that could address the unfulfilled needs in controlling C. difficile infection.
Auranofin, Baicalin, Clostridioides difficile, Diarrhea, Spores, Toxin, Vancomycin
Clostridioides difficile infection (CDI) constitutes the most serious etiology of antibiotic-associated diarrhea, which can eventually invoke a fatal outcome if improperly treated. Meanwhile, the treatment of CDI is an arduous challenge; with infections being mostly hospital-acquired, typically in the elderly patients who are infection-vulnerable due to comorbid conditions.1,2 An additional challenge for treatment is the emergence of hypervirulent strains e.g. the PCR-ribotype 027 and the North American pulsotype 1 (NAP 1) that produce higher amounts of toxin A and toxin B; the main virulence factors of C. difficile. Furthermore, C. difficile spores are highly resistant to adverse conditions. Being able to survive in the patients’ intestines until antibiotic cessation, these spores act as springboards that elicit relapses of infection.3
Even with adequate therapy, the recurrence rates of CDI are high. Within just a month of completing an antibiotic course for an initial episode, almost 15 – 30% of the patients will experience a recurrence and, of these, up to 60% will sustain further relapses.4,5 In addition to being debilitating and undermining the patients’ life quality, such relapses pertain to higher mortalities and uprising health care expenditures.6,7
The commonly employed drugs for treatment of CDIs include vancomycin and fidaxomicin, the former being toxic and the latter being overly expensive (150 times the cost of metronidazole).7 The challenge of treatment is further compounded by the worldwide emanation of C. difficile resistance to these drugs.8 Altogether, such challenges imperatively call for state-of-the-art anti-clostridial therapy that provides durable treatment outcomes.
Nonetheless, de novo drug discovery is a tedious process; where the development of a single new drug could take several years and cost billions. This has consequently directed researchers toward exploring alternative avenues for the treatment of CDI.
On the one hand, drug repurposing approaches have been gaining applause in various disease conditions. In this context, auranofin, an FDA-approved gold-containing anti-rheumatic drug has been investigated in the treatment of infections caused by Staphylococcus aureus, as well as by vancomycin-resistant enterococci.7 In addition to being a potential anti-cancer agent, auranofin has been identified in some recent research as having anti-CDI activity. Possessing a thiol ligand with a high affinity to selenium, auranofin exhibits potency against selenium-consuming microorganisms such as C. difficile. Displacing the sulfur with selenium in that thiol ligand of auranofin deprives C. difficile from a key micronutrient. It has also been reported that auranofin inhibits redox enzymes which are indispensable for various cellular processes.9 Meanwhile, previous reports have revealed that auranofin suppresses toxin production in several pathogens, such as Staphylococcus aureus, Corynebacterium diphtheriae, Clostridium tetani, Clostridium botulinum, and most recently, C. difficile.10-13
On the other hand, the overwhelming global drug resistance has revived the interest in herbal extracts for the treatment of a range of microbial infections. Some reports have shown that the multi-purpose herbs, Scutellaria baicalensis, displays a powerful in vitro antimicrobial action.14 Baicalin, a flavone glycoside found in Scutellaria baicalensis has been employed in traditional medicine for the treatment of dysentery, hepatitis, pneumonia, and scarlet fever.15 Moreover, earlier research has demonstrated an inhibitory effect of baicalin against Staphylococcus saprophyticus,16 Helicobacter pylori,17 Gram-negative pathogens,14 and Mycobacterium tuberculosis.18 The antibacterial activity of baicalin is postulated to involve the disruption of bacterial cell membranes; as well as inhibiting bacterial DNA, RNA, and protein synthesis.19
As a step in the pathway of probing novel anti-clostridial agents, the objective of this study was to compare between the in vitro antibacterial and antivirulence potential of each of auranofin and baicalin with relevance to the standard-of-care drug, vancomycin, against C. difficile.
Bacterial Strain
Clostridioides difficile (reference number: ATCC® BAA-1870™) was procured from Microbiologics (Minnesota, USA) representing an epidemic strain with increased sporulation. The strain is of ribotype 027, toxinotype IIIb, and has been identified by PCR as tcdA-positive, tcdB-positive and cdtB-positive. The strain was grown on brain heart infusion (BHI) agar (Himedia, Mumbai, India) at 37°C in anaerobic conditions (Figure 1).
