ISSN: 0973-7510
E-ISSN: 2581-690X
Aims of the study were to compare liver markers in T2DM patients with that in non-diabetic healthy volunteers and also to find the correlation between insulin resistance (IR) and liver markers. The objective of the study was also to find out whether PON1 can be an alternative liver marker. The cross-sectional study was conducted in the Clinical Biochemistry laboratory. 114 type 2 DM patients in the age group 18-65 years, diagnosed as per ADA guidelines were recruited in the study.100 age and gender-matched non-diabetics, healthy volunteers or those having health packages were taken as controls. The blood sample was collected and fasting blood glucose, transaminases and Alkaline phosphatase, bilirubin (total and direct), total protein, albumin, and insulin were assayed. HOMA-IR was calculated. Statistical analysis was done by using SPSS 16. A significant elevation was seen in AST, ALT, ALP, GGT, TB, DB, TP, A: G ratio in diabetics. A lowered albumin and A: G ratio were observed in diabetics as compared to controls. Fasting insulin levels were 1.7 times higher in diabetics compared controls, suggesting hyperinsulinemia in cases. Homeostatic model for assessment of insulin resistance (insulin based) was 2.7 times greater in T2DM compared to controls. A significant positive correlation was found between insulin levels and total and direct bilirubin, (r=0.279, P=0.003, and r=0.233, P=0.014 respectively). ALP, total and direct bilirubin had a significant positive correlation with HOMA-IR (r=0.228, P=0.033,; r=0.231,P=0.030 ; r=0.242. P=0.023 respectively). A very significant negative correlation was found between albumin and HOMA- IR (r= -0.306, P=0.004). A significant positive correlation was observed between PON1 and HOMA-IR (P=0.000), PON and insulin (P=0.015). It can be concluded from that diabetics had high liver enzymes as compared to non-diabetics. An association was found between T2DM, liver markers, and IR. It was observed that PON1 was not a good liver marker in T2DM.
Insulin resistance, diabetes mellitus, liver markers, Paraoxanase 1.
Liver disease is reported to be one of the important causes of death in diabetes mellitus (DM). A report by De Marco et al suggests that cirrhosis accounted for 4.4% of diabetes-related deaths, in a population based study1. A study by Balkau B et al reported that cirrhosis was the cause of deaths in DM in 12.5% of population2. Various reports suggest that diabetes has merged as one of the commonest causes of liver disease. A spectrum of liver disorders can occur in DM. Trombetta et al suggest that prevalence of diabetes in cirrhotics is 12.3 -57%3. These suggest a higher prevalence of DM in liver diseases. The relationship between diabetes mellitus and liver disorders are yet to be established in our settings. Since this is a less explored area, we focused to establish an association between liver markers, insulin resistance (IR) and T2DM.
The rationale of the study: As IR is associated with DM as well as liver disorders, it is justifiable to measure liver markers in diabetics. As traditional liver markers proved to be nonspecific in identifying liver disorders, there is a need to explore, a better non-invasive marker. Hence this is an attempt to find out whether paraoxonase 1(PON1) can be a better marker compared to traditional liver markers. It is very much essential to establish a relationship between insulin resistance, liver markers, and diabetes mellitus.
IR and DM
IR is a condition where cells are non-responsive to insulin. Insulin resistance is associated with T2DM4.
IR and liver disorders
IR is independently associated with NAFLD and a close association was found between NAFLD and metabolic syndrome5. NAFLD is in turn consistently associated with DM.
Since IR is associated with both DM and liver disorders, liver markers could be elevated in DM.
DM & Liver markers
A clinical trials report suggests that serum transaminases or alkaline phosphatase were 1-2.5 times elevated in type 2 DM6. In a retrospective study, we found ALT and AST were 1.3 and 1.4 times respectively higher in diabetics. It has been suggested that diabetics may be more prone for alterations of liver enzymes7. Enhanced activity of the liver enzymes is associated with IR.
From the literature review, an association between IR &DM, DM & liver disorders, liver disorders, and IR is evident. However, the cause and effect relationship between these is not well established. This necessitates a study which explores an association between insulin resistance and liver markers. It has been widely accepted that standard biochemical tests which assess liver functions have low sensitivities. Histopatho-logical study of liver biopsy specimen is the gold standard. Invasive procedure and complications are its limitations. Hence, an accurate, reliable, and noninvasive hepatic marker is needed. Under such circumstances parameter of choice appears to be paraoxanase 1 (PON1), which originates from the liver and its gene expression is confined to the liver.
