ISSN: 0973-7510
E-ISSN: 2581-690X
Diabetes mellitus (DM) has been considered as one of the predisposing factors for candiduria and Candida urinary tract infections. The study determined the socio-demographic characteristics, risk factors of DM patients with asymptomatic candiduria and ascertained the prevalence, virulence factors and antifungal susceptibility of Candida isolated. Socio-demographic and risk factors were obtained via questionnaires. Microscopic, macroscopic and chemical analysis of mid-stream urine (MSU) samples were determined by microbiological method and dipsticks. The characterization, virulence factors, antibiotic susceptibility of Candida isolates were determined by conventional, mycological media and disc diffusion techniques, respectively. Of the 51 MSU samples, ≥ 31.4% were amber and clear in colour, contained yeast cells and leukocytes; between 5.9 to 25.5% had hyaline casts, urobilinogen, epithelial cells, red blood cells, pus cells and nitrite, while the specific gravity was ≥ 1.015. The prevalence of candiduria among subjects with respect to age, types and duration of diabetes, gender, tobacco and alcohol consumption were not significant (p ≥ 0.005). Candida dubliniensis and C. parapsilosis prevalence was highest in subjects with random blood sugar (mg/dL) of ≥ 400 and 300-399, respectively. Of the 39 isolates, 64.1% were Fluconazole sensitive, 10.3% were dose dependent susceptible to Ketoconazole, 74.4% exhibited Voriconazole sensitivity, 100% C. dubliniensis were Clotrimazole sensitive, ≤ 28.6% C. tropicalis and C. glabrata were resistant to Amphotericin B and Itraconazole, while between 23.1% and 71.8% isolates produced hydrolytic enzymes and biofilm. This study revealed the socio-demographic characteristics and risk factors among subjects and the necessity to continuously investigate pathogenic Candida against antifungal agents for effective treatments of asymptomatic candiduria in diabetes mellitus patients.
Candiduria, Diabetes, Risk Factors, Antifungal, Susceptibility, Virulence Factors
Diabetes mellitus, chronic metabolic and degenerative disorder characterized by hyperglycemia, is an increasing global health problem with numerous complications (Lotfi-Kamran et al., 2009; Zahra et al., 2016). Human behavioural changes, lifestyle, genetics and environment factors such as high intake of refined carbohydrates, smoking and heavy consumption of alcohol have been implicated in the burgeoning prevalence of diabetes mellitus (Hu, 2011). The two types of diabetes mellitus are type 1 and type 2 (Mukhtar et al., 2019). The type 1 diabetes mellitus is a heterogeneous disorder characterized by the destruction of pancreatic beta cells, resulting in absolute insulin deficiency (Maahs et al., 2010; Mukhtar et al., 2019), while type 2 consists of dysfunctions characterized by hyperglycemia and resulting from the combination of resistance to insulin action and an inadequate compensatory insulin secretory response (Mukhtar et al., 2019). Diabetes mellitus, immunosuppression, gender, age, exposure to antimicrobial agents and organ transplantation have long been considered as a predisposing factor for Candida urinary tract infections (Kauffman, 2005; Sobel et al., 2011).
The urinary tract consists of organs of the body involved in the production, storage and excretion of urine (Beer et al., 2006). The urinary tract is sterile, devoid of microorganisms but may be invaded by pathogenic organisms (Akinjogunla et al., 2010). Several studies have revealed increase in urinary tract infections (UTIs), attributable to Candida spp (Yashavanth et al., 2013). Candida species are thin-walled, dimorphic fungi (8 to 12 µm in diameter), that reproduce by budding (Talaro and Talaro, 1996). Although, >150 Candida spp have been reported, only few cause human diseases (Kwon-Chung et al., 1992). Candiduria, the presence of Candida spp such as C. albicans, C. glabrata, C. krusei, C. tropicalis and C. parapsilosis in urine, is classified into asymptomatic and symptomatic forms. Candiduria can lead to morbidity and mortality in immunocompromised and ill-patients (Sanglard and Odds, 2002). Candida spp promptly react to environmental changes and take advantage of compromised immunity for establishment of diseases (Akinjogunla et al., 2017). The emergence of drug‑resistant Candida spp is attributable to the use of prolonged and inappropriate empirical therapy which has further complicated the management of patients (Chakrabarti et al., 1996). Thus, it’s important to appropriately identify the causal agents of the infection and administer proper treatment so as to prevent the disease from becoming chronic.
The virulence factors that enhance the pathogenicity of Candida spp are the: phenotypic switching (Soll, 1992); adhesins and invasins on the cell surface (Akinjogunla et al., 2019); yeast hyphal morphogenetic transformation and hydrolytic enzymes e.g. proteases, phospholipases, haemolysins and lipase (François et al., 2013). These hydrolytic enzymes aid in adherence, tissue penetration, invasion and destruction of host tissues (Silva et al., 2011; Akinjogunla et al., 2019). Biofilms, communities of microorganisms embedded in an extracellular matrix, similarly contribute to the pathogenicity of Candida spp and confer substantial resistance to antifungal therapy (Donlan and Costerton, 2002). The study determined the socio-demographic characteristics and risk factors of diabetic patients with asymptomatic candiduria and as well ascertained the prevalence, virulence markers / factors (amylase, lipase, phospholipase, caseinase, gelatinase, coagulase, biofilm, haemolysin) and susceptibility profiles of Candida isolated to antifungal drugs (Ketoconazole, Clotrimazole, Voriconazole, Itraconazole, Fluconazole and Amphotericin) using disc diffusion technique.
Collection of Samples
Mid-stream urine (MSU) samples were aseptically collected using sterile wide-necked, leak-proof containers from 51 diabetic patients (aged < 40 yrs and ≥ 41 yrs), with random blood sugar ranging between 200 and ≥ 400 mg/dL, attending some hospitals / clinics in Uyo, Akwa Ibom State. The Ethics Review Committee approval and verbal informed consent of diabetic patients were obtained prior to samples collection. The MSU samples were properly labelled and transported in a cooler box (4oC) to microbiology laboratory for analysis.