Figure 1. A) KWIK STIK device that contains lyophilized Clostridioides difficile pellet, an ampoule of hydrating fluid, and an inoculating swab. B) Early growth of C. difficile on brain heart infusion (BHI) agar supplemented with 0.1% Na+ taurocholate
Antimicrobial Agents
Auranofin, baicalin, and vancomycin powders (Figure 2) were obtained from Cayman Europe (Tallinn, Estonia). Auranofin and baicalin were dissolved in dimethyl sulfoxide (DMSO), while vancomycin was reconstituted in saline. All drugs were preserved as stock solutions.
Determining the Minimum Inhibitory Concentrations (MICs) of the Employed Agents
The broth microdilution assay was employed.20 C. difficile broth suspension that was equivalent to 0.5 McFarland was added to the microplate wells containing auranofin, baicalin, and vancomycin at serial dilutions. Following anaerobic incubation for 24 hours, MICs were recorded as the minimum concentration of each agent that visually halted C. difficile growth.
Quantitation of Viable Bacterial Cells
Overnight C. difficile colonies were suspended in brain heart infusion (BHI) broth to yield a concentration of 0.5 McFarland.
Each of auranofin, baicalin, and vancomycin was added to the broth suspension at its reported MIC. Following anaerobic incubation of the broth with and without the drugs, serial dilutions (10-2 and 10-3) were prepared at 24 and 48 hours.
From each dilution (neat, 10-2 and 10-3), 100 μL were spread onto BHI agar plates. After anaerobic incubation for 24 hours, the average colony forming unit (CFU) per mL was calculated for each plate.9,13
ELISA
ELISA RIDASCREEN kit procured from R-Biopharm (Darmstadt, Germany) was employed. In addition to a control tube, auranofin, baicalin and vancomycin were added at subinhibitory concentrations (concentrations below the MIC) to C. difficile broth cultures which were then incubated in anaerobic environment. After 48 hours, the broth cultures were centrifuged at 10,000 rpm for five minutes, and the supernatant was used to perform the assay as per the manufacturer’s booklet. The optical density corresponding to the total toxins concentration was measured at 450 nm wavelength and a reference filter of 630 nm, and compared for the three antimicrobial agents.21
Quantitation of Viable Bacterial Spores
In addition to a control tube, drugs at subinhibitory concentrations were added to C. difficile broth cultures, which were then incubated in an anaerobic environment at 37°C.13
At 24 and 48 hours post-incubation, a plate was cultivated from the control tube to determine the total count of vegetative cells plus spores. All tubes were then centrifuged twice, and in each time, the medium was replaced with phosphate buffered saline (PBS).22 In order to destroy the vegetative cells, the tubes were then subjected to heat shock at 65°C for 45 minutes.
Dilutions were prepared from all tubes, and from each dilution, 100 μL were plated onto BHI agar supplemented with 0.1% sodium taurocholate (Sigma Aldrich, Missouri, USA); the latter being a potent inducer of spore germination. This was ensued by anaerobic incubation for 48 hours. The average colony forming unit (CFU) per mL was then calculated for each plate.
Statistical Analysis
Analysis was performed via SPSS version 24 and Graph pad prism version 9. One-way ANOVA and Kruskal Wallis tests were employed to assess the difference between drugs; followed by pairwise comparison by Bonferroni and Dunn test as appropriate. All of the P-values were two-sided. P-values ≤0.05 were regarded significant.
The relative change was defined as the difference between the final value and the initial value divided by the initial value, with one-fold change equivalent to 100% change.
All endeavors were replicated for at least three times.
Minimum Inhibitory Concentrations
The minimum inhibitory concentration of vancomycin against hypervirulent C. difficile ATCC BAA-1870 (ribotype 027) was revealed to be 2 μg/mL, while that of auranofin was 3 μg/mL, and that of baicalin was 800 μg/mL (0.08%).
Effect of the Tested Agents on Viable Cell Count
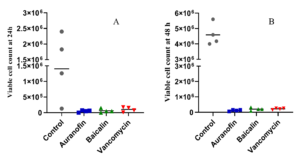
Each of auranofin and baicalin exhibited a pronounced reduction of C. difficile cell count following 24-hour incubation (P-values 0.031 and 0.033 respectively), as well as after 48-hour incubation (P-value <0.001 for each). These results were quite close to the 24 and 48-hour performance of vancomycin (P-values 0.037 and <0.001 respectively).