PON1 is an antioxidant enzyme, associated with DM, IR as well as liver disorders.
PON1, IR, and DM
PON1 is HDL bound antioxidant, found to be significantly reduced in diabetics with insulin resistance. It has also been suggested that PON1 activity is positively correlated to IR, as assessed by HOMA index8.
PON1 & liver disorders
PON1 has been reported to be reduced significantly in acute viral hepatitis, chronic hepatitis, cirrhosis, and sepsis9. These findings suggest that PON1 may serve as a useful additional marker in the evaluation of liver conditions.
All the published data available are international, there are only a few Indian studies which focus on IR, liver markers and PON1 to the best of our knowledge.
Objectives
Aims of the study were to
compare liver markers in T2DM patients with that in non-diabetic healthy volunteers
find the correlation between insulin resistance (insulin based) and liver markers
find out the effectiveness of PON1 activity as a liver biomarker as compared to traditional liver parameters.
Study design
The cross-sectional study was carried out in the Department of Biochemistry, KS Hegde Medical Academy, Mangalore, Karnataka. Institutional ethics committee approval was obtained to conduct the study. Informed consent was taken from the study subjects.
Inclusion criteria
114 type 2 diabetics in the age group of 18-65 years, diagnosed as per ADA 2016 guidelines were included as cases. Hundred age and gender-matched nondiabetics, healthy volunteers were considered as controls.
Exclusion criteria
Alcoholics, diagnosed cases of acute and chronic hepatitis, other liver disorders.
Sample collection and analysis
Five ml of fasting venous blood sample was collected using aseptic precaution. The blood sample was centrifuged at 3000rpm for 20 min and serum was separated. Fasting blood glucose, AST, ALT, ALP, GGT, bilirubin, total protein and albumin were estimated using fully automated chemistry analyzer, Cobas C-311.
Insulin levels were assayed using hormone analyzer, Cobas e411 which works on the principle of electrochemiluminescence.
Insulin resistance was calculated by the homeostasis model assessment (HOMA).
HOMA -IR= fasting glucose X fasting insulin/22.5; insulin expressed in ל U/L, glucose in mmol/l.
PON1 activity was assayed using the spectrophotometric method 10.
Statistical analysis
Statistical analysis was done using the software, SPSS version 16.
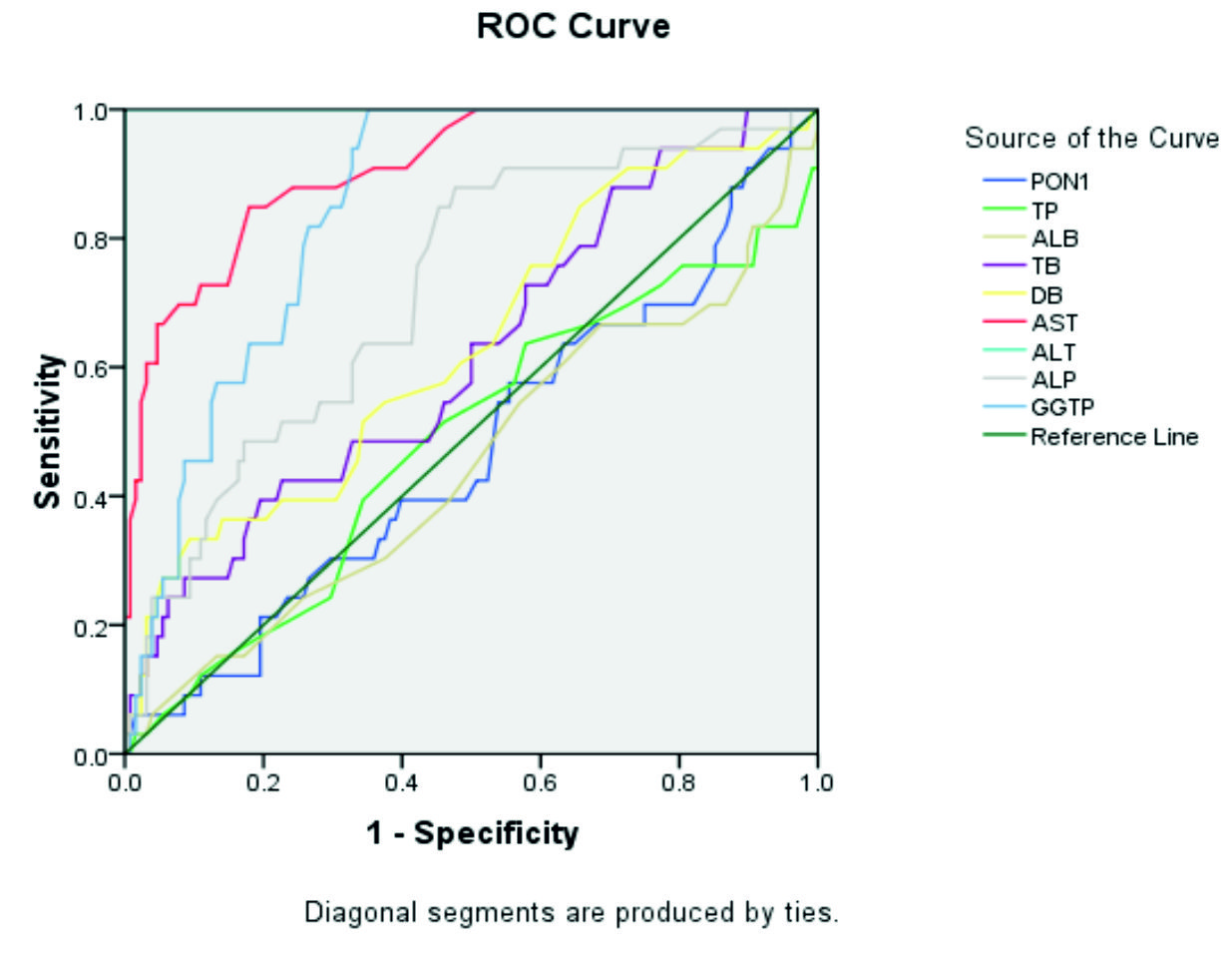