Administration of Questionnaires
Structured questionnaires reflecting the socio-demographic characteristics and associated risk factors were administered to the participants after their verbal informed consent.
Inclusion Criteria
The diabetic patients who agreed and gave verbal consent to participate in the study.
Exclusion Criteria
The non-diabetic patients and diabetic patients who were on antibiotics within one week prior to enrolment and/or those that declined to participate in the study.
Microscopic and Macroscopic Examination of Mid-stream Urine Samples
Two loopful of each uncentrifuged MSU sample was aseptically placed on a clean grease-free slide and covered with a cover slip. The presence of epithelial cell, pus cell, yeast cell, granular cast, crystals, hyaline cast and red blood cells were observed using 10 x and 40 x objectives with condenser iris sufficiently closed. Macroscopic examination was done by observing colour of the MSU samples as previously described by Akinjogunla and Divine-Anthony (2013).
Chemical (Dipstick) Analysis of Mid-stream Urine Samples
The chemical analysis of MSU samples was carried out using dipsticks (Medi-Test Combi 10 SGL, Macherey-Nagel, Germany). The parameters evaluated were: urobilinogen, leukocytes, protein, ketone, bilirubin, nitrites, specific gravity and blood. The dipstick was dipped into each fresh uncentrifuged MSU sample and removed after 5-10 secs. A colour change of the dipstick within 2 mins was observed and recorded according to the manufacturer’s instructions.
Mycological Analysis of Mid-stream Urine Samples
A loopful of each MSU sample was aseptically inoculated onto each plate of Sabouraud Dextrose Agar (SDA, Oxoid UK) supplemented with chloramphenicol and aerobically incubated at 35°C for 48 hrs. After incubation, the plates were observed for yeast growth and were subcultured onto fresh plates of SDA and incubated at 35°C ± 2 for 48 hr. After incubation, the pure isolates were subcultured onto plates of CHROMagar Candida (Difco BBL., USA), aerobically incubated at 35°C ± 2 for 48 hr and their colonial morphology and pigmentation on CHROMagar Candida were used for identification. The Candida species were further identified using Gram staining, temperature tolerance, chlamydospore and germ tube formation, sugar fermentation and assimilation tests. The Candida isolates were maintained on SDA slant at 4°C.
In vitro Antifungal Susceptibility of Candida Isolates
In vitro antifungal susceptibility of Candida isolates to Ketoconazole (KET, 10 µg), Clotrimazole (CLO, 10 µg), Voriconazole (VOR, 1µg), Itraconazole (ITR, 10 µg), Fluconazole (FLU, 25 µg), and Amphotericin B (AMP, 20 µg) was carried out using disc diffusion technique (CLSI, 2009). Ten microlitre of inoculum suspension, adjusted to turbidity of 0.5 McFarland standards, was inoculated onto the plate of Mueller Hilton Agar supplemented with 2 % glucose and 0.5 g/mL methylene blue (GM-MHA). The GM-MHA plates were allowed to dry for 15 mins before antifungal discs were aseptically placed on the surfaces of the culture plates using sterile forceps. The plates were aerobically incubated at 35°C ± 2 for 24 hr, inhibition zones after incubation were observed, measured in millimeters (mm) using a ruler and interpreted as sensitive (S), dose dependent susceptible (DDS) and resistant (R) was made as follows: ITR, AMP and VOR (S: ≥ 16, DDS: 10-15, R ≤ 9), FLU (S: ≥19, DDS: 15-18, R ≤ 14), KET (S: ≥ 30, DDS: 23-29, R ≤ 22) and CLO (S: ≥ 20, DDS: 12-19, R ≤ 11).
Detection of Phospholipase, Lipase, Gelatinase, Caseinase and Hemolysin Producing Candida isolates
Phospholipase, lipase, gelatinase, caseinase and hemolysin producing Candida isolates were detected using egg yolk agar (SDA, 2% egg yolk), tributyrin agar (SDA, 1% tributyrin), gelatin agar (SDA, 1% gelatin), skim milk agar (SDA, 1% skim milk) and human blood – Sabouraud Dextrose Agar (SDA, 3 % glucose, 5% human blood) respectively (Deorukhkar et al., 2014; Akinjogunla et al., 2107). Ten microliters of Candida isolate suspension, adjusted to turbidity of 0.5 McFarland standards, was inoculated onto each plate of egg yolk agar, tributyrin agar, gelatin agar, skim milk agar and human blood-SDA. All the plates were aerobically incubated at 35°C ± 2 for 72 hrs. After incubation, transparent zones around the colonies were observed and considered positive for production of phospholipase, lipase, gelatinase, caseinase and hemolysin, respectively.
Detection of Amylase producing Candida isolates
Amylase producing Candida isolates was detected using starch agar (SDA, 2% starch). The Candida isolates were streaked onto starch agar plates and aerobically incubated for 48-72 hr at 35°C ± 2. After incubation, 3 drops of 10 % Lugol iodine was put on the culture plates and allowed to react for 10 min. Clear zone around the colony indicated the production of amylase (Akinjogunla et al., 2107).
Detection of Biofilm Producing Candida Isolates
A loopful of each Candida isolate from overnight agar plate was aseptically inoculated onto each tube of Glucose-Sabouraud Dextrose Broth (10 ml SDB, 2% glucose). Each tube was incubated aerobically for 48 hrs at 35°C ± 2. After incubation, the contents of each tube were poured out, each tube washed with phosphate buffer saline (PBS, pH 7.2), dried and stained with safranin (1%). Excess stain in each tube was rinsed with PBS and tubes dried in an inverted position. A biofilm formation was indicated when a visible film lined the wall and bottom of the tube (Deorukhkar et al., 2014; Akinjogunla et al., 2107).