Figure 3. A) Number of viable vegetative cells from Clostridioides difficile cultures treated with auranofin, baicalin, or vancomycin assessed at 24-hours post-inoculation. B) Number of viable vegetative cells from Clostridioides difficile cultures treated with auranofin, baicalin, or vancomycin assessed at 48-hours post-inoculation. Numbers represent the colony forming unit (CFU) per mL
After 24-hour incubation, auranofin-treated culture demonstrated 0.97 fold decrease in C. difficile cell count, while baicalin-treated culture demonstrated 0.95 fold decrease, and vancomycin-treated culture demonstrated 0.93 fold decrease (Figure 3A).
After 48-hour incubation, auranofin-treated culture demonstrated 0.91 fold decrease in the cell count, while baicalin-treated culture demonstrated 0.85 fold decrease, and vancomycin-treated culture demonstrated 0.84 fold decrease (Figure 3B).
In comparison to vancomycin, auranofin exhibited a pronounced reduction in viable cell count 48-hours post-inoculation (0.026), but not after 24 hours (0.215). On the other hand, baicalin did not exhibit a significant reduction compared to vancomycin, either after 24 hours or after 48 hours.
Meanwhile, no difference was noticeable between auranofin and baicalin in reducing C. difficile viable cell count, either after 24 hours or after 48 hours.
Effect of the Tested Agents on Viable Spore Count
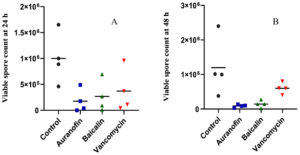
Both auranofin and baicalin markedly reduced C. difficile viable spore count after 24-hour incubation (P-values 0.023 and 0.045 respectively), as well as after 48-hour incubation (P-values 0.041 and 0.05 respectively). On the other hand, vancomycin could not demonstrate a significant reduction of viable spore count either after 24 hours or after 48 hours (P-values 0.099 and 0.219 respectively).
Figure 4. A) Number of viable spores from Clostridioides difficile cultures treated with auranofin, baicalin, or vancomycin assessed at 24-hours post-inoculation. B) Number of viable spores from Clostridioides difficile cultures treated with auranofin, baicalin, or vancomycin assessed at 48-hours post-inoculation. Numbers represent the colony forming unit (CFU) per mL.
After 24-hour incubation, auranofin-treated culture demonstrated 0.82 fold decrease in the spore count, while baicalin-treated culture demonstrated 0.73 fold decrease, and vancomycin-treated culture caused 0.63 fold decrease (Figure 4A).
After 48-hour incubation, auranofin-treated culture demonstrated 12.31 fold decrease in the spore count, while baicalin-treated culture demonstrated 11.68 fold decrease, and vancomycin-treated culture demonstrated 6.63 fold decrease (Figure 4B).
In comparison to vancomycin, both auranofin and baicalin exhibited a pronounced reduction in viable spore count 48-hours post inoculation (P-values 0.001 and 0.003 respectively), but not after 24 hours.
Meanwhile, no difference was noticeable between auranofin and baicalin in reducing C. difficile viable spore count, either after 24 hours or after 48 hours.
Effect of the Tested Agents on Toxin Production
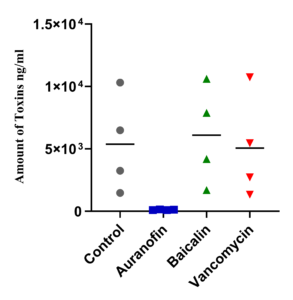
Auranofin demonstrated a striking reduction in the total toxin production (toxins A and B) by C. difficile in comparison to the untreated control (P-value 0.021). In contrast, neither baicalin nor vancomycin could reduce the total amount of toxin produced (P-values 0.76 and 0.94 respectively). Of note, the amount of toxin was slightly elevated in the presence of baicalin (Figure 5).
Figure 5. Effects of auranofin, baicalin, and vancomycin on C. difficile ATCC BAA 1870 total toxin production
In comparison to baicalin and vancomycin, it was noticeable that auranofin resulted in a remarkable reduction in total toxin production (P-values 0.009 and 0.026 respectively).
Clostridioides difficile infection (CDI) has been enlisted by the CDC as one of the five urgent threats that mandate aggressive measures. Despite this fact, drug development against CDI has been proceeding at a much slower pace than coveted.23
Only three drugs are in current use; metronidazole and vancomycin, both discovered in the 1950s, and fidaxomicin, which gained approval in 2011. Nonetheless, the use of these drugs is hindered by a number of limitations. For instance, the estimated treatment failure rates are 14% and 22% for vancomycin and metronidazole, respectively. Additionally, 25-30% of those treated with either drug will experience recurrence.24 Regrettably, the therapeutic outcome is still unsatisfactory even after fidaxomicin discovery.25 Altogether, this underpins the compelling need for avant-garde effective therapeutics against CDI.