Mann Whitney U test was applied to compare liver markers in diabetics and non-diabetics. Spearman’s correlation coefficient was used to find the correlation between liver markers and insulin resistance. Receiver Operative characteristic curve (ROC) was constructed to find out whether PON1 can be used as an alternative liver marker.
Insulin levels were raised 1.76 times in diabetics compared to controls. In liver profile, total and direct bilirubin, liver enzymes like AST, ALT, ALP, GGT were higher 1.2, 1.12, 1.63, 1.43, 1.09, 1.59 times respectively in cases compared to controls. Albumin levels were decreased and total protein and globulins were increased significantly in cases compared to control. Homeostatic model for assessment of insulin resistance was 2.7 times higher in T2DM.
Insulin level showed a significant positive correlation with total and direct bilirubin, ( r=0.279, P=0.003 and r=0.233, P=0.014 respectively). ALP, total and direct bilirubin had a significant positive correlation with HOMA-IR (r=0.228, P=0.033; r=0.231,P=0.030; r=0.242. P=0.023 respectively). Albumin and HOMA- IR showed a very significant negative correlation (r= -0.306, P=0.004).
A significant increase in Bilirubin, liver transaminases and total proteins were observed in diabetics compared to non-diabetics (Table 1).
Table (1):
Comparison of Liver markers and insulin resistance in diabetics and non-diabetics
CASES(T2DM) |
CONTROLS (Non diabetics) |
P VALUE |
|
|---|---|---|---|
INSULIN (μU/L) |
20.51±3.37 |
11.66±1.34 |
0.001** |
TP (G/dl) |
7.47±0.06 |
7.22±0.06 |
0.01* |
ALB(G/dl) |
4.1±0.04 |
4.2±0.057 |
0.026* |
Globulin (G/dl) |
3.2 ± 0.78 |
1.4± 0.49 |
0.000** |
A:G ratio |
1.29±0.03 |
1.48±0.03 |
<0.0001** |
TB (mg/dl) |
0.94±0.066 |
0.78±0.96 |
0.026* |
DB (mg/dl) |
0.37±0.03 |
0.33±0.05 |
0.016* |
AST (U/L) |
52.28±5.75 |
32.1±3.6 |
0.0179* |
ALT (U/L) |
40.17±3.74 |
28.1±3.845 |
0.0001*** |
ALP (IU/L) |
94.54± 2.96 |
86.5± 3.91 |
0.04* |
GGTP(U/L) |
68.09±13.44 |
42.89±5.2 |
0.011* |
GLU (mg/dl) |
192.7±9.12 |
105.97±2.29 |
0.000*** |
GLU (mmol/l) |
10.69±0.50 |
5.88±0.12 |
0.000*** |
HOMA IR |
8.17±1.25 |
3.01±0.36 |
0.000*** |
PON 1 (nmol/ml/min) |
0.84±0.03 |
0.69±0.04 |
0.003** |
*P<0.05 is significant
**P< 0.01 highly significant
***P<0.001 very highly significant
When the utility of PON1 as a biomarker of liver disease, it was observed that , PON1 was not a good liver marker in T2DM.Liver transaminases, especially ALT and AST were good markers ( AUC =1 and 0.908 respectively whereas that for PON1 was 0.472).GGT was a better marker compared to ALP (AUC 0.848 VS 0.720) .