Detection of Coagulase Producing Candida Isolates
The production of coagulase by Candida isolates was determined using EDTA-human plasma. Ten microlitre of each Candida isolate from overnight agar plate was aseptically inoculated onto each tube of EDTA-human plasma. Each tube was incubated at 35°C ± 2 and observed for clot formation after 2, 4, 6 and 24 hr. The presence of clot that could not be resuspended by gentle shaking indicated positive for coagulase production (Rodrigues et al., 2003).
Statistical Analysis
The Statistical Package for Social Sciences (IBM SPSS Version 22.0. Armonk, NY: IBM Corp.) was used for data analysis. The significant difference in the socio-demographic characteristics and associated risk factors among the diabetic patients at p ≤0.05 were determined using chi-square (χ2) test. Descriptive data were presented as charts and percentages.
The microscopic analysis of MSU samples based on age and gender of subjects are shown on Table 1. Of the 51 samples collected, 13.7%, 25.5%, 35.3%, 9.8%, 13.7%, 5.9% and 11.8% had epithelial cells, pus cells, yeast cells, granular cast, crystals, hyaline casts and red blood cells (RBCs), respectively. The occurrence of epithelial cells, pus cells, yeast cells, crystals, hyaline casts and RBCs was higher in female subjects and those aged ≥ 40 yrs than male subjects and those aged < 40 yrs. Only 5.9% MSU samples of subjects aged ≥ 40 yrs contained hyaline casts, while none of the samples of subjects within age group < 40 yrs had hyaline casts (Table 1).
Table (1):
Microscopic Analysis of Urine Samples of Diabetic Patients Based on Age and Gender.
| Parameters | No of Samples Collected | No (%) of Occurrence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Epithelial Cell | Pus Cell | Yeast Cell | Granular Cast | Crystals | Hyaline Cast | RBC | ||||
|
Age (yrs) |
< 40 | 13 | 1 (7.7) | 3 (23.1) | 4 (30.8) | 2 (15.4) | 1 (7.7) | 0 (0.0) | 1 (7.7) | |
| ≥ 40 | 38 | 6 (15.8) | 10 (26.3) | 14 (36.8) | 3 (7.9) | 6 (15.8) | 3 (7.9) | 5 (13.2) | ||
| Total | 51 | 7 (13.7) | 13 (25.5) | 18 (35.3) | 5 (9.8) | 7 (13.7) | 3 (5.9) | 6 (11.8) | ||
|
Gender
|
Male | 29 | 3 (10.3) | 5 (17.2) | 8 (27.6) | 4 (13.8) | 3 (10.3) | 1 (3.4) | 1 (3.4) | |
| Female | 22 | 4 (18.2) | 8 (36.4) | 10 (45.5) | 1 (4.5) | 4 (18.2) | 2 (9.1) | 5 (22.7) | ||
| Total | 51 | 7 (13.7) | 13 (25.5) | 18 (35.3) | 5 (9.8) | 7 (13.7) | 3 (5.9) | 6 (11.8) | ||
Keys: RBC: Red Blood Cell. values in parentheses expressed the percentages.
The results of macroscopic and dipstick analysis of MSU samples are shown in Table 2. The colour of MSU samples was: pale yellow and clear 11(21.6%), pale yellow and turbid 6(11.8%), yellow and turbid 3(5.9%), colourless and clear 7(13.7%); amber and clear 10 (35.3%); amber and turbid 6(11.8%). Of the 51 MSU samples collected, 6 samples had leukocytes (+), 12 samples had leukocytes (++); 19.6% were positive for proteins; 15.7% contained ketone (+), 15.7% had ketone (++), 4.0% contained urobilinogen (+), 2.0% had urobilinogen (++), 11.8% contained blood; 5.9% had specific gravity (SG) of 1.000; 27.5% had SG of 1.005; 13.7% had SG of 1.010, SG of 52.9% was 1.015, while nitrites and bilirubin were present in 11.8% and 7.8%, respectively.
Table (2):
Macroscopic and Chemical Analysis of Urine Samples of Diabetic Patients (n=51).
| Variables | Categories | No (%) of Occurrence | Total No (%) | |
|---|---|---|---|---|
| Male (29) | Female (22) | |||
| Appearance | PYC | 7 (24.1) | 4 (18.2) | 11 (21.6) |
| PYT | 3 (10.3) | 3 (13.6) | 6 (11.8) | |
| YT | 2 (6.9) | 1 (4.5) | 3 (5.9) | |
| CC | 4 (13.8) | 3 (13.6) | 7 (13.7) | |
| AC | 11 (37.9) | 7 (31.8) | 18 (35.3) | |
| AT | 2 (6.9) | 4 (18.2) | 6 (11.8) | |
|
Protein |
++ | 2 (6.9) | 1 (4.5) | 3 (5.9) |
| + | 4 (13.8) | 3 (13.6) | 7 (13.7) | |
| – | 23 (79.3) | 18 (81.8) | 41(80.4) | |
|
Ketone |
++ | 5 (17.2) | 3 (13.6) | 8 (15.7) |
| + | 4 (13.8) | 4 (18.2) | 8 (15.7) | |
| – | 20 (69.0) | 15 (68.2) | 35 (68.6) | |
|
Bilirubin |
++ | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| + | 1 (3.4) | 3 (13.6) | 4 (7.8) | |
| – | 28 (96.6) | 19 (86.4) | 47 (92.2) | |
|
Nitrite |
+ | 5 (17.2) | 1 (4.5) | 6 (11.8) |
| – | 24 (82.8) | 21 (95.5) | 45 (88.2) | |
| Urobilinogen
|
++ | 1 (3.4) | 0 (0.0) | 1 (2.0) |
| + | 1 (3.4) | 1 (4.5) | 2 (4.0) | |
| – | 27 (93.1) | 21 (95.5) | 48 (94.1) | |
Leukocytes |
++ | 3 (10.3) | 3 (13.6) | 6 (11.8) |
| + | 5 (17.2) | 7 (31.8) | 12 (23.5) | |
| – | 21 (72.4) | 12 (54.5) | 33 (64.7) | |
|
Blood |
++ | 0 (0.0) | 1 (4.5) | 1 (2.0) |
| + | 1 (3.4) | 4 (18.2) | 5 (9.8) | |
| – | 28 (96.6) | 17 (77.3) | 45 (88.2) | |
|
Specific Gravity |
1.000 | 2 (6.9) | 1 (4.5) | 3 (5.9) |
| 1.005 | 8 (27.6) | 6 (27.3) | 14 (27.5) | |
| 1.010 | 4 (13.8) | 3 (13.6) | 7 (13.7) | |
| 1.015 | 15 (51.7) | 12 (54.5) | 27 (52.9) | |
Keys: -: Negative; +: Present in low amount; ++: Present in high amount; PYC: Pale yellow and clear; PYT: Pale yellow and turbid; YT: Yellow and turbid; CC: Colourless and clear; AC: Amber and clear; AC: Amber and turbid.