The discovery of novel antibacterial agents is a very lengthy and costly process. Meanwhile, the repositioning of an FDA-approved agent for a novel indication is an encouraging strategy for drug discovery. Owing to a meticulous clinical investigation, the pharmacodynamics, pharmacokinetics, and safety profile of such drugs have been well-investigated, which will unarguably lessen both the time and expenses pertinent to drug discovery.26,27 Auranofin is an FDA-approved anti-rheumatic drug with a well-studied safety profile and limited adverse effects.28 Owing to its poor absorption via the intestinal tract, auranofin possesses ideal pharmacokinetics as a therapeutic against CDI. With only 15-25% absorbability, almost 85% of the administered dose would reach the distal colon to be excreted in feces,29 implying that auranofin can reach therapeutic concentrations at the infection site.29,30
On the other hand, antivirulence agents are currently gaining a foothold in halting resistant pathogens. Such therapeutic modality attenuates the pathogen’s virulence rather than its growth.31 Consequently, the pathogen’s tendency toward developing resistance would be diminished; and moreover, the possibility of perturbing the beneficial microflora would be minimal.21
Baicalin is a plant-derived compound that has long been used in the treatment of various bacterial and viral infections.15 In earlier research, it has been demonstrated as an inhibitor of bacterial protein synthesis, quorum sensing, and the expression of virulence genes.32
This study has been endeavored to assess the in vitro antibacterial and antivirulence activity of each of auranofin and baicalin against a hypervirulent C. difficile strain (ATCC® BAA-1870™), in comparison to the standard therapeutic agent, vancomycin.
In concordance with previous studies, auranofin was found to have a low MIC of 3 μg/mL against C. difficile; a concentration comparable to that of vancomycin MIC which was recorded as 2 μg/mL. These findings were consistent with those of Roder and Athan9 who reported auranofin MIC as 4 μg/mL, as well as with those of AbdelKhalek et al.13 who reported auranofin MIC range against C. difficile as 0.25-4 μg/mL and vancomycin MIC range as being 0.25-2 μg/mL. Meanwhile, Hutton et al.18 reported auranofin MIC as 1.7 μg/mL, while another study revealed that both auranofin and vancomycin had the same MIC of 1 μg/mL against C. difficile.33 On the other hand, baicalin MIC was found to be 800 μg/mL (0.08%). As previously demonstrated by Zhao et al.,14 baicalin had an MIC of 4000 μg/mL against E. coli, while Wang et al.16 reported baicalin MIC against Staphylococcus saprophyticus as 500 μg/mL.
In this study, each of auranofin and baicalin exhibited a pronounced reduction in the viable cell count of hypervirulent C. difficile (P-values 0.03 for each). This finding corroborates those of earlier research,9,13 which revealed the superiority of auranofin in inhibiting C. difficile growth.
Meanwhile, C. difficile ability to sporulate is a fundamental virulence component in the disease process. Hypervirulent C. difficile possesses a higher ability to form spores that tolerate the standard disinfection procedures. This rationalizes the tendency of these strains to spread more readily in the environment. Following the conclusion of a treatment course, such spores can germinate in the intestine resulting in relapses.34,35
Remarkably, each of auranofin and baicalin resulted in a noticeable inhibition of C. difficile sporulation (P-values 0.023 and 0.045 respectively). These findings reiterate those of Roder and Athan,9 and AbdelKhalek et al.13 who reported an anti-spore activity of auranofin, as well those of Pellissery et al.21 who demonstrated an anti-spore activity of baicalin.
It has been demonstrated that auranofin disrupts several protein synthesis pathways in Staphylococcus aureus. Baicalin has also been reported as an inhibitor of bacterial DNA and protein biosynthesis.19 Hence, it can be conjectured that auranofin and baicalin inhibit the synthesis of the coat proteins in C. difficile spores.36
Noteworthy, the ability of auranofin and baicalin to suppress sporulation would be reflected as improved treatment outcomes, lower rates of CDI relapses, and reduced infection transmission.
In the meantime, vancomycin caused a much less reduction of C. difficile sporulation. This was consistent with the observation reported by Baines et al.37 and AbdelKhalek et al.13 who detected almost no reduction in the spore count with vancomycin. Contradicting this finding, Roder and Athan9 reported an ability of vancomycin to reduce C. difficile spore count. Of note, the response to vancomycin can vary in different strains; while C. difficile BAA-1870 was used in this study and in the study by AbdelKhalek et al., Roder and Athan used C. difficile M7404.