PON1 was found to bear a significant positive correlation with HOMA-IR and insulin (P=0.000 and P=0.015 respectively).
Elevation of ALT is commonly reported in patients with type 2 diabetes, while uncommon in apparently normal subjects5. A clinical trial by Belcher et al suggests that 2- 24% of screened type 2 DM patients had transaminase levels higher than normal limit11. Another report by Lebovitz et al involving multiple clinical trials with DM suggests that diabetics had higher levels of serum transaminases and ALP6. The liver plays a key role in the carbohydrate metabolism and plasma glucose maintainance. It is the key organ for glycogenesis and gluconeogenesis. This role of the liver makes it susceptible in DM12.
ALP and bilirubin showed a significant positive correlation with HOMA-IR. This finding is supported by the increased activity of the liver enzymes associated with Insulin resistance13. The relationship between diabetes mellitus and liver diseases, the cause and effect aspect are yet to be established. This is the less explored area in the field of research in our settings.
In our previous study, serum transaminases levels were in the normal range, but AST levels were 1.3 times high in diabetes patients as compared to non-diabetics. ALT levels were 1.4 times high in diabetics. These findings suggest that diabetics may be more prone for an altered hepatic transaminases9. However our previous study had a few limitations that insulin resistance was not studied.
There are several studies which suggest an elevation in serum transaminases in diabetics. In a clinical trial report by Yamada et al, hepatic enzymes were 1-2.5 times in DM. Serum ALT values were 1 – 2.5 times higher than normal range in 5.6% patients8. A mild elevations of transaminases in asymptomatic individuals could be due to fatty liver disease or chronic hepatitis13. Non-alcoholic fatty liver disease is the most common cause of a mild elevation of serum ALT and it is the most prevalent hepatic disorder in T2DM14,15. Findings of our study are supported by a review report by Paola et al, which opined that type 2 diabetics are more prone for non-alcoholic steatohepatitis (NASH), even though they have a normal liver enzyme levels16. Comparatively elevated hepatic enzymes suggest a probable risk of hepatic disorder in the future. As the histopathology of liver biopsy specimens were not analyzed in the study, it is not possible to specify whether a fatty change is involved or which type of liver disease is likely.
An elevation in total proteins and a decline in albumin levels in diabetics found in the study could be attributed to a low rate of synthesis of albumin due to insulin deficiency. A study by Rehman et al also reported a lowered albumin levels in diabetes mellitus patients17. A study by Mohammed et al observed an elevated total proteins in diabetics18.
Our previous study report suggests an elevation of total protein in diabetics as compared to non-diabetics. Globulin was extremely significantly high in diabetics. Lowered albumin levels found in diabetics was insignificant. A/G ratio was lowered in an extremely significant manner in diabetics19.
Comparatively elevated total proteins observed in our present study is supported by various reports20,21. This could be due to the elevation of various acute phase proteins, fibrinogen, and globulins in T2DM which in turn rise plasma proteins. Studies suggest an elevation in acute phase proteins CRP, a1-acid glycoprotein, plasminogen, complement C3, ceruloplasmin in type 2 diabetes mellitus22-25. Elevated fibrinogen levels in diabetics could be attributed to increased hepatic synthesis25. A study by Ardavi and colleagues reported a hypergamma-globulinemia in diabetes mellitus26.