The socio-demographic characteristics and risk factors of subjects are presented in Table 3. Of the 51 MSU samples from subjects, 35.3 % had Candida isolates growth, indicating candiduria, while 64.7 % had no growth of Candida isolates. Of 18 subjects with candiduria, 9(50.0%) were males and 9(50.0 %) were females, giving a gender ratio of 1:1. The hypertensive subjects had higher prevalence of candiduria (8/20; 40.0%) than non-hypertensive subjects (10/31; 32.3%). The prevalence of candiduria was lower among subjects with type I diabetes, family history of diabetes and duration of diabetes < 5 yrs than among subjects with type 2 diabetes, duration of diabetes ≥ 5 yrs and without family history of diabetes. There was no statistically significant relationship between the prevalence of candiduria among subjects with respect to age (c2 = 1.14; p = 0.286), marital status (p = 0.921), occupations (p = 0.869), educational status (p = 0.693), type of diabetes (p = 0.534), duration of diabetes (p = 0.510), family history of diabetes (p = 0.530) and alcohol consumption (p = 0.534). The prevalence of candiduria was higher among subjects that smoked tobacco (42.9%) than non-tobacco smokers (34.1%), but there was no statistically significant difference (c2 = 0.203, p = 0.652) (Table 3).
Table (3):
Socio-Demographic Characteristics and Risk Factors of Diabetic Patients.
| Socio-Demographic Characteristics /Risk Factors | No of Samples Collected | No (%) of Subjects | χ2 | p-value | |
|---|---|---|---|---|---|
| with Candiduria | without Candiduria | ||||
| Age (yrs) | |||||
| < 40 | 13 | 3 (23.1) | 10 (76.9) | 1.14 | 0.286 |
| ≥ 40 | 38 | 15 (39.5) | 23 (60.5) | ||
| Gender | |||||
| Male | 29 | 9 (31.0) | 20 (69.0) | 0.534 | 0.465 |
| Female | 22 | 9 (40.9) | 13 (59.1) | ||
| Marital Status | |||||
| Single | 7 | 2 (28.6) | 5 (71.4) | ||
| Married | 30 | 11 (36.7) | 19 (63.3) | 0.164 | 0.921 |
| Widowed / Divorced | 14 | 5 (35.7) | 9 (62.3) | ||
| Occupation | |||||
| Teaching | 8 | 2 (25.0) | 6 (75.0) | ||
| Civil / Public Service | 21 | 7 (33.3) | 14 (66.7) | 0.717 | 0.869 |
| Self -Employed | 12 | 5 (41.7) | 7 (58.3) | ||
| Unemployed | 10 | 4 (40.0) | 6 (60.0) | ||
| Educational Status | |||||
| Primary | 10 | 4 (40.0) | 6 (60.0) | ||
| Secondary | 9 | 3 (33.3) | 6 (66.7) | 1.45 | 0.693 |
| Tertiary | 19 | 5 (26.3) | 14 (73.7) | ||
| None | 13 | 6 (46.2) | 7 (53.8) | ||
| Type of Diabetes | |||||
| Type 1 | 17 | 5 (29.4) | 12 (70.6) | 0.386 | 0.534 |
| Type 2 | 34 | 13 (38.2) | 21 (61.8) | ||
| Duration of Diabetes | |||||
| < 5 yrs | 23 | 7 (30.4) | 16 (69.6) | 0.433 | 0.510 |
| ≥ 5 yrs | 28 | 11 (39.3) | 17 (60.7) | ||
| Family History of DM | |||||
| Yes | 11 | 3 (27.3) | 8 (72.7) | 0.395 | 0.530 |
| No | 40 | 15 (37.5) | 25 (62.5) | ||
| Alcohol Consumption | |||||
| Yes | 17 | 7 (41.2) | 10 (58.8) | 0.386 | 0.534 |
| No | 34 | 11 (32.4) | 23 (67.6) | ||
| Tobacco Intake | |||||
| Yes | 7 | 3 (42.9) | 4 (57.1) | 0.203 | 0.652 |
| No | 44 | 15 (34.1) | 29 (65.9) | ||
| Blood Pressure | |||||
| Hypertensive | 20 | 8 (40.0) | 12 (60.0) | 0.319 | 0.572 |
| Non-Hypertensive | 31 | 10 (32.3) | 21 (67.7) | ||
Keys: DM: Diabetes Mellitus
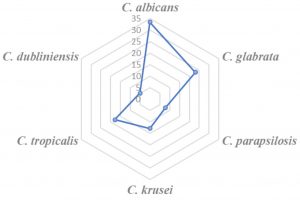
The percentage occurrence of Candida isolates in MSU samples of subjects in deceasing order was: C. albicans (33.3%) > C. glabrata (23.1%) > C. tropicalis (17.9%) > C. krusei (12.8%) > C. parapsilosis (7.7%) > C. dubliniensis (5.1 %) (Fig 1). With regards to total Candida isolates (n=39) obtained, subjects aged ≥ 40 yrs had 79.5 % isolates; subjects aged < 40 yrs had 20.5 % isolates; male subjects (46.2%) and female subjects (53.8%) isolates. The predominant candida isolate in female and male subjects was C. albicans and C. parapsilosis, respectively (Table 4). C. dubliniensis prevalence was found to be highest (100%) in subjects with random blood sugar (RBS) of ≥ 400 mg/dL, while C. krusei and C. parapsilosis was predominant candida isolate in subjects with RBS (mg/dL) of 200-299 and 300-399, respectively (Table 4).