On the other hand, toxins A and B are pivotal for C. difficile to provoke a disease. Furthermore, toxin production is augmented by environmental stresses; consequently, some anti-clostridial agents (e.g. vancomycin) could promote C. difficile toxin secretion.38
Hence, an additional approach in this study was to assess the activity of the three drugs at subinhibitory concentrations against toxin production by C. difficile.
In concordance with earlier studies,9,13 auranofin exerted a substantial inhibition of toxin production by C. difficile. Nonetheless, baicalin at a subinhibitory concentration did not significantly affect toxin production. This was in contrast to a study by Pellissery et al.21 who reported 70-85 % reduction in C. difficile toxin concentration in the presence of baicalin. Corroborating earlier studies,13,39 vancomycin could not reduce the amount of toxins produced by C. difficile in the present study.
Further in vitro research is justified to elucidate the mechanisms by which auranofin and baicalin interfere with C. difficile sporulation. It is also rationalized to evaluate the effect of baicalin on toxin production in a wider panel of C. difficile strains. Another fundamental factor that ought to be investigated is the impact of each of auranofin and baicalin on the gut microbiome; where a possibility of dysbiosis could elevate the risk of CDI recurrence.40
Auranofin demonstrated a substantial reduction in C. difficile viable cell count as well as viable spore count. Additionally, auranofin markedly reduced the amount of toxin produced by C. difficile.
Meanwhile, baicalin proved to be non-inferior to vancomycin in inhibiting C. difficile growth and spore formation; but at subinhibitory concentration, it could not reduce toxin production.
Although auranofin proved superior to baicalin in reducing toxin secretion, the possibility of auranofin-induced resistance should be thoroughly investigated. Collectively, these results underpin that auranofin and baicalin represent potential safe and effective additions to the current pipeline of CDI treatment. Nonetheless, follow-up in vivo investigations are warranted for dose standardization and long-term safety. This can be ensued by clinical trials in human subjects.
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Nasiri MJ, Goudarzi M, Hajikhani B, Ghazi M, Goudarzi H, Pouriran R. Clostridium (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: a systematic review and meta-analysis. Anaerobe. 2018; 50:32-37.
Crossref - Donskey CJ. Clostridium difficile in older adults. Infect Dis Clin North Am. 2017;31(4):743-756.
Crossref - Kuehne SA, Cartman ST, Minton NP. Both, toxin A and toxin B, are important in Clostridium difficile infection. Gut Microbes. 2011;2(4):252-255.
Crossref - Leffler DA, Lamont JT. Treatment of Clostridium difficile-associated disease. Gastroenterology. 2009;136(6):1899-1912.
Crossref - Maroo S, Lamont JT. Recurrent Clostridium difficile. Gastroenterology. 2006;130(4):1311-1316.
Crossref - Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447.
Crossref - Abutaleb NS, Seleem MN. In vivo efficacy of auranofin in a hamster model of Clostridioides difficile infection. Sci Rep. 2021;11(1):1-7.
Crossref - Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154-161.
Crossref - Roder C, Athan E. In Vitro Investigation of Auranofin as a Treatment for Clostridium difficile Infection. Drugs R D. 2020;20(3):209-216. doi: 10.1007/s40268-020-00306-3
- Thangamani S, Mohammad H, Abushahba MF, et al. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci Rep. 2016;6:22571.
Crossref - Schnell L, Dmochewitz-Kuck L, Feigl P, Montecucco C, Barth H. Thioredoxin reductase inhibitor auranofin prevents membrane transport of diphtheria toxin into the cytosol and protects human cells from intoxication. Toxicon. 2016;116:23-28.
Crossref - Pirazzini M, Bordin F, Rossetto O, Shone CC, Binz T, Montecucco C. The thioredoxin reductase-thioredoxin system is involved in the entry of tetanus and botulinum neurotoxins in the cytosol of nerve terminals. FEBS Lett. 2013;587(2):150-155.
Crossref - AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. Antibacterial and antivirulence activities of auranofin against Clostridium difficile. Int J Antimicrob Agents. 2019;53(1):54-62
Crossref - Zhao QY, Yuan FW, Liang T, et al. Baicalin inhibits Escherichia coli isolates in bovine mastitic milk and reduces antimicrobial resistance. Int J Dairy Sci. 2018;101(3):2415-2422.