Albumin had a significant negative correlation with C-peptide based insulin resistance in the present study. Studies reported that an elevated albumin level was associated with IR27-33. However we couldnot establish an independent association of albumin on the development of diabetes. Although the causal relationship between IR and serum albumin levels is not clear, our results suggest that IR may affect serum albumin levels. IR is by definition associated to hyperinsulinemia which was observed in our study34.
ROC curve is the graphical plot which is used to compare the diagnostic ability of two diagnostic tests. If a ROC curve follows the left-hand border and top border of ROC space, it suggests that the test could be accurate. If an area under the curve (AUC) equal to 1, it suggests that the test is perfect. If AUC lies between 0.9 -1, the test is said to be excellent. The AUC value of 0.80-0.90 suggests a good accuracy.
Based on our results, transaminases have an excellent area under the curve implying that they are the better markers of liver disease compared to PON1 which has the AUC of 0.472.AUC for GGT is in the acceptable range (AUC = 0.848).
Our finding is in contradictory to the reports by Pyati et al, which compared the diagnostic accuracy of PON1 versus routine liver markers. The study showed that PON1 had an area under the curve 0.990 which was in agreement with the other parameters like, ALT (AUC = 0.999), TB (AUC = 0.977) and ALP (AUC = 0.904)(35).
However, a significant increase (1.25 times) in PON1 levels was observed in diabetics compared to non-diabetics. Our results are in agreement with the study by Suvarna et al, which reported an elevated PON1 in uncomplicated diabetics compared to non-diabetics36. PON1 is an antioxidant enzyme, it’s elevation could be a compensatory increase so as to fight the enhanced oxidative stress in diabetics.
A positive correlation was established between the Homeostasis Model Assessment (HOMA) index and PON1 activity in non-diabetic Japanese subjects by Yamada et al.8 Tabur et al reported in Turkish population that PON1 activities were not different between non-diabetic subjects with and without metabolic syndrome37.
Beer et al reported that PON1 concentration and activity were same in diabetic patients, impaired glucose tolerant patients and non-diabetics. However postprandial hyper-lipidemia was associated with alterations in PON1 activity in diabetic subjects38. In the same study, it was observed that there was no difference in the postprandial PON1 response between diabetics and non-diabetics. Kopprasch et al suggested that PON1 activity was not significantly different in normoglycemic subjects, glucose-intolerant subjects and newly ascer-tained diabetics39. It may be concluded from these studies that PON1 activity may be lost in the course of diabetes mellitus and hyperglycemia, rather than in the initial stage of IR. Studies suggest a significantly lowered serum PON1 activity in diabetics compared to the healthy subjects40-42.
A study by Pyati et al suggests that PON1 activity has a better diagnostic accuracy compared to other liver markers. It also reports that assay of PON1 activity may improve the efficiency significantly, of a laboratory’s evaluation system35. However, we could not establish the role of PON1 as an effective liver marker in predicting liver diseases associated with T2DM.
Table (2):
Area under the curve for PON1 and liver markers
Test Variable |
The area under the curve |
|---|---|
AST |
0.908 |
ALT |
1 |
ALP |
0.720 |
GGT |
0.848 |
TB |
0.617 |
DB |
0.634 |
TP |
0.484 |
Albumin |
0.450 |
PON1 |
0.472 |
It could be concluded from the study that diabetics had elevated serum transaminases, bilirubin and total protein as compared to non-diabetic controls. An association was found between type 2 diabetes mellitus, liver markers and insulin resistance. Paraoxonase 1 activity may not be a good marker to predict liver disease in diabetes mellitus.
Acknowledgements
We sincerely thank Dr. Sukanya Shetty, HOD Biochemistry for the support during the course of this work.
Conflict Of Interest
The authors declare that there is no conflict of interest.
Authors’ Contribution
UA designed the study, wrote the protocol and did statistical analysis. KP helped in sample collection. NP helped in standardization of procedure. UA wrote the manuscript.
Funding
The study was supported by grant no:NUFR1/2017/06/05 dated 10.06.2017 from NITTE-Deemed to be University, Mangaluru, Karnataka, India.
Data Availability
Data was collected from Department of Biochemistry, KS Hegde Medical Academy, NITTE-Deemed to be University, Mangaluru, Karnataka, India. All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
The study was approved by Institutional Ethics committee of KS Hegde Medical Academy, NITTE-Deemed to be University, Mangaluru, Karnataka, India. Approval Number: INST.EC/EC/021/2017-18.