Table (4):
Distribution of Candida Isolates Based on Age, Gender and RBS Values of Diabetic (Candiduric) Patients.
| Candida Isolates | No of Isolates | No (%) of Occurence | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (yrs) | Gender | Random Blood Sugar (mg/dL) | ||||||
| < 40 | ≥ 40 | Male | Female | 200-299 | 300-399 | ≥ 400 | ||
| C. albicans | 13 | 3 (23.1) | 10 (76.9) | 4 (30.8) | 9 (69.2) | 3 (23.1) | 4 (30.8) | 6 (46.2) |
| C. glabrata | 9 | 2 (22.2) | 7 (77.8) | 5 (55.6) | 4 (44.4) | 2 (22.2) | 5 (55.6) | 2 (22.2) |
| C. krusei | 3 | 0 (0.0) | 3 (100) | 1 (33.3) | 2 (66.7) | 3 (100) | 0 (0.0) | 0 (0.0) |
| C. parapsilosis | 5 | 2 (40.0) | 3 (60.0) | 3 (60.0) | 2 (40.0) | 0 (0.0) | 4 (80.0) | 1 (20.0) |
| C. tropicalis | 7 | 1 (14.3) | 6 (85.7) | 4 (57.1) | 3 (42.9) | 1 (14.3) | 2 (28.6) | 4 (57.1) |
| C. dubliniensis | 2 | 0 (0.0) | 2 (100) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 2 (100) |
| Total | 39 | 8 (20.5) | 31 (79.5) | 18 (46.2) | 21 (53.8) | 9 (23.1) | 15 (38.5) | 15 (38.5) |
Keys: RBS: Random Blood Sugar; Values in parentheses expressed the percentage of Candida isolates
The results showed that 64.1% Candida isolates were Fluconazole (FLU) sensitive, while 24.5% were FLU resistant (Table 5). Of the 39 Candida isolates, 71.8 %, 10.3 % and 17.9 % were sensitive, dose dependent susceptible (DDS) and resistant to Ketoconazole (KET) respectively;74.4% isolates were sensitive to Voriconazole (VOR), 12.8% were DDS, while 12.8% isolates were resistant. All (100%) C. dubliniensis were sensitive to Clotrimazole (CLO), KET and FLU; between 22.2% and 28.6% C. tropicalis and C. glabrata were resistant to Amphotericin B and Itraconazole (ITR); ≤ 23.1% C. parapsilosis and C. albicans were CLO resistant, while 33.3% C. krusei were DDS to VOR and ITR (Table 5).
Table (5):
Antifungal Susceptibility of Candida Isolates from Diabetic (Candiduric) Patients (n = 39).
| Antifungal Drug | Candida Isolates | Total No (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans | C. glabrata | C. krusei | C. parapsilosis | C. tropicalis | C. dubliniensis | |||
| No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | |||
|
Fluconazole |
S | 7 (53.8) | 6 (66.7) | 2 (66.7) | 4 (80.0) | 4 (57.1) | 2 (100) | 25 (64.1) |
| DDS | 3 (23.1) | 1 (11.1) | 0 (0.0) | 1 (20.0) | 1 (14.3) | 0 (0.0) | 6 (15.4) | |
| R | 3 (23.1) | 2 (22.2) | 1 (33.3) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 8 (20.5) | |
|
Ketoconazole |
S | 10 (76.9) | 6 (66.7) | 3 (100) | 3 (60.0) | 5 (71.4) | 1 (50.0) | 28 (71.8) |
| DDS | 0 (0.0) | 2 (22.2) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (50.0) | 4 (10.3) | |
| R | 3 (23.1) | 1 (11.1) | 0 (0.0) | 1 (20.0) | 2 (28.6) | 0 (0.0) | 7 (17.9) | |
|
Voriconazole |
S | 9 (69.2) | 8 (88.9) | 2 (66.7) | 3 (60.0) | 5 (71.4) | 2 (100) | 29 (74.4) |
| DDS | 1 (7.7) | 0 (0.0) | 1 (33.3) | 2 (40.0) | 1 (14.3) | 0 (0.0) | 5 (12.8) | |
| R | 3 (23.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 5 (12.8) | |
|
Amphotericin B |
S | 10 (76.9) | 6 (66.7) | 2 (66.7) | 4 (80.0) | 5 (71.4) | 1 (50.0) | 28 (71.8) |
| DDS | 0 (0.0) | 1 (11.1) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (50.0) | 3 (7.7) | |
| R | 3 (23.1) | 2 (22.2) | 1 (33.3) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 8 (20.5) | |
| Itraconazole | S | 11 (84.6) | 5 (55.6) | 2 (66.7) | 3 (60.0) | 4 (57.1) | 0 (0.0) | 25 (64.1) |
| DDS | 1 (7.7) | 2 (22.2) | 1 (33.3) | 0 (0.0) | 1 (14.3) | 1 (50.0) | 6 (15.4) | |
| R | 1 (7.7) | 2 (22.2) | 0 (0.0) | 2 (40.0) | 2 (28.6) | 1 (50.0) | 8 (20.5) | |
| Clotrimazole | S | 9 (69.2) | 8 (88.9) | 3 (100) | 3 (60.0) | 5 (71.4) | 2 (100) | 30 (76.9) |
| DDS | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (14.3) | 0 (0.0) | 3 (7.7) | |
| R | 3 (23.1) | 1 (11.1) | 0 (0.0) | 1 (20.0) | 1 (14.3) | 0 (0.0) | 6 (15.4) | |
Keys: S: Sensitive; DDS: Dose Dependent Susceptible; R: Resistant; Values in parentheses expressed the percentage of Candida isolates
Table 6 shows the distribution of virulence factors in Candida isolates from MSU samples of subjects. The percentage occurrence of each virulence factor was as follows: 38.5% amylase, 30.8% lipase, 25.6% coagulase and 23.1% biofilm. A higher prevalence, > 50%, was observed for haemolysin, phospholipase, gelatinase and caseinase (71.8%, 59.0%, 61.5% and 64.1%, respectively). Among candida isolates showing biofilm formation were C. albicans (n=5), C. glabrata (n=2), C. parapsilosis (n=1) and C. tropicalis (n=1) while none of the C. dubliniensis and C. krusei were amylase, coagulase and biofilm producers (Table 6).