Crossref - Liu IX, Durham DG, Richards RM. Baicalin synergy with beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other beta-lactam-resistant strains of S. aureus. J Pharm Pharmacol. 2000;52(3):361-366.
Crossref - Wang J, Qiao M, Zhou Y, et al. In vitro synergistic effect of baicalin with azithromycin against Staphylococcus saprophyticus isolated from francolins with ophthalmia. Poult Sci. 2019;98(1):373-380
Crossref - Huang YQ, Huang GR, Wu MH, et al. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: treatment of Helicobacter pylori-induced multidrug resistance. World J Gastroenterol. 2015;21(14):4225-4231.
Crossref - Hutton ML, Pehlivanoglu H, Vidor CJ, James ML, Thomson MJ, Lyras D. Repurposing auranofin as a Clostridioides difficile therapeutic. J Antimicrob Chemother. 2020;75(2):409-417
Crossref - Huang YQ, Huang GR, Wu MH, et al. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: treatment of Helicobacter pylori-induced multidrug resistance. World J Gastroenterol. 2015; 21(14):4225.
Crossref - CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 8th Edition. M11-A8. 2012.
- Pellissery AJ, Vinayamohan PG, Venkitanarayanan K. In vitro antivirulence activity of baicalin against Clostridioides difficile. J Med Microbiol. 2020;69(4):631-639.
Crossref - Casas VDL, Miller S, DuPont H, Zhi-Dong J. In vitro Effects of Probiotics on Clostridium difficile Toxin Production and Sporulation. Int Arch Public Health Community Med. 2020;4:040.
- Budi N, Godfrey JJ, Safdar N, Shukla SK, Rose WE. Omadacycline compared to vancomycin when combined with germinants to disrupt the life cycle of Clostridioides difficile. Antimicrob Agents Chemother. 2021;65(5):e01431-e01420.
Crossref - Vardakas KZ, Polyzos KA, Patouni K, Rafailidis PI, Samonis G, Falagas ME. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int J Antimicrob Agents. 2012;40(1):1-8.
Crossref - Orenstein R. Fidaxomicin failures in recurrent Clostridium difficile infection: a problem of timing. Clin Infect Dis. 2012;55(4):613-614.
Crossref - Younis W, AbdelKhalek A, S Mayhoub A, N Seleem M. In vitro screening of an FDA-approved library against ESKAPE pathogens. Curr Pharm Des. 2017;23(14):2147-2157.
Crossref - Mohammad H, AbdelKhalek A, Abutaleb NS, Seleem MN. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. Int J Antimicrob Agents. 2018;51(6):897-904.
Crossref - Roder C, Thomson MJ. Auranofin: repurposing an old drug for a golden new age. Drugs R D. 2015;15(1):13-20.
Crossref - Kean WF, Kean IR. Clinical pharmacology of gold. Inflammopharmacology. 2008;16(3):112-125.
Crossref - Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9(4):233-243.
Crossref - Khodaverdian V, Pesho M, Truitt B, et al. Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemoth. 2013;57(8):3645-3652.
Crossref - Peng LY, Yuan M, Wu ZM, et al. Anti-bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Sci Rep. 2019;9(1):4063.
Crossref - Abutaleb NS, Seleem MN. Auranofin, at clinically achievable dose, protects mice and prevents recurrence from Clostridioides difficile infection. Sci Rep. 2020;10(1):7701.
Crossref - Vedantam G, Clark A, Chu M, McQuade R, Mallozzi M, Viswanathan VK. Clostridium difficile infection: toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut microbes. 2012;3(2):121-134
Crossref - Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin Infect Dis. 2011;53(10):1003-1006.
Crossref - Paredes-Sabja D, Shen A, and Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol. 2014;22(7):406-416
Crossref - Baines SD, O’Connor R, Saxton K, Freeman J, Wilcox MH. Activity of vancomycin against epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother. 2009;63(3):520-525
Crossref - Onderdonk AB, Lowe BR, Bartlett JG. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Appl Environ Microbiol. 1979;38(4):637-641.
Crossref - Abutaleb NS, Seleem MN. Repurposing the antiamoebic drug diiodohydroxyquinoline for treatment of Clostridioides difficile infections. Antimicrob Agents Chemother. 2020;64(6):e02115-19.
Crossref - Dupuy B, Sonenshein AL. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol. 1998;27(1):107-120.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.