- De Marco R., Locatelli F., Zoppini G., Verlato G., Bonora E., Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care, 1999; 22(5): 756-61.
- Balkau B., Eschwטge E., Ducimetiטre P., Richard J.L., Warnet J.M. The high risk of death by alcohol related diseases in subjects diagnosed as diabetic and impaired glucose tolerant: the Paris Prospective Study after 15 years of follow-up. J. Clin. Epidemiol., 1991; 44(6): 465-74.
- Trombetta M., Spiazzi G., Zoppini G., Muggeo M. Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study. Aliment Pharmacol. Ther., 2005; 22(2): 24-7.
- Olokoba A.B., Obateru O.A.,Olokoba L.B. Type 2 diabetes mellitus: A review of current trends. Oman medical Journal, 2012; 27(4): 269-273.
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R. et al. Non-alcoholic fatty liver, steato hepatitis and the metabolic syndrome, 2003; 37: 917-923.
- Lebovitz H.E., Kreider M., Freed M.I. Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care, 2002; 25(5): 815-21
- Adiga U., Malawadi B.N. Alterations in liver enzymes in type 2 diabetes mellitus. Journal of Basic and Applied Medical Sciences, 2016; 3(1): 13-16.
- Yamada A., Shoji T., Tahara H., Emoto M., Nishizawa Y. Effect of insulin resistance on serum paraoxanase activity in non diabetic poplation. Metabolism, 2001; 50(7): 805-11.
- Kedage V., Manjunath S., Shetty M., Suvarna R., Soumya S. et al. Serum PON 1 activity status in patients with liver disorders. Saudi J. of gastroenterology, 2010; 16(2): 79-83.
- Ferre N., Camps J., Prats E., Vilella E., Paul A. Serum paraoxonaseactivity:a new additional test for the improved evaluation of chronic liver disease.Clin. chem., 2002; 48(2): 261-8.
- Belcher G., Schernthaner G. Changes in liver tests during 1-year treatment of patients with Type 2 diabetes with pioglitazone, metformin or gliclazide. Diabet Med., 2005; 22(8): 973-9.
- Levinthal GN, TavillAJ. Liver disease and diabetes mellitus. Clin. Diabetes, 1999; 17: 73.
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A.J., Natale S., Forlani G., Melchionda N. Non-alcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes, 2001; 50(8): 1844-50.
- Hultcrantz R., Glaumann H., Lindberg G., Nilsson L.H. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J. Gastroenterol, 1986; 21(1): 109-13.
- Harris M.I., Flegal K.M., Cowie C.C., Eberhardt M.S., Goldstein D.E., Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care, 1998; 21(4): 518-24.
- Paola P.S.,Kenneth C. Treatment of NAFLD in patients with type 2 diabetes mellitus. Clinical Diabetes & Endocrinology, 2016; 2: 9.
- Rehman A., Zamir S., Bhatti A., Jan S.S., Ali S., Wazir F. Evaluation of albuminuria, total plasma proteins and serum albumin in diabetics. Gomal. J. Med. Sci., 2012; 10: 198-200.
- Taj Mohammad, Akbar Khoja, Khem A. Karira, Abdur Harman. Comparison of Plasma Protein Concentration and Hematological parameters in type-1 and type-2 Diabetics of short and long duration. Med. channel, 2001; 7(4): 51-4.
- B.N. Malawadi, Usha Adiga. Plasma Proteins in Type 2 Diabetes Mellitus. IOSR Journal of Biotechnology and Biochemistry, 2016; 2(5): 1-3.
- Al Ghamdi K. Micro albuminuria among patients with diabetes type 1 and type 2 at the Armed Forces Hospital in Jubail. Annals Saudi Med., 2001; 21: 236-8.
- Kawai T., Clinical Aspects of the Plasma Proteins. J.B. Lippincott, Philadelphia, 1973, section IV.
- Sun T, Lien Y Y, Gross S. Clinical application of a high-resolution electrophoresis system, Ann. Clin. Lab. Sci., 1978; 8: 219.
- Ritchie R.F. Automated nephelometric analysis apecific serum proteins: clinical applications, in Protides of the Biological Fluids (21st colloquium 1973), Peeters, H., Ed., Pergamon Press, Oxford, 1974, 593.
- Laurell C.B. Is Eletrophoretic analysis of plasma proteins becoming out-dated? Scand. J. Clin. Lab. Invest., 1972; 30: 233.