Table (6):
Virulence Factors of Candida Isolates from Diabetic (Candiduric) Patients.
| Candida Isolates | No of Isolates | No (%) of Occurence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AMY | LIP | PHO | CAS | GEL | COA | HAE | BIO | ||
| C. albicans | 13 | 7 (53.8) | 5 (38.5) | 8 (61.5) | 10 (76.9) | 9 (69.2) | 6 (46.2) | 11 (84.6) | 5 (38.5) |
| C. glabrata | 9 | 4 (44.4) | 3 (33.3) | 5 (55.6) | 6 (66.7) | 5 (55.6) | 2 (22.2) | 5 (55.6) | 2 (22.2) |
| C. krusei | 3 | 0 (0.0) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 3 (100) | 0 (0.0) | 2 (66.7) | 0 (0.0) |
| C. parapsilosis | 5 | 1 (20.0) | 0 (0.0) | 2 (40.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 3 (60.0) | 1 (20.0) |
| C. tropicalis | 7 | 3 (42.9) | 2 (28.6) | 4 (57.1) | 4 (57.1) | 3 (42.9) | 1 (14.3) | 5 (71.4) | 1 (14.3) |
| C. dubliniensis | 2 | 0 (0.0) | 1 (50.0) | 2 (100) | 1 (50.0) | 2 (100) | 0 (0.0) | 2 (100) | 0 (0.0) |
| Total | 39 | 15 (38.5) | 12 (30.8) | 23 (59.0) | 25 (64.1) | 24 (61.5) | 10 (25.6) | 28 (71.8) | 9 (23.1) |
Keys: AMY: Amylase; LIP: Lipase; PHO: Phospholipase; CAS: Caseinase; GEL: Gelatinase; COA: Coagulase; BIO: Biofilm; HAE: Haemolysin; Values in parentheses expressed the percentage occurrences of Candida isolates.
Candida infections have increased vastly among immuno-compromised patients especially those with diabetic mellitus (DM). In this study, microscopic examinations of MSU of DM patients revealed the presence of epithelial cells, pus cells, yeast cells, crystals, hyaline casts and RBCs. The 13.7% MSU with epithelial cells and crystals obtained in this study was ≤ 27.5% epithelial cells and crystals obtained by Abebe et al. (2019) among DM patients at University of Gondar Hospital, Ethiopia. The presence of pus cells and RBCs in MSU had been reported by Akinjogunla and Divine-Anthony (2013) and Matulewicz and Meeks (2016), respectively. The presence of RBCs in urine may be attributed to lower UTI (cystitis), upper UTI (pyelonephritis) and cancer of the urinary tract, which are directly or indirectly related to DM (Matulewicz and Meeks, 2016). In our study, 25.5% DM patients had pus cells in their MSU and this value was < 35.5% reported by Abdulla et al. (2015). The pus cells are white blood cells that have succumbed in defense of the body against pathogens that invade it, therefore their presence indicates microbial infections. The MSU of DM patients in this study had specific gravity of ≤ 1.015 and also contained leukocytes, proteins, ketone, urobilinogen, blood, nitrites and bilirubin. The percentage of MSU with protein (19.6%), ketone (31.4%) and leukocytes (35.3%) in our study was higher than 11.3%, 4.5% and 24.9% for protein, ketone and leukocytes, respectively obtained by Abebe et al. (2019) among DM patients at University of Gondar Hospital, Ethiopia. The presence of nitrite has a predictive value for UTIs (Akinjogunla and Divine-Anthony, 2013) and the value obtained for nitrite (11.8%) in this study was lower than 21.5 % MSU with nitrite reported by Fernandes et al. (2018).
The prevalence of candiduria was more in subjects aged ≥ 40 than in subjects < 40 yrs and this substantiated the earlier report of Hassaneen et al. (2014) and Abebe et al. (2019) that candiduria was more frequent in elderly patients. Several reports have showed frequency of candiduria to be more in diabetic women than in diabetic men (Janifer et al., 2009; Abdulla et al., 2015) and this study similarly confirmed these reports. The hypertensive subjects had higher prevalence of candiduria than non-hypertensive subjects in our study and this was in conformity with a study conducted by Muller et al. (2007) in which diabetic patients were more at risk for candiduria / UTI than hypertensive patients without diabetes. Other predisposing factors such as duration of diabetes, family history of diabetes, alcohol in-take and tobacco consumption were observed to be associated with occurrence of candiduria in this study. The prevalence of candiduria was more in subjects with duration of diabetes ≥ 5 yrs than those subjects with duration of diabetes < 5 yrs and this was similar to the findings of Janifer et al. (2009).
Candida albicans, C. krusei, C. tropicalis, C. glabrata, C. dubliniensis and C. parapsilosis were isolated from MSU of DM patients in our study with random blood sugar ranging from 200 to ≥ 400 mg/dL. Isolation of non albicans Candida (NAC) from MSU of DM patients in our study corroborated the previous findings of Pandey and Pandey (2013) who reported the emergence of NAC in MSU of diabetic patients at Gwalior, India. The percentage occurrence of NAC (66.7%) in MSU of DM patients was higher than C. albicans (33.3%) and this agreed with the results of Ekpo et al. (2017) in Cameroon. Our observation also concurred with Kauffmann (2005) who reported that >50% of urinary Candida isolates were NAC.
The susceptibility of Candida spp to antifungal agents is not often predictable, hence, investigating pathogenic Candida isolates against appropriate antifungal agents is required for effective treatments of patients. In our study, 20.5% Fluconazole and 20.5% Itraconazole resistant Candida spp were obtained and these values were > 13% Fluconazole and 18.5% Itraconazole resistant Candida spp reported by Sojakova et al. (2004). The resistance of Candida spp to Ketoconazole in this study correlated with the reports of Hugo and Russell (2007). Over-expression of CtERG11 or CDR1/CDR2 gene (Sanglard and Odds, 2002), over-expression of efflux proteins which act by pumping the drug out of the cell at a rate faster rate than how the drug enters the cell (Akinjogunla and Eghafona, 2012) and modifications of target enzymes have been attributed to the resistance of Candida spp to azole group of antifungal agents (Silva et al., 2011). A high percentage of Amphotericin B sensitive C. albicans, C. parapsilosis and C. tropicalis was observed and this substantiated the findings of Metin et al. (2011).
The transition of Candida isolate from a harmless commensal to potent pathogen is determined by several host predisposing factors and virulence attributes of organisms. The pathogenicity of Candida isolates is attributable to secretion of extracellular hydrolytic enzymes that act synergistically under favourable conditions (Silva et al., 2011). The occurrence of haemolysin, lipase and phospholipase producing Candida spp in this study corroborated the reports of Saha (2018) and Akinjogunla et al. (2019). Phospholipase facilitates Candida invasion by damaging host cellular contents and cleaves phospholipids of host cell membrane (Deorukhkar et al., 2014). Haemolysin aids in lysis of host erythrocytes and strips iron from haemoglobin molecules (Manns et al. 1994). Candida albicans and NAC exhibited biofilm formation and the occurrence of biofilm producing Candida spp agreed with Seneviratne et al. (2008). In our study, 14.3% coagulase producing C. tropicalis obtained was similar to 14.6 % by Deorukhkar et al. (2014), but < 82.6% reported by Rodrigues et al. (2003). Coagulase binds plasma fibrinogen and activates a cascade of reactions that induce clotting of plasma (Rodrigues et al., 2003).
This study has shown the socio-demographic characteristics and risk factors in diabetic mellitus patients with or without candiduria; revealed the high sensitivity of Candida isolates to azole drugs and varied percentages of haemolysin, coagulase, biofilm, phospholipase and lipase producing Candida isolates in MSU samples and as well showed the necessity to continuously investigate pathogenic candida isolates against appropriate antifungal agents for effective treatments of asymptomatic candiduria in type 1 and 2 diabetes mellitus patients.
ACKNOWLEDGMENTS
The authors vastly acknowledged all the participants and support staff who participated in this study.
CONFLICT OF INTEREST
The authors declares that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Author AOJ designed the study, wrote the protocol and wrote the first draft of the manuscript. Authors DO and AYO wrote part of the manuscript and managed the analyses of the study. Authors EIU and EIJ managed the literature searches and performed the statistical analysis. All authors read and approved the final manuscript.
FUNDING
None.
ETHICS STATEMENT
The study protocol was in accordance with ethical procedures and recommendations of State Hospitals Management Boards guidelines.
AVAILABILITY OF DATA
The dataset used and/or analyzed during the current study are included in the manuscript.
- Abdulla MC, Jenner FP, Alungal J. Urinary tract infection in type 2 diabetic patients: risk factors and antimicrobial pattern. J Res Med Sci. 2015;3(10):2576-2579.
- Abebe M, Adane T, Kefyalew K, et al. Variation of urine parameters among diabetic patients: A cross-sectional study. Ethiopians Journal of Health Sciences. 2019;29 (1): 877-882.
- Akinjogunla OJ, Odeyemi AT, Olasehinde GI. Epidemiological studies of urinary tract infection (UTI) among post-menopausal women in Uyo Metropolis, South-South, Nigeria. Journal of American Science. 2010;6(12):1674-1681.
- Akinjogunla OJ, Divine-Anthony O. Asymptomatic bacteriuria among apparently healthyundergraduate students in Uyo, South-South, Nigeria. Annual Review and Research in Biology. 2013;3(3):213-225.
- Akinjogunla OJ, Eghafona NO. Mycological investigation in patients with acute otitis media. Scientific Journal of Microbiology. 2012;1(1):19-26.
- Akinjogunla OJ, Divine-Anthony O, Akpan MM, Ogbonna FC. Virulence markers and antifungal susceptibility of vaginal yeast isolates from contraceptive and non-contraceptive users in Uyo, Nigeria. Journal of Experimental Research. 2019;7(1):33- 40.
- Akinjogunla OJ, Divine-Anthony O, Nwadialor J. Candidaisolates from high vaginal swabs of women of child bearing age: species distributions, virulence factors and antifungal susceptibility profile, Nigerian Journal of Scientific Research. 2017;16(4):50-58.