- Coppola G., Corrado E., Tantillo R., Vitale G., Lo Coco L., Novo S. Increased levels of C-reactive protein and fibrinogen influence the risk of vascular events in patients with NIDDM. Int. J. Cardiol., 2006; 106(1): 16-20.
- Ardavi M.S., Nasrat H.A., Bahnassy A.A. Serum Immunoglobulin concentrations in diabetic patients. Diabetic Med., 1994; 11: 384-87.
- Sohee Kim, Shinae Kang.Serum albumin levels: A simple answer to complex Problem?Are we on right track of assessing metabolic syndrome. Endocrinol. Metab., 2013; 18(1): 17-19
- Ji Cheol Bae, Sung Hwan Seo, Kyu Yeon Hur, Jae Hyeon Kim, Myung-Shik Lee, Moon Kyu Lee, Won Young Lee, Eun Jung Rhee, Ki Won Oh. Association between Serum Albumin, Insulin Resistance, and Incident Diabetes in Nondiabetic Subjects. Endocrinol. Metab., 2013; 28: 26-32.enm
- Kahn C.R. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes, 1994; 43: 1066-84.
- Ishizaka N., Ishizaka Y., Nagai R., Toda E., Hashimoto H., Yamakado M. Association between serum albumin, carotid atherosclerosis, and metabolic syndrome in Japanese individuals. Atherosclerosis, 2007; 193: 373-9.
- Hostmark A.T., Tomten S.E., Berg .JE. Serum albumin and blood pressure: a population-based, cross-sectional study. J. Hypertens, 2005; 23: 725-30.
- Danesh J., Muir J., Wong Y.K., Ward M., Gallimore J.R., Pepys M.B. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J., 1999; 20: 954-9.
- Saito I., Yonemasu K., Inami F. Association of body mass index, body fat, and weight gain with inflammation markers among rural residents in Japan. Circ J., 2003; 67: 323-9.
- Shanik M.H., Xu Y., Skrha J., Dankner R., Zick Y., Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care, 2008; 31 (Suppl 2): S262-8.
- Pyati A.K., Halappa C.K., Pyati S.A., Nagaraj, Wali V. Serum Basal Paraoxonase 1 Activity as an Additional Liver Function Test for the Evaluation of Patients with Chronic Hepatitis. JCDR 2015; 9(11): BC12-BC15.
- Suvarna R., Rao S, Joshi C., Kedage V., Muttigi M.S., Shetty J.K., Mungli P. Paraoxonase Activity In Type 2 Diabetes Mellitus Patients With And Without Complications. Journal of Clinical and Diagnostic Research, 2011; 5(1): 63-65.
- Tabur S., Torun A.N., Sabuncu T., Turan M.N., Celik H., Ocak A.R., et al. Nondiabetic metabolic syndrome and obesity do not affect serum paraoxonase and arylesterase activities but do affect stress and inflammation. Eur. J. Endocrinol., 2010; 162: 535-41.
- Beer S., Moren X., Ruiz J., James R.W. Postprandial modulation of serum paraoxonase activity and concentration in diabetic and non-diabetic subjects. Nutr. Metab. Cardiovasc. Dis., 2006; 16: 457-65.
- Kopprasch S., Pietzsch J., Kuhlisch E., Graessler J. Lack of association between paraoxonase 1 activities and increased oxidized low-density lipoprotein levels in impaired glucose tolerance and newly diagnosed diabetes mellitus. J. Clin. Endocrinol. Metab., 2003; 88: 1711-6.
- Ferretti G., Bacchetti T., Busni D., Rabini R.A., Curatola G. Protective effect of paraoxonase activity in high-density lipoproteins against erythrocyte membranes peroxidation: A comparison between healthy subjects and type 1 diabetic patients. J. Clin. Endocrinol. Metab., 2004; 89: 2957-62.
- Karabina S.A., Lehner A.N., Franke E., Parthasara S., Santanam N. Oxidative inactivation of para-oxonase-implications in diabetes mellitus and atherosclerosis. Biochim Biophys Acta, 2005; 1725: 21321.
- Flekac M., Skrha J., Zidkova K., Lacinova Z., Hilgertova J. Paraoxonase 1 gene polymorphisms and enzyme activities in diabetes mellitus. Physiol Res., 2008;57: 717-26.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.