- Beers MH, Porter RS, Jones TV, Kaplan JL, Berkwits M. The Merck Manual of Diagnosis and Therapy. 18th edition. New Jersey: Merck Research Laboratories. 2006;1-85
- Chakrabarti A, Ghosh A, Batra R, Kaushal A, Roy P, H Singh H. Antifungal susceptibility pattern of non-albicans Candida species and distribution of species isolated from candidaemia cases over 5-year period. Indian J Microbiol. 1996;104:171-176.
- Clinical Laboratory Standards Institute. Method for antifungal disk diffusion Susceptibility testing of Yeasts; Approved Guideline-(2nd Ed). CLSI document M44-A2. Clinical Laboratory Standard Institute, Wayne: Pennsylvania. 2009.
- Deorukhkar SC, Saini S, Mathew S. Virulence factors contributing to pathogenicity of Candida tropicalis and its antifungal susceptibility profile. International Journal of Microbiology. 2014;(ID 456878):1-6.
Crossref - Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167-193.
Crossref - Ekpo IT, Kechia FA, Iwewe YS, Ngueguim AD, Nangwat NC, Dzoyem JP. Species distribution and antifungal susceptibility profile of Candida spp isolated from urine of hospitalized patients in Dschang District Hospital, Cameroon. International Journal Biological and Chemical Sciences. 2017;11(3):1212-1221.
Crossref - Fernandes DJ, Jaidev MD, Dipthi NC. Utility of dipstick test (nitrite and leukocyte esterase) and microscopic analysis of urine when compared to culture in the diagnosis of urinary tract infection in children. International Journal of Contemporary Pediatrics. 2018;5(1):156-160.
Crossref - Francois LM, Duncan W, Bernhard H. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119-128.
Crossref - Hassaneen AM, Ghonaim RA, Hassanin HM, Salama NA, Elgohary T. Different aspects of candiduria as an important nosocomial infection. Medical Journal of Cairo University. 2014;82(1):199-204.
- Hu FB. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249-1257.
Crossref - Hugo WB, Russell AD. Pharmaceutical Microbiology, (7th Ed). Blackwell Science. 2007;481.
- Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian Journal of Nephrology. 2009;19:107-111.
Crossref - Kauffman CA. Candiduria. Clinical Infectious Diseases. 2005;41(Suppl 6):S371-376.
Crossref - Lotfi-Kamran MH, Jafari AA, Falah-Tafti A, Tavakoli E, Falahzadeh MH. Candida colonization on the denture of diabetic and non-diabetic patients. Dental Research Journal. 2009;6(1):23-27.
- Maahs D, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of Type 1 Diabetes. Endocrinology and Metabolism Clinics of North America. 2010;39(3):481-497.
Crossref - Manns JM, Mosser DM, Buckley HR. Production of a haemolytic factor by Candida albicans. Infect Immun. 1994;62:5154-5156.
Crossref - Matulewicz RS, Meeks JJ. Blood in the urine (Hematuria). JAMA. 2016;316(14):1-2.
Crossref - Metin DY, Hilmioglu-Polat S, Samlioglu P, Doganay-Oflazoglu B, Inci R, Tumbay E. Evaluation of antifungal susceptibility testing with microdilution and Etest methods of Candida blood isolates. Mycopathologia. 2011;172(3):187-199.
Crossref - Mukhtar Y, Galalain AM, Yunusa UM. A modern overview on diabetes mellitus: a chronic endocrine disorder. European Journal of Biology. 2019; 4(1):1-14.
- Muller V, Viemann D, Schmidt M, et al. Candida albicans triggers activation of distinct signaling pathways to establish a proinflammatory gene expression program in primary human endothelial cells. Journal of Immunology. 2007;179:8435-8445.
Crossref - Pandey M, Pandey A. Emergence of Non-albicans Candida in urine of diabetic patients at Gwalior (M.P.), India. IOSR Journal of Dental and Medical Sciences. 2013;4(5):11-14.
Crossref - Rodrigues AG, Pina-Vaz C, Costa-de-Oliveira S, Tavares C. Expression of plasma coagulase among pathogenic Candida Species. J Clin Microbiol. 2003;41(12):5792-5793.
Crossref - Saha S. Species distribution, virulence factors and antifungal susceptibility profile of Candida isolates from various clinical samples. Indian Journal of Basic and Applied Medical Research. 2018;7(3):206-212.
- Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2:73-85.
Crossref - Seneviratne CJ, Jin LJ, Samaranayake YH, Samaranayake LP. Cell density and cell aging as factors modulating antifungal resistance of Candida albicansbiofilms. Antimicrob Agents Chemother. 2008;52:3259-3266.
Crossref - Silva S, Negri M, Henriques M, Oliveira R, David WW, Azeredo J. Adherence and biofilm formation of non-Candida albicans species. Trends in Microbiology. 2011;19(5):241-247.
Crossref - Sobel JD, Fisher JF, Kauffman CA, Newman CA. Candida urinary tract infections: epidemiology. Clin Infect Dis. 2011;52(6):S433-S436.
Crossref - Sojakova M, Liptajova D, Borovsky M, Subik J. Fluconazole and itraconazole susceptibility of vaginal yeast isolates from Slovakia. Mycopathologia. 2004;157:163-169.
Crossref - Soll DR. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183-203.
Crossref - Talaro KP, Talaro A. Foundations in Microbiology. (4th edn). McGraw-Hill companies, Inc, New York. 2002;899.
- Yashavanth R, Shiju M, Bhaskar U, Ronald R, Anita K. Candiduria: prevalence and trends in antifungal susceptibility in a tertiary care hospital of Mangalore. Journal of Clinical Diagnostics Research. 2013;7(11):2459-2461.
- Zahra N, Rehman K, Aqeel R, Parveen A, Aka MSM. Assessment of urinary tract infection and their resistance to antibiotics in diabetic and non-diabetic patients. Bangabandhu Sheikh Mujib Medical University Journal. 2016;9:151-155.